Drug Information
Drug (ID: DG00069) and It's Reported Resistant Information
| Name |
Fluorouracil
|
||||
|---|---|---|---|---|---|
| Synonyms |
5-Fluorouracil; 51-21-8; fluorouracil; 5-FU; Fluoroplex; Adrucil; Efudex; Carac; Fluracil; Fluoroblastin; 5-fluoropyrimidine-2,4(1H,3H)-dione; Kecimeton; Timazin; Carzonal; Efudix; Arumel; Fluril; Queroplex; Fluracilum; Ulup; 5-Fluoracil; Phthoruracil; Fluro Uracil; 5-Fluoro-2,4(1H,3H)-pyrimidinedione; Ftoruracil; Fluorouracilum; Efurix; Fluri; 5 Fluorouracil; Effluderm (free base); 5-fluoro-1H-pyrimidine-2,4-dione; Fluorouracilo; Fluroblastin; Phtoruracil; 2,4-Dihydroxy-5-fluoropyrimidine; 2,4(1H,3H)-Pyrimidinedione, 5-fluoro-; Adrucil; Effluderm; Fluorouracile; Fluoruracil; Fluracedyl; Flurodex; Neofluor; Onkofluor; Ribofluor; Tetratogen; URF; Allergan Brand of Fluorouracil; Biosyn Brand of Fluorouracil; CSP Brand of Fluorouracil; Cinco FU; Dakota Brand of Fluorouracil; Dermatech Brand of Fluorouracil; Dermik Brandof Fluorouracil; Ferrer Brand of Fluorouracil; Fluoro Uracile ICN; Fluorouracil GRY; Fluorouracil Mononitrate; Fluorouracil Monopotassium Salt; Fluorouracil Monosodium Salt; Fluorouracil Potassium Salt; Fluorouracil Teva Brand; Fluorouracile Dakota; Fluorouracile [DCIT]; Fluorouracilo Ferrer Far; Gry Brand of Fluorouracil; Haemato Brand of Fluorouracil; Haemato fu; Hexal Brand of Fluorouracil; ICN Brand of Fluorouracil; Inhibits thymilidate synthetase; Medac Brand of Fluorouracil; Neocorp Brand of Fluorouracil; Onkoworks Brand of Fluorouracil; Ribosepharm Brand of Fluorouracil; Riemser Brand of Fluorouracil; Roche Brand of Fluorouracil; Teva Brand of Fluorouracil; F 6627; F0151; IN1335; U 8953; Adrucil (TN); Carac (TN); Dakota, Fluorouracile; Efudex (TN); Fluoro-Uracile ICN; Fluoro-uracile; Fluoro-uracilo; Fluoroplex (TN); Fluorouracil-GRY; Fluorouracilo [INN-Spanish]; Fluorouracilum [INN-Latin]; Haemato-fu; Ro 2-9757; U-8953; Ro-2-9757; Fluorouracil (JP15/USP/INN); Fluorouracil [USAN:INN:BAN:JAN]; 1-fluoro-1h-pyrimidine-2,4-dione; 2,4-Dioxo-5-fluoropryimidine; 2,4-Dioxo-5-fluoropyrimidine; 5 FU Lederle; 5 FU medac; 5 Fluorouracil biosyn; 5 HU Hexal; 5-FU (TN); 5-FU Lederle; 5-FU medac; 5-Faracil; 5-Fluor-2,4(1H,3H)-pyrimidindion; 5-Fluor-2,4(1H,3H)-pyrimidindion [Czech]; 5-Fluor-2,4-dihydroxypyrimidin; 5-Fluor-2,4-dihydroxypyrimidin [Czech]; 5-Fluor-2,4-pyrimidindiol; 5-Fluor-2,4-pyrimidindiol [Czech]; 5-Fluoracil [German]; 5-Fluoracyl; 5-Fluoro-2,4-pyrimidinedione; 5-Fluoropyrimidin-2,4-diol; 5-Fluoropyrimidine-2,4-dione; 5-Fluorouracil-biosyn; 5-Fluoruracil; 5-Fluoruracil [German]; 5-Ftouracyl; 5-HU Hexal; 5-fluoro uracil; 5FU
Click to Show/Hide
|
||||
| Indication |
In total 2 Indication(s)
|
||||
| Structure |
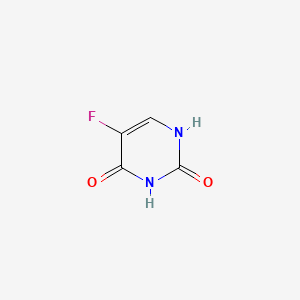
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Clinically Reported Resistance for This Drug
(11 diseases)
[3]
[5]
[6]
[7]
[8]
[9]
[10]
[11]
[13]
[14]
[15]
Disease(s) with Resistance Information Discovered by Cell Line Test for This Drug
(13 diseases)
[12]
[16]
[17]
[18]
[19]
[20]
[21]
[22]
[23]
[24]
[17]
[25]
|
||||
| Target | Candida Thymidylate synthase (Candi TMP1) | TYSY_CANAL | [1] | ||
| Dihydrothymine dehydrogenase (DPYD) | DPYD_HUMAN | [1] | |||
| TERT messenger RNA (TERT mRNA) | TERT_HUMAN | [1] | |||
| Thymidylate synthase messenger RNA (TYMS mRNA) | TYSY_HUMAN | [1] | |||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
C4H3FN2O2
|
||||
| IsoSMILES |
C1=C(C(=O)NC(=O)N1)F
|
||||
| InChI |
1S/C4H3FN2O2/c5-2-1-6-4(9)7-3(2)8/h1H,(H2,6,7,8,9)
|
||||
| InChIKey |
GHASVSINZRGABV-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
| VARIDT ID | |||||
| INTEDE ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-02: Benign/in-situ/malignant neoplasm
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Mast/stem cell growth factor receptor Kit (KIT) | [26] | |||
| Sensitive Disease | Colorectal cancer [ICD-11: 2B91.1] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Colorectal cancer [ICD-11: 2B91] | |||
| The Specified Disease | Colorectal cancer | |||
| The Studied Tissue | Blood | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 9.75E-30 Fold-change: -3.66E-01 Z-score: -1.26E+01 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell migration | Inhibition | hsa04670 | ||
| Cell proliferation | Inhibition | hsa05200 | ||
| In Vitro Model | SW480 cells | Colon | Homo sapiens (Human) | CVCL_0546 |
| DLD1 cells | Colon | Homo sapiens (Human) | CVCL_0248 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
A real-time cell analyzer assay | |||
| Mechanism Description | c-KIT was shown to mediate chemo-resistance (kike 5-FU) in ovarian tumor initiating cells, miR-34a inhibits Erk signaling and colony formation by down-regulation of c-kit, miR-34a can inhibit this effect via down-regulation of c-kit and therefore sensitize cells to chemotherapeutic treatment. | |||
| Key Molecule: Neurogenic locus notch homolog protein 1 (NOTCH1) | [27] | |||
| Sensitive Disease | Colorectal cancer [ICD-11: 2B91.1] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Colorectal cancer [ICD-11: 2B91] | |||
| The Specified Disease | Colorectal cancer | |||
| The Studied Tissue | Blood | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 3.67E-06 Fold-change: -9.64E-02 Z-score: -4.72E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell proliferation | Inhibition | hsa05200 | ||
| IGF-1R/AKT/S6 signaling pathway | Inhibition | hsa05225 | ||
| In Vitro Model | HCT116 cells | Colon | Homo sapiens (Human) | CVCL_0291 |
| HCT-8 cells | Colon | Homo sapiens (Human) | CVCL_2478 | |
| LOVO cells | Colon | Homo sapiens (Human) | CVCL_0399 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | Ectopic expression of miR-139-5p sensitized CRC cells to 5-FU by increasing 5-FU-induced apoptosis. In addition, miR-139-5p inhibited the expression of the miR-139-5p target gene NOTCH-1 and its downstream molecules MRP-1 and BCL-2, two key MDR-associated genes. Furthermore, silencing NOTCH-1 expression promoted the chemotherapeutic effects of 5-FU, and up-regulation of NOTCH-1 abrogated miR-139-5p-mediated sensitization to 5-FU in LoVo and HCT-116 cells. | |||
| Key Molecule: Neurogenic locus notch homolog protein 1 (NOTCH1) | [29] | |||
| Sensitive Disease | Colorectal carcinoma [ICD-11: 2B91.3] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Colorectal cancer [ICD-11: 2B91] | |||
| The Specified Disease | Colorectal carcinoma | |||
| The Studied Tissue | Blood | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 3.67E-06 Fold-change: -9.64E-02 Z-score: -4.72E+00 |
|||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | HCT116 cells | Colon | Homo sapiens (Human) | CVCL_0291 |
| HCT8 cells | Colon | Homo sapiens (Human) | CVCL_2478 | |
| In Vivo Model | Mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay; Colony formation assay | |||
| Mechanism Description | miR139-5p reverses CD44+/CD133+-associated multidrug resistance by downregulating NOTCH1 in colorectal carcinoma cells. | |||
| Key Molecule: Cadherin-1 (CDH1) | [30] | |||
| Sensitive Disease | Colorectal cancer [ICD-11: 2B91.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Colorectal cancer [ICD-11: 2B91] | |||
| The Specified Disease | Colorectal cancer | |||
| The Studied Tissue | Blood | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 8.45E-01 Fold-change: 6.54E-03 Z-score: 1.95E-01 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | AKT signaling pathway | Inhibition | hsa04151 | |
| In Vitro Model | HCT116 cells | Colon | Homo sapiens (Human) | CVCL_0291 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay; Annexin V/ PI staining; Caspase-3 activity assay | |||
| Mechanism Description | Levels of PTEN and E-cadherin were reduced by knockdown of miR200c in HCT-116 cells, PTEN inactivate the AkT signaling pathway, and E-cadherin is one of the major downstream regulators of miRNA-200c contributing to EMT, which is also important to inhibit tumor invasion and proliferation as well as to induce cell apoptosis. | |||
|
|
||||
| Key Molecule: AT-rich interactive domain-containing protein 4B (ARID4B) | [28] | |||
| Sensitive Disease | Colorectal cancer [ICD-11: 2B91.1] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Colorectal cancer [ICD-11: 2B91] | |||
| The Specified Disease | Colorectal cancer | |||
| The Studied Tissue | Blood | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 2.69E-05 Fold-change: -1.63E-01 Z-score: -4.24E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell proliferation | Inhibition | hsa05200 | ||
| Cell viability | Inhibition | hsa05200 | ||
| In Vitro Model | SW480 cells | Colon | Homo sapiens (Human) | CVCL_0546 |
| HCT116 cells | Colon | Homo sapiens (Human) | CVCL_0291 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay; Flow cytometry assay | |||
| Mechanism Description | miR-519b-3p mimics promoted HCT116 and SW480 cells more sensitive to chemoradiation treatment while ectopic expression of ARID4B in the meantime decreased the sensitivity. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Metalloproteinase inhibitor 3 (TIMP3) | [31] | |||
| Sensitive Disease | Renal carcinoma [ICD-11: 2C90.2] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Kidney cancer [ICD-11: 2C90] | |||
| The Specified Disease | Renal carcinoma | |||
| The Studied Tissue | Blood | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.59E-02 Fold-change: 2.68E+00 Z-score: 3.16E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | PI3K/AKT signaling pathway | Inhibition | hsa04151 | |
| In Vitro Model | 786-O cells | Kidney | Homo sapiens (Human) | CVCL_1051 |
| ACHN cells | Pleural effusion | Homo sapiens (Human) | CVCL_1067 | |
| HK-2 cells | Kidney | Homo sapiens (Human) | CVCL_0302 | |
| RCC10 cells | Kidney | Homo sapiens (Human) | CVCL_6265 | |
| RCC4 cells | Kidney | Homo sapiens (Human) | CVCL_0498 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Celltiter96 Aqueous Non Radioactive Cell Proliferation Assay | |||
| Mechanism Description | Tumor suppressor genes like PTEN, PDCD4 and TIMP3, are target genes of miR21. PTEN is a potent inhibitor of PI3k/Akt pathway, as well as a direct target of miR21. | |||
| Key Molecule: Wee1-like protein kinase (WEE1) | [36] | |||
| Sensitive Disease | Kidney cancer [ICD-11: 2C90.1] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Kidney cancer [ICD-11: 2C90] | |||
| The Specified Disease | Kidney cancer | |||
| The Studied Tissue | Kidney | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.32E-08 Fold-change: -6.91E-01 Z-score: -6.01E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell proliferation | Inhibition | hsa05200 | ||
| WEE1/Cdc2 signaling pathway | Activation | hsa04110 | ||
| In Vitro Model | 786-O cells | Kidney | Homo sapiens (Human) | CVCL_1051 |
| HK-2 cells | Kidney | Homo sapiens (Human) | CVCL_0302 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miR-381 increases sensitivity of 786-O cells to 5-FU by inhibitory WEE1 and increase of Cdc2activity. | |||
| Key Molecule: Programmed cell death protein 4 (PDCD4) | [31] | |||
| Sensitive Disease | Renal carcinoma [ICD-11: 2C90.2] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Kidney cancer [ICD-11: 2C90] | |||
| The Specified Disease | Renal carcinoma | |||
| The Studied Tissue | Kidney | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 8.62E-01 Fold-change: 6.66E-03 Z-score: 1.74E-01 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | PI3K/AKT signaling pathway | Inhibition | hsa04151 | |
| In Vitro Model | 786-O cells | Kidney | Homo sapiens (Human) | CVCL_1051 |
| ACHN cells | Pleural effusion | Homo sapiens (Human) | CVCL_1067 | |
| HK-2 cells | Kidney | Homo sapiens (Human) | CVCL_0302 | |
| RCC10 cells | Kidney | Homo sapiens (Human) | CVCL_6265 | |
| RCC4 cells | Kidney | Homo sapiens (Human) | CVCL_0498 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Celltiter96 Aqueous Non Radioactive Cell Proliferation Assay | |||
| Mechanism Description | Tumor suppressor genes like PTEN, PDCD4 and TIMP3, are target genes of miR21. PTEN is a potent inhibitor of PI3k/Akt pathway, as well as a direct target of miR21. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: SET and MYND domain containing 2 (SMYD2) | [10] | |||
| Resistant Disease | Kidney cancer [ICD-11: 2C90.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Kidney cancer [ICD-11: 2C90] | |||
| The Specified Disease | Kidney cancer | |||
| The Studied Tissue | Kidney | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 8.07E-35 Fold-change: 6.59E-01 Z-score: 1.61E+01 |
|||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | HEK293T cells | Kidney | Homo sapiens (Human) | CVCL_0063 |
| HK-2 cells | Kidney | Homo sapiens (Human) | CVCL_0302 | |
| In Vivo Model | Balb/c athymic nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blotting assay | |||
| Experiment for Drug Resistance |
MTS assay | |||
| Mechanism Description | SMYD2 is a histone methyltransferase.The estimated IC50 values of cisplatin, doxorubicin, or 5-FU (but not docetaxel) for AZ505-treated RCC cells were significantly lower than those for the control cells, indicating that the SMYD2 inhibition enhanced the drug sensitivity in renal cancer cells. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Proprotein convertase subtilisin/kexin type 9 (PCSK9) | [32] | |||
| Metabolic Type | Lipid metabolism | |||
| Resistant Disease | Hepatocellular carcinoma [ICD-11: 2C12.02] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Liver cancer [ICD-11: 2C12] | |||
| The Specified Disease | Hepatocellular carcinoma | |||
| The Studied Tissue | Liver tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 5.96E-09 Fold-change: 4.92E-01 Z-score: 5.99E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cholesterol metabolism | Activation | hsa04979 | |
| In Vivo Model | Hepa1-6 hepatocellular carcinoma transplanted tumor model mice | Mice | ||
| Experiment for Molecule Alteration |
Western blot analysis and immunohistochemical assays | |||
| Experiment for Drug Resistance |
Tumor volume assay | |||
| Mechanism Description | ARBU significantly inhibited the proliferation of Hepa1-6 in vivo and in vitro, regulated cholesterol metabolism, and promoted the M1-type polarization of macrophages in the tumor microenvironment. ARBU inhibits cholesterol synthesis in the TME through the PCSK9/LDL-R signaling pathway, thereby blocking macrophage M2 polarization, promoting apoptosis of the tumor cells, and inhibiting their proliferation and migration. | |||
| Key Molecule: Proprotein convertase subtilisin/kexin type 9 (PCSK9) | [32] | |||
| Metabolic Type | Lipid metabolism | |||
| Resistant Disease | Hepatocellular carcinoma [ICD-11: 2C12.02] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Liver cancer [ICD-11: 2C12] | |||
| The Specified Disease | Hepatocellular carcinoma | |||
| The Studied Tissue | Liver tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 5.96E-09 Fold-change: 4.92E-01 Z-score: 5.99E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cholesterol metabolism | Activation | hsa04979 | |
| In Vitro Model | Hepa1-6 cells | Liver | Mus musculus (Mouse) | CVCL_0327 |
| Experiment for Molecule Alteration |
Western blot analysis and immunohistochemical assays | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | ARBU significantly inhibited the proliferation of Hepa1-6 in vivo and in vitro, regulated cholesterol metabolism, and promoted the M1-type polarization of macrophages in the tumor microenvironment. ARBU inhibits cholesterol synthesis in the TME through the PCSK9/LDL-R signaling pathway, thereby blocking macrophage M2 polarization, promoting apoptosis of the tumor cells, and inhibiting their proliferation and migration. | |||
|
|
||||
| Key Molecule: Ubiquitin carboxyl-terminal hydrolase 22 (USP22) | [41] | |||
| Resistant Disease | Hepatocellular carcinoma [ICD-11: 2C12.2] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Liver cancer [ICD-11: 2C12] | |||
| The Specified Disease | Hepatocellular carcinoma | |||
| The Studied Tissue | Liver tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.48E-08 Fold-change: 3.19E-01 Z-score: 5.81E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Huh-7 cells | Liver | Homo sapiens (Human) | CVCL_0336 |
| HepG2 cells | Liver | Homo sapiens (Human) | CVCL_0027 | |
| Hep3B cells | Liver | Homo sapiens (Human) | CVCL_0326 | |
| SMMC7721 cells | Uterus | Homo sapiens (Human) | CVCL_0534 | |
| PLC cells | Liver | Homo sapiens (Human) | CVCL_0485 | |
| L02 cells | Liver | Homo sapiens (Human) | CVCL_6926 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | miR6825-5p, miR6845-5p and miR6886-3p could decrease the level of USP22 protein by binding to the 3'-untranlated region of USP22 mRNA. All the three microRNAs (miRNAs) were downregulated by HULC, which resulted in the elevation of USP22. The pathway 'HULC/USP22/Sirt1/ protective autophagy' attenuates the sensitivity of HCC cells to chemotherapeutic agents. | |||
| Key Molecule: High mobility group protein HMGI-C (HMGA2) | [43] | |||
| Resistant Disease | Liver cancer [ICD-11: 2C12.6] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Liver cancer [ICD-11: 2C12] | |||
| The Specified Disease | Liver cancer | |||
| The Studied Tissue | Liver tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 2.13E-05 Fold-change: 1.02E+00 Z-score: 4.33E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell proliferation | Activation | hsa05200 | ||
| In Vitro Model | BEL-7402 cells | Liver | Homo sapiens (Human) | CVCL_5492 |
| HepG2 cells | Liver | Homo sapiens (Human) | CVCL_0027 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
RT-qPCR; Luciferase activity assay | |||
| Experiment for Drug Resistance |
MTT assay; Flow cytometry assay | |||
| Mechanism Description | Downregulated LncRNA CRNDE could up-regulate miR-33a expression and inhibit HMGA2 expression, thus it could significantly promote apoptosis of liver cancer drug-resistant cells on different chemotherapeutic drugs (ADM, DDP, 5-FU)and inhibit its proliferation, migration, invasion and drug resistance. | |||
| Key Molecule: Nuclear receptor subfamily 2 group C2 (NR2C2) | [44] | |||
| Resistant Disease | Hepatocellular carcinoma [ICD-11: 2C12.2] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Liver cancer [ICD-11: 2C12] | |||
| The Specified Disease | Liver cancer | |||
| The Studied Tissue | Liver tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 2.03E-05 Fold-change: 7.29E-02 Z-score: 4.71E+00 |
|||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell proliferation | Activation | hsa05200 | ||
| In Vitro Model | Huh-7 cells | Liver | Homo sapiens (Human) | CVCL_0336 |
| BEL-7402 cells | Liver | Homo sapiens (Human) | CVCL_5492 | |
| SMMC7721 cells | Uterus | Homo sapiens (Human) | CVCL_0534 | |
| Skhep1 cells | Liver | Homo sapiens (Human) | CVCL_0525 | |
| MHCC97-H cells | Liver | Homo sapiens (Human) | CVCL_4972 | |
| HCC-LM3 cells | Liver | Homo sapiens (Human) | CVCL_6832 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8; Flow cytometry assay; EdU assay | |||
| Mechanism Description | Ectopic expression of SNHG6-003 in HCC cells promoted cell proliferation and induced drug resistance, whereas SNHG6-003 knockdown promoted apoptosis. Moreover, SNHG6-003 functioned as a competitive endogenous RNA (ceRNA), effectively becoming sponge for miR-26a/b and thereby modulating the expression of transforming growth factor-beta-activated kinase 1 (TAk1). Importantly, expression analysis revealed that both SNHG6-003 and TAk1 were upregulated in human cancers, exhibiting a co-expression pattern. In HCC patients, high expression of SNHG6-003 closely correlated with tumor progression and shorter survival. | |||
| Key Molecule: Eukaryotic translation initiation factor 4E (EIF4E) | [58] | |||
| Resistant Disease | Hepatocellular carcinoma [ICD-11: 2C12.2] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Liver cancer [ICD-11: 2C12] | |||
| The Specified Disease | Liver cancer | |||
| The Studied Tissue | Liver tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 3.85E-01 Fold-change: -1.87E-02 Z-score: -8.76E-01 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | BEL-7402 cells | Liver | Homo sapiens (Human) | CVCL_5492 |
| HepG2 cells | Liver | Homo sapiens (Human) | CVCL_0027 | |
| SMMC7721 cells | Uterus | Homo sapiens (Human) | CVCL_0534 | |
| L02 cells | Liver | Homo sapiens (Human) | CVCL_6926 | |
| Experiment for Molecule Alteration |
Western blot analysis; RT-qPCR | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miR503 inhibits proliferation making human hepatocellular carcinoma cells susceptible to 5 fluorouracil by targeting EIF4E. | |||
| Key Molecule: Dual specificity protein phosphatase 6 (DUSP6) | [65] | |||
| Resistant Disease | Hepatocellular carcinoma [ICD-11: 2C12.2] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Liver cancer [ICD-11: 2C12] | |||
| The Specified Disease | Liver cancer | |||
| The Studied Tissue | Liver tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 5.96E-02 Fold-change: -3.20E-02 Z-score: -1.92E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Hep3B cells | Liver | Homo sapiens (Human) | CVCL_0326 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTS assay; Flow cytometric analysis; Colony forming assay | |||
| Mechanism Description | miR200a-3p enhances anti-cancer drug resistance by decreasing DUSP6 expression. | |||
| Key Molecule: Phosphatase and tensin homolog (PTEN) | [11] | |||
| Resistant Disease | Hepatocellular carcinoma [ICD-11: 2C12.2] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Liver cancer [ICD-11: 2C12] | |||
| The Specified Disease | Liver cancer | |||
| The Studied Tissue | Liver tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.86E-04 Fold-change: -4.60E-02 Z-score: -3.95E+00 |
|||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell viability | Activation | hsa05200 | |
| In Vitro Model | Huh-7 cells | Liver | Homo sapiens (Human) | CVCL_0336 |
| HepG2 cells | Liver | Homo sapiens (Human) | CVCL_0027 | |
| PLC/PRF/5 cells | Liver | Homo sapiens (Human) | CVCL_0485 | |
| HLE cells | Liver | Homo sapiens (Human) | CVCL_1281 | |
| HLF cells | Liver | Homo sapiens (Human) | CVCL_2947 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Hepatocellular carcinoma cells transfected with pre-miR-21 were significantly resistant to IFN-alpha/5-FU. Transfection of anti-miR-21 rendered HCC cells sensitive to IFN-alpha/5-FU, and such sensitivity was weakened by transfection of siRNAs of target molecules, PETN and PDCD4, miR-21 induces chemoresistance to IFN-alpha and 5-FU, mediated through PETN and PDCD4. | |||
|
|
||||
| Key Molecule: Small nucleolar RNA host gene 6 (SNHG6) | [44] | |||
| Resistant Disease | Hepatocellular carcinoma [ICD-11: 2C12.2] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Liver cancer [ICD-11: 2C12] | |||
| The Specified Disease | Liver hepatocellular carcinoma | |||
| The Studied Tissue | Liver | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 9.03E-20 Fold-change: 1.59E+00 Z-score: 9.61E+00 |
|||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell proliferation | Activation | hsa05200 | ||
| In Vitro Model | Huh-7 cells | Liver | Homo sapiens (Human) | CVCL_0336 |
| BEL-7402 cells | Liver | Homo sapiens (Human) | CVCL_5492 | |
| SMMC7721 cells | Uterus | Homo sapiens (Human) | CVCL_0534 | |
| Skhep1 cells | Liver | Homo sapiens (Human) | CVCL_0525 | |
| MHCC97-H cells | Liver | Homo sapiens (Human) | CVCL_4972 | |
| HCC-LM3 cells | Liver | Homo sapiens (Human) | CVCL_6832 | |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
CCK8; Flow cytometry assay; EdU assay | |||
| Mechanism Description | Ectopic expression of SNHG6-003 in HCC cells promoted cell proliferation and induced drug resistance, whereas SNHG6-003 knockdown promoted apoptosis. Moreover, SNHG6-003 functioned as a competitive endogenous RNA (ceRNA), effectively becoming sponge for miR-26a/b and thereby modulating the expression of transforming growth factor-beta-activated kinase 1 (TAk1). Importantly, expression analysis revealed that both SNHG6-003 and TAk1 were upregulated in human cancers, exhibiting a co-expression pattern. In HCC patients, high expression of SNHG6-003 closely correlated with tumor progression and shorter survival. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Bcl-2-associated agonist of cell death (BAD) | [39], [40] | |||
| Sensitive Disease | Hepatocellular carcinoma [ICD-11: 2C12.2] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Liver cancer [ICD-11: 2C12] | |||
| The Specified Disease | Hepatocellular carcinoma | |||
| The Studied Tissue | Liver tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 3.19E-09 Fold-change: -2.62E-01 Z-score: -6.15E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell proliferation | Inhibition | hsa05200 | ||
| Mitochondrial signaling pathway | Activation | hsa04217 | ||
| In Vitro Model | BEL-7402 cells | Liver | Homo sapiens (Human) | CVCL_5492 |
| HepG2 cells | Liver | Homo sapiens (Human) | CVCL_0027 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay; MTT assay | |||
| Mechanism Description | Let-7b increased 5 FU sensitivity by repressing Bcl xl expression in HCC cells. And miR-133a and miR-326 share a common target gene, Bcl-xl. Expression levels of miR-133a and miR-326 are significantly upregulated subsequent to transfection. miR-133a and miR-326 downregulate the mRNA expression of Bcl-xl. miR-133a and miR-326 sensitize HepG2 cells to 5-FU and DDP. | |||
| Key Molecule: Kelch-like ECH-associated protein 1 (KEAP1) | [47] | |||
| Sensitive Disease | Hepatocellular carcinoma [ICD-11: 2C12.2] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Liver cancer [ICD-11: 2C12] | |||
| The Specified Disease | Liver cancer | |||
| The Studied Tissue | Liver tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.51E-03 Fold-change: 3.81E-02 Z-score: 3.34E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell viability | Inhibition | hsa05200 | ||
| Nrf2 signaling pathway | Inhibition | hsa05208 | ||
| In Vitro Model | HepG2 cells | Liver | Homo sapiens (Human) | CVCL_0027 |
| SMMC7721 cells | Uterus | Homo sapiens (Human) | CVCL_0534 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay; Flow cytometry assay | |||
| Mechanism Description | Cells with kRAL overexpression exhibited a reversal in the resistance against 5-FU, with a significant decrease in the IC50 and a dramatic increase in cellular apoptosis, while silencing keap1 or ectopically expressing miR-141 partially rescued this effect. | |||
| Key Molecule: High mobility group protein HMGI-C (HMGA2) | [59] | |||
| Sensitive Disease | Hepatocellular carcinoma [ICD-11: 2C12.2] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Liver cancer [ICD-11: 2C12] | |||
| The Specified Disease | Liver cancer | |||
| The Studied Tissue | Liver tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 3.56E-02 Fold-change: -2.08E-02 Z-score: -2.15E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell colony | Activation | hsa05200 | ||
| Cell cycle | Activation | hsa04110 | ||
| In Vitro Model | Bel-7402/5-Fu cells | Liver | Homo sapiens (Human) | CVCL_5493 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay; Flow cytometry assay | |||
| Mechanism Description | Let-7g microRNA contributed to an increase of 5-Fu-induced cell cycle inhibit in human hepatoma cell and sensitized cells to 5-Fu, leading to increased the effectiveness of the drug in treating hepatoma cancer. | |||
| Key Molecule: Bcl-2-like protein 2 (BCL2L2) | [64] | |||
| Sensitive Disease | Hepatocellular carcinoma [ICD-11: 2C12.2] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Liver cancer [ICD-11: 2C12] | |||
| The Specified Disease | Liver cancer | |||
| The Studied Tissue | Liver tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 9.79E-03 Fold-change: -3.17E-02 Z-score: -2.69E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | BEL-7402 cells | Liver | Homo sapiens (Human) | CVCL_5492 |
| Bel-7402/5-Fu cells | Liver | Homo sapiens (Human) | CVCL_5493 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miR-195 antisense oligonucleotide induced drug resistance in BEL-7402/5-FU cells. miR-195 overexpression repressed Bcl-w protein level. miR-195, one of the down-regulated miRNAs in BEL-7402/5-FU cells, was demonstrated to play a role in the development of drug resistance in hepatocellular carcinoma cells by targeting the antiapoptotic gene, Bcl-w. | |||
| Key Molecule: Suppressor of cytokine signaling 6 (SOCS6) | [67] | |||
| Sensitive Disease | Hepatocellular cancer [ICD-11: 2C12.4] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Liver cancer [ICD-11: 2C12] | |||
| The Specified Disease | Liver cancer | |||
| The Studied Tissue | Liver tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 3.24E-04 Fold-change: -7.85E-02 Z-score: -3.85E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell proliferation | Inhibition | hsa05200 | |
| miR183/IDH2/SOCS6/HIF1alpha feedback loop signaling pathway | Regulation | N.A. | ||
| In Vitro Model | BEL-7402 cells | Liver | Homo sapiens (Human) | CVCL_5492 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | IDH2 knockdown resulted in significantly increased HIF-1alpha expression in both BEL-7402 and BEL-7402/5-FU cells. knockdown of SOCS6 had similar but stronger effect as miR-183 in promoting MRP2, P-gp, p-STAT3 and HIF-1alpha expression in BEL-7402 cells, while SOCS6 overexpression also showed similar but stronger effect as miR-183 inhibition in reducing MRP2, P-gp, p-STAT3 and HIF-1alpha levels in BEL-7402/5-FU cells. Both SOCS6 overexpression and miR-183 knockdown significantly increased the sensitivity of BEL-7402/5-FU cells to 5-FU. miR-183 overexpression partly abrogated the effect of SOCS6 in enhancing 5-FU sensitivity. | |||
|
|
||||
| Key Molecule: Metastasis associated lung adenocarcinoma transcript 1 (MALAT1) | [52] | |||
| Sensitive Disease | Liver cancer [ICD-11: 2C12.6] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Liver cancer [ICD-11: 2C12] | |||
| The Specified Disease | Cholangiocarcinoma | |||
| The Studied Tissue | Bile duct | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.43E-07 Fold-change: 2.16E+00 Z-score: 6.36E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell colony | Activation | hsa05200 | ||
| Cell viability | Activation | hsa05200 | ||
| In Vitro Model | Huh-7 cells | Liver | Homo sapiens (Human) | CVCL_0336 |
| BEL-7402 cells | Liver | Homo sapiens (Human) | CVCL_5492 | |
| HepG2 cells | Liver | Homo sapiens (Human) | CVCL_0027 | |
| HCCLM3 cells | Liver | Homo sapiens (Human) | CVCL_6832 | |
| Hep3B cells | Liver | Homo sapiens (Human) | CVCL_0326 | |
| SMMC7721 cells | Uterus | Homo sapiens (Human) | CVCL_0534 | |
| MHCC97-L cells | Liver | Homo sapiens (Human) | CVCL_4973 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
qPCR | |||
| Experiment for Drug Resistance |
CCK8 analysis; EdU analysis; Boyden chamber assay; Transwell assay; Flow cytometry assay | |||
| Mechanism Description | MALAT1 deficiency related increase in sensitivity of liver cancer cells was associated with regulation of NF-kB. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Integrin beta-5 (ITGB5) | [33] | |||
| Metabolic Type | Redox metabolism | |||
| Resistant Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Lung cancer [ICD-11: 2C25] | |||
| The Specified Disease | Lung adenocarcinoma | |||
| The Studied Tissue | Lung tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 3.87E-14 Fold-change: 4.20E-01 Z-score: 8.33E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | A5419 cells | Lung | Homo sapiens (Human) | N.A. |
| LLC cells | Lung | Homo sapiens (Human) | CVCL_A9AW | |
| Experiment for Molecule Alteration |
qRT-PCR; Western blot analysis | |||
| Experiment for Drug Resistance |
IC50 assay | |||
| Mechanism Description | Mechanistically, our proteomic analysis reveals a consistent up-regulation of sphingolipid metabolic enzyme ASAH2 and beta5-integrin expression in GemR pancreatic and lung cancer cells as well as stable beta5-integrin-expressing cells. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Glucose-6-phosphate dehydrogenase (G6PD) | [34] | |||
| Metabolic Type | Glucose metabolism | |||
| Resistant Disease | Pancreatic ductal adenocarcinoma [ICD-11: 2C10.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Pancreatic cancer [ICD-11: 2C10] | |||
| The Specified Disease | Pancreatic ductal adenocarcinoma | |||
| The Studied Tissue | Pancreas | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 3.01E-17 Fold-change: 4.09E-01 Z-score: 9.54E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Adrenergic signaling in cardiomyocytes | Activation | hsa04261 | |
| In Vivo Model | Female SCID mice of 6-week-old, with fresh tissue from patient | Mice | ||
| Experiment for Molecule Alteration |
qRT-PCR; Western blot analysis | |||
| Experiment for Drug Resistance |
Tumor volume assay | |||
| Mechanism Description | Glucomet-PDACs are more resistant to chemotherapy than lipomet-PDACs, and patients with glucomet-PDAC have a worse prognosis. Integrated analyses reveal that the GLUT1/aldolase B (ALDOB)/glucose-6-phosphate dehydrogenase (G6PD) axis induces chemotherapy resistance by remodeling glucose metabolism in glucomet-PDAC. Increased glycolytic flux, G6PD activity, and pyrimidine biosynthesis are identified in glucomet-PDAC with high GLUT1 and low ALDOB expression, and these phenotypes could be reversed by inhibiting GLUT1 expression or by increasing ALDOB expression. | |||
| Key Molecule: Solute carrier family 2 member 1 (SLC2A1) | [34] | |||
| Metabolic Type | Glucose metabolism | |||
| Resistant Disease | Pancreatic ductal adenocarcinoma [ICD-11: 2C10.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Pancreatic cancer [ICD-11: 2C10] | |||
| The Specified Disease | Pancreatic ductal adenocarcinoma | |||
| The Studied Tissue | Pancreas | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 2.33E-08 Fold-change: 5.47E-01 Z-score: 5.83E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Adrenergic signaling in cardiomyocytes | Activation | hsa04261 | |
| In Vivo Model | Female SCID mice of 4-week-old, with fresh tissue from patient | Mice | ||
| Experiment for Molecule Alteration |
qRT-PCR; Western blot analysis | |||
| Experiment for Drug Resistance |
Tumor volume assay | |||
| Mechanism Description | Glucomet-PDACs are more resistant to chemotherapy than lipomet-PDACs, and patients with glucomet-PDAC have a worse prognosis. Integrated analyses reveal that the GLUT1/aldolase B (ALDOB)/glucose-6-phosphate dehydrogenase (G6PD) axis induces chemotherapy resistance by remodeling glucose metabolism in glucomet-PDAC. Increased glycolytic flux, G6PD activity, and pyrimidine biosynthesis are identified in glucomet-PDAC with high GLUT1 and low ALDOB expression, and these phenotypes could be reversed by inhibiting GLUT1 expression or by increasing ALDOB expression. | |||
| Key Molecule: N-acylsphingosine amidohydrolase 2 (ASAH2) | [33] | |||
| Metabolic Type | Redox metabolism | |||
| Resistant Disease | Pancreatic ductal adenocarcinoma [ICD-11: 2C10.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Pancreatic cancer [ICD-11: 2C10] | |||
| The Specified Disease | Pancreatic ductal adenocarcinoma | |||
| The Studied Tissue | Pancreas | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 3.44E-01 Fold-change: 1.83E-01 Z-score: 9.68E-01 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Panc1 cells | Pancreas | Homo sapiens (Human) | CVCL_0480 |
| TB32048 cells | N.A. | Homo sapiens (Human) | N.A. | |
| Experiment for Molecule Alteration |
qRT-PCR; Western blot analysis | |||
| Experiment for Drug Resistance |
IC50 assay | |||
| Mechanism Description | Mechanistically, our proteomic analysis reveals a consistent up-regulation of sphingolipid metabolic enzyme ASAH2 and beta5-integrin expression in GemR pancreatic and lung cancer cells as well as stable beta5-integrin-expressing cells. | |||
| Key Molecule: Integrin beta-5 (ITGB5) | [33] | |||
| Metabolic Type | Redox metabolism | |||
| Resistant Disease | Pancreatic ductal adenocarcinoma [ICD-11: 2C10.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Pancreatic cancer [ICD-11: 2C10] | |||
| The Specified Disease | Pancreatic ductal adenocarcinoma | |||
| The Studied Tissue | Pancreas | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.91E-26 Fold-change: 7.66E-01 Z-score: 1.30E+01 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Panc1 cells | Pancreas | Homo sapiens (Human) | CVCL_0480 |
| TB32048 cells | N.A. | Homo sapiens (Human) | N.A. | |
| Experiment for Molecule Alteration |
qRT-PCR; Western blot analysis | |||
| Experiment for Drug Resistance |
IC50 assay | |||
| Mechanism Description | Mechanistically, our proteomic analysis reveals a consistent up-regulation of sphingolipid metabolic enzyme ASAH2 and beta5-integrin expression in GemR pancreatic and lung cancer cells as well as stable beta5-integrin-expressing cells. | |||
| Key Molecule: Glucose-6-phosphate dehydrogenase (G6PD) | [34] | |||
| Metabolic Type | Glucose metabolism | |||
| Resistant Disease | Pancreatic ductal adenocarcinoma [ICD-11: 2C10.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Pancreatic cancer [ICD-11: 2C10] | |||
| The Specified Disease | Pancreatic ductal adenocarcinoma | |||
| The Studied Tissue | Pancreas | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 3.01E-17 Fold-change: 4.09E-01 Z-score: 9.54E+00 |
|||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Adrenergic signaling in cardiomyocytes | Activation | hsa04261 | |
| In Vivo Model | HCC patients | Homo Sapiens | ||
| Experiment for Molecule Alteration |
qRT-PCR; Western blot analysis | |||
| Mechanism Description | Glucomet-PDACs are more resistant to chemotherapy than lipomet-PDACs, and patients with glucomet-PDAC have a worse prognosis. Integrated analyses reveal that the GLUT1/aldolase B (ALDOB)/glucose-6-phosphate dehydrogenase (G6PD) axis induces chemotherapy resistance by remodeling glucose metabolism in glucomet-PDAC. Increased glycolytic flux, G6PD activity, and pyrimidine biosynthesis are identified in glucomet-PDAC with high GLUT1 and low ALDOB expression, and these phenotypes could be reversed by inhibiting GLUT1 expression or by increasing ALDOB expression. | |||
| Key Molecule: Solute carrier family 2 member 1 (SLC2A1) | [34] | |||
| Metabolic Type | Glucose metabolism | |||
| Resistant Disease | Pancreatic ductal adenocarcinoma [ICD-11: 2C10.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Pancreatic cancer [ICD-11: 2C10] | |||
| The Specified Disease | Pancreatic ductal adenocarcinoma | |||
| The Studied Tissue | Pancreas | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 2.33E-08 Fold-change: 5.47E-01 Z-score: 5.83E+00 |
|||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Adrenergic signaling in cardiomyocytes | Activation | hsa04261 | |
| In Vivo Model | HCC patients | Homo Sapiens | ||
| Experiment for Molecule Alteration |
qRT-PCR; Western blot analysis | |||
| Mechanism Description | Glucomet-PDACs are more resistant to chemotherapy than lipomet-PDACs, and patients with glucomet-PDAC have a worse prognosis. Integrated analyses reveal that the GLUT1/aldolase B (ALDOB)/glucose-6-phosphate dehydrogenase (G6PD) axis induces chemotherapy resistance by remodeling glucose metabolism in glucomet-PDAC. Increased glycolytic flux, G6PD activity, and pyrimidine biosynthesis are identified in glucomet-PDAC with high GLUT1 and low ALDOB expression, and these phenotypes could be reversed by inhibiting GLUT1 expression or by increasing ALDOB expression. | |||
| Key Molecule: Glucose-6-phosphate dehydrogenase (G6PD) | [34] | |||
| Metabolic Type | Glucose metabolism | |||
| Resistant Disease | Pancreatic ductal adenocarcinoma [ICD-11: 2C10.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Pancreatic cancer [ICD-11: 2C10] | |||
| The Specified Disease | Pancreatic ductal adenocarcinoma | |||
| The Studied Tissue | Pancreas | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 3.01E-17 Fold-change: 4.09E-01 Z-score: 9.54E+00 |
|||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Adrenergic signaling in cardiomyocytes | Activation | hsa04261 | |
| In Vivo Model | HCC patients | Homo Sapiens | ||
| Experiment for Molecule Alteration |
qRT-PCR; Western blot analysis | |||
| Mechanism Description | Glucomet-PDACs are more resistant to chemotherapy than lipomet-PDACs, and patients with glucomet-PDAC have a worse prognosis. Integrated analyses reveal that the GLUT1/aldolase B (ALDOB)/glucose-6-phosphate dehydrogenase (G6PD) axis induces chemotherapy resistance by remodeling glucose metabolism in glucomet-PDAC. Increased glycolytic flux, G6PD activity, and pyrimidine biosynthesis are identified in glucomet-PDAC with high GLUT1 and low ALDOB expression, and these phenotypes could be reversed by inhibiting GLUT1 expression or by increasing ALDOB expression. | |||
| Key Molecule: Solute carrier family 2 member 1 (SLC2A1) | [34] | |||
| Metabolic Type | Glucose metabolism | |||
| Resistant Disease | Pancreatic ductal adenocarcinoma [ICD-11: 2C10.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Pancreatic cancer [ICD-11: 2C10] | |||
| The Specified Disease | Pancreatic ductal adenocarcinoma | |||
| The Studied Tissue | Pancreas | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 2.33E-08 Fold-change: 5.47E-01 Z-score: 5.83E+00 |
|||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Adrenergic signaling in cardiomyocytes | Activation | hsa04261 | |
| In Vivo Model | HCC patients | Homo Sapiens | ||
| Experiment for Molecule Alteration |
qRT-PCR; Western blot analysis | |||
| Mechanism Description | Glucomet-PDACs are more resistant to chemotherapy than lipomet-PDACs, and patients with glucomet-PDAC have a worse prognosis. Integrated analyses reveal that the GLUT1/aldolase B (ALDOB)/glucose-6-phosphate dehydrogenase (G6PD) axis induces chemotherapy resistance by remodeling glucose metabolism in glucomet-PDAC. Increased glycolytic flux, G6PD activity, and pyrimidine biosynthesis are identified in glucomet-PDAC with high GLUT1 and low ALDOB expression, and these phenotypes could be reversed by inhibiting GLUT1 expression or by increasing ALDOB expression. | |||
|
|
||||
| Key Molecule: Protein salvador homolog 1 (SAV1) | [38] | |||
| Resistant Disease | Pancreatic cancer [ICD-11: 2C10.3] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Pancreatic cancer [ICD-11: 2C10] | |||
| The Specified Disease | Pancreatic cancer | |||
| The Studied Tissue | Pancreas | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 7.75E-14 Fold-change: -5.70E-01 Z-score: -8.32E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Hippo signaling pathway | Regulation | N.A. | ||
| In Vitro Model | BxPC-3 cells | Pancreas | Homo sapiens (Human) | CVCL_0186 |
| PANC-1 cells | Pancreas | Homo sapiens (Human) | CVCL_0480 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Flow cytometry assay | |||
| Mechanism Description | miR-181c directly repressed MST1, LATS2, MOB1 and SAV1 expression in human pancreatic cancer cells. Overexpression of miR-181c induced hyperactivation of the YAP/TAZ and (+) expression of the Hippo signaling downstream genes CTGF, BIRC5 and BLC2L1, leading to pancreatic cancer cell survival and chemoresistance in vitro and in vivo. Importantly, high miR-181c levels were significantly correlated with Hippo signaling inactivation in pancreatic cancer samples, and predicted a poor patient overall survival. | |||
| Key Molecule: Programmed cell death protein 4 (PDCD4) | [70], [71] | |||
| Resistant Disease | Pancreatic cancer [ICD-11: 2C10.3] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Pancreatic cancer [ICD-11: 2C10] | |||
| The Specified Disease | Pancreatic cancer | |||
| The Studied Tissue | Pancreas | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.56E-02 Fold-change: -2.17E-01 Z-score: -2.68E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell invasion | Activation | hsa05200 | ||
| Cell migration | Activation | hsa04670 | ||
| Cell proliferation | Activation | hsa05200 | ||
| PI3K/AKT/mTOR signaling pathway | Regulation | N.A. | ||
| In Vitro Model | PANC-1 cells | Pancreas | Homo sapiens (Human) | CVCL_0480 |
| PATU8988 cells | Pancreas | Homo sapiens (Human) | CVCL_1846 | |
| 293TN cells | Pancreas | Homo sapiens (Human) | CVCL_UL49 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay; Wound Healing assay; Matrigel transmembrane invasion assay | |||
| Mechanism Description | miR-21 regulates 5-FU drug resistance in pancreatic cancer by reducing the expression of its targets, PTEN and PDCD4. And PTEN and PDCD4, as tumor suppressors, not only can inhibit tumor growth and invasion, but also can downregulate the 5-FU resistance induced by miR-21 in pancreatic cancer cells. | |||
|
|
||||
| Key Molecule: Urothelial cancer associated 1 (UCA1) | [45] | |||
| Resistant Disease | Pancreatic ductal adenocarcinoma [ICD-11: 2C10.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Pancreatic cancer [ICD-11: 2C10] | |||
| The Specified Disease | Pancreatic adenocarcinoma | |||
| The Studied Tissue | Pancreas | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 2.90E-08 Fold-change: 5.54E+00 Z-score: 5.81E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | AKT signaling pathway | Activation | hsa04151 | |
| Cell apoptosis | Inhibition | hsa04210 | ||
| Cell invasion | Activation | hsa05200 | ||
| Cell migration | Activation | hsa04670 | ||
| Cell proliferation | Activation | hsa05200 | ||
| ERK signaling pathway | Activation | hsa04210 | ||
| In Vitro Model | BxPC-3 cells | Pancreas | Homo sapiens (Human) | CVCL_0186 |
| MIA PaCa-2 cells | Pancreas | Homo sapiens (Human) | CVCL_0428 | |
| PANC-1 cells | Pancreas | Homo sapiens (Human) | CVCL_0480 | |
| Capan-1 cells | Pancreas | Homo sapiens (Human) | CVCL_0237 | |
| AsPC-1 cells | Pancreas | Homo sapiens (Human) | CVCL_0152 | |
| SW1990 cells | Pancreas | Homo sapiens (Human) | CVCL_1723 | |
| CFPAC1 cells | Pancreas | Homo sapiens (Human) | CVCL_1119 | |
| HPAC cells | Pancreas | Homo sapiens (Human) | CVCL_3517 | |
| In Vivo Model | BALB/c nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
RT-qPCR | |||
| Experiment for Drug Resistance |
MTT assay; Flow cytometry assay; Wound-healing assay | |||
| Mechanism Description | CUDR overexpression inhibits cell apoptosis and promotes drug resistance in PDAC and CUDR overexpression in Panc-1 cells significantly increased phosphorylated (p-) focal adhesion kinase (FAk) and p-AkT levels, whereas the total FAk and AkT were not altered compared with in Panc-1 cells transfected with an empty vector. | |||
| Key Molecule: Growth arrest specific 5 (GAS5) | [72] | |||
| Resistant Disease | Pancreatic cancer [ICD-11: 2C10.3] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Pancreatic cancer [ICD-11: 2C10] | |||
| The Specified Disease | Pancreatic adenocarcinoma | |||
| The Studied Tissue | Pancreas | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 6.05E-15 Fold-change: -8.27E-01 Z-score: -8.25E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Hippo signaling pathway | Inhibition | hsa04390 | |
| In Vitro Model | SW1990 cells | Pancreas | Homo sapiens (Human) | CVCL_1723 |
| 5-FU cells | Colon | Homo sapiens (Human) | CVCL_1846 | |
| PATU8988 | Pancreas | Homo sapiens (Human) | CVCL_1847 | |
| PATU8988 cells | Pancreas | Homo sapiens (Human) | CVCL_1846 | |
| SW1990/GEM cells | Pancreas | Homo sapiens (Human) | CVCL_ZW98 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | GAS5 regualtes Hippo signaling pathway via miR181c-5p to antagonize the development of multidrug resistance in pancreatic cancer cells. GAS5 regulated chemoresistance and Hippo pathway of pancreatic cancer cells via miR181c-5p/Hippo. | |||
| Key Molecule: DiGeorge syndrome critical region gene 5 (DGCR5) | [15] | |||
| Resistant Disease | Pancreatic ductal adenocarcinoma [ICD-11: 2C10.0] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Pancreatic cancer [ICD-11: 2C10] | |||
| The Specified Disease | Pancreatic adenocarcinoma | |||
| The Studied Tissue | Pancreas | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 7.83E-10 Fold-change: -1.00E+00 Z-score: -6.33E+00 |
|||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell migration | Activation | hsa04670 | |
| Cell proliferation | Activation | hsa05200 | ||
| In Vitro Model | PANC-1 cells | Pancreas | Homo sapiens (Human) | CVCL_0480 |
| HAPC cells | Pancreas | Homo sapiens (Human) | N.A. | |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | DGCR5 and miR320a regulate each other in a reciprocal manner and that DGCR5 reverses the inhibition of PDCD4 by miR320a, which is involved in the regulation of the PDAC cell phenotype and response to 5-FU. miR320a is involved in 5-FU resistance modulated by DGCR5. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Histone deacetylase 4 (HDAC4) | [35] | |||
| Sensitive Disease | Colon cancer [ICD-11: 2B90.1] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Colon cancer [ICD-11: 2B90] | |||
| The Specified Disease | Colon cancer | |||
| The Studied Tissue | Colon tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 9.80E-14 Fold-change: -3.79E-01 Z-score: -8.17E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell proliferation | Inhibition | hsa05200 | |
| In Vitro Model | HCT116 cells | Colon | Homo sapiens (Human) | CVCL_0291 |
| Experiment for Molecule Alteration |
Western blot analysis; Immunofluorescence analysis | |||
| Experiment for Drug Resistance |
WST-1 assay | |||
| Mechanism Description | miR-140 is involved in the chemoresistance by reduced cell proliferation via G1 and G2 phase arrest mediated in part. | |||
| Key Molecule: Dihydropyrimidine dehydrogenase [NADP(+)] | [61] | |||
| Sensitive Disease | Colon cancer [ICD-11: 2B90.1] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Colon cancer [ICD-11: 2B90] | |||
| The Specified Disease | Colon cancer | |||
| The Studied Tissue | Colon tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.29E-04 Fold-change: -2.76E-02 Z-score: -3.90E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | 5-Fu catabolic signaling pathway | Regulation | N.A. | |
| Cell apoptosis | Activation | hsa04210 | ||
| In Vitro Model | HCT116 cells | Colon | Homo sapiens (Human) | CVCL_0291 |
| LOVO cells | Colon | Homo sapiens (Human) | CVCL_0399 | |
| HCT8 cells | Colon | Homo sapiens (Human) | CVCL_2478 | |
| HT-29 cells | Colon | Homo sapiens (Human) | CVCL_0320 | |
| HCT15 cells | Colon | Homo sapiens (Human) | CVCL_0292 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miR-494 also negatively regulated endogenous DPYD expression in SW480 cells. Overexpression or knockdown of DPYD could attenuate miR-494 mediated 5-Fu sensitivity regulation, suggesting the dependence of DPYD regulation in miR-494 activity. miR-494 inhibited SW480/5-Fu derived xenograft tumors growth in vivo at present of 5-Fu. | |||
| Key Molecule: Heat shock protein beta-1 (HSPB1) | [62] | |||
| Sensitive Disease | Colon cancer [ICD-11: 2B90.1] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Colon cancer [ICD-11: 2B90] | |||
| The Specified Disease | Colon cancer | |||
| The Studied Tissue | Colon tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.98E-05 Fold-change: -2.80E-02 Z-score: -4.32E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell viability | Inhibition | hsa05200 | ||
| In Vitro Model | LOVO cells | Colon | Homo sapiens (Human) | CVCL_0399 |
| HT-29 cells | Colon | Homo sapiens (Human) | CVCL_0320 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay; TUNEL assay | |||
| Mechanism Description | miR-214 targeted heat shock protein 27 and could sensitize non-resistant colon cancer cells and 5-FU-resistant colon cancer cellsto 5-FU while overexpression of Hsp27 could block miR-214 with an effect on the sensitivity of colon cancer cells to 5-FU. | |||
| Key Molecule: Insulin-like growth factor 1 receptor (IGF1R) | [63] | |||
| Sensitive Disease | Colon cancer [ICD-11: 2B90.1] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Colon cancer [ICD-11: 2B90] | |||
| The Specified Disease | Colon cancer | |||
| The Studied Tissue | Colon tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 5.32E-03 Fold-change: -3.07E-02 Z-score: -2.81E+00 |
|||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | PI3K/AKT signaling pathway | Inhibition | hsa04151 | |
| In Vitro Model | HT29 Cells | Colon | Homo sapiens (Human) | CVCL_A8EZ |
| HCT116 cells | Colon | Homo sapiens (Human) | CVCL_0291 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Trypan blue dye-exclusion assay; Annexin V-FITC apoptosis assay; Flow cytometer | |||
| Mechanism Description | Both miR 302a and si IGF 1R inhibited Akt signaling. MiR 302a targeted IGF 1R and enhanced 5 FU induced cell death and viability inhibition in human colon cancer cells. | |||
| Key Molecule: Prominin-1 (PROM1) | [68] | |||
| Sensitive Disease | Colon cancer [ICD-11: 2B90.1] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Colon cancer [ICD-11: 2B90] | |||
| The Specified Disease | Colon cancer | |||
| The Studied Tissue | Colon tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 4.04E-12 Fold-change: -9.18E-02 Z-score: -7.18E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | CaCo2 cells | Colon | Homo sapiens (Human) | CVCL_0025 |
| SW1116 cells | Colon | Homo sapiens (Human) | CVCL_0544 | |
| In Vivo Model | HT-29 xenograft mouse model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Annexin V assay | |||
| Mechanism Description | The miR-142-3p was markedly decreased in coloncancer specimens, in which it was negatively correlated withthe expression of CD133, Lgr5, and ABCG2. Transfection of miR-142-3p mimics in colon cancer cells downregulated cyclin D1expression, induced G1phase cell cycle arrest, and elevatedthe sensitivity of the cells to 5-fluorouracil. Furthermore,OCT4 suppressed miR-142-3p, and hypomethylation of theOCT4promoter was associated with a reduction in miR-142-3p. | |||
|
|
||||
| Key Molecule: E3 ubiquitin-protein ligase XIAP (XIAP) | [42] | |||
| Sensitive Disease | Colon cancer [ICD-11: 2B90.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Colon cancer [ICD-11: 2B90] | |||
| The Specified Disease | Colon cancer | |||
| The Studied Tissue | Colon tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 3.85E-04 Fold-change: 1.37E-01 Z-score: 3.61E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell migration | Inhibition | hsa04670 | |
| In Vitro Model | DLD1 cells | Colon | Homo sapiens (Human) | CVCL_0248 |
| SW620 cells | Colon | Homo sapiens (Human) | CVCL_0547 | |
| HCT116 cells | Colon | Homo sapiens (Human) | CVCL_0291 | |
| NCM460 cells | Colon | Homo sapiens (Human) | CVCL_0460 | |
| SW1116 cells | Colon | Homo sapiens (Human) | CVCL_0544 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
CellTiter-Glo Luminescent Cell Viability Assay; CCK8 assay; Flow cytometric analysis | |||
| Mechanism Description | Overexpression of XIAP decreases the inhibitory effects of miR15b-5p on drug resistance in colon cancer cells. miR15b-5p mediates NF- B regulation by targeting the anti-apoptosis protein XIAP in vitro. | |||
|
|
||||
| Key Molecule: ATP-binding cassette sub-family G2 (ABCG2) | [68] | |||
| Sensitive Disease | Colon cancer [ICD-11: 2B90.1] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Colon cancer [ICD-11: 2B90] | |||
| The Specified Disease | Colon cancer | |||
| The Studied Tissue | Colon tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.83E-95 Fold-change: -9.70E-01 Z-score: -4.32E+01 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | CaCo2 cells | Colon | Homo sapiens (Human) | CVCL_0025 |
| SW1116 cells | Colon | Homo sapiens (Human) | CVCL_0544 | |
| In Vivo Model | HT-29 xenograft mouse model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Annexin V assay | |||
| Mechanism Description | The miR-142-3p was markedly decreased in coloncancer specimens, in which it was negatively correlated withthe expression of CD133, Lgr5, and ABCG2. Transfection of miR-142-3p mimics in colon cancer cells downregulated cyclin D1expression, induced G1phase cell cycle arrest, and elevatedthe sensitivity of the cells to 5-fluorouracil. Furthermore,OCT4 suppressed miR-142-3p, and hypomethylation of theOCT4promoter was associated with a reduction in miR-142-3p. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Programmed cell death protein 4 (PDCD4) | [37] | |||
| Resistant Disease | Colon cancer [ICD-11: 2B90.1] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Colon cancer [ICD-11: 2B90] | |||
| The Specified Disease | Colon cancer | |||
| The Studied Tissue | Colon tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.69E-35 Fold-change: -7.24E-01 Z-score: -1.54E+01 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| PI3K/AKT signaling pathway | Regulation | N.A. | ||
| In Vitro Model | RkO cells | Colon | Homo sapiens (Human) | CVCL_0504 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miR-21 can mediate the drug resistance to 5-FU by inhibiting its target PDCD4, which can regulate the expression of ABCC5 and CD44 genes. | |||
| Key Molecule: Serine/threonine-protein kinase Chk1 (CHK1) | [49] | |||
| Resistant Disease | Colon cancer [ICD-11: 2B90.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Colon cancer [ICD-11: 2B90] | |||
| The Specified Disease | Colon cancer | |||
| The Studied Tissue | Colon tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 6.28E-72 Fold-change: 2.53E-01 Z-score: 2.53E+01 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | HCT116 cells | Colon | Homo sapiens (Human) | CVCL_0291 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay; Flow cytometric analysis | |||
| Mechanism Description | Inhibition of miR195 sensitized resistant cells to 5-FU by downregulating WEE1 and CHk1. | |||
| Key Molecule: Transcription factor E2F3 (E2F3) | [5] | |||
| Resistant Disease | Colon cancer [ICD-11: 2B90.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Colon cancer [ICD-11: 2B90] | |||
| The Specified Disease | Colon cancer | |||
| The Studied Tissue | Colon tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.02E-82 Fold-change: 1.51E-01 Z-score: 2.75E+01 |
|||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | PI3K/AKT signaling pathway | Activation | hsa04151 | |
| In Vitro Model | DLD1 cells | Colon | Homo sapiens (Human) | CVCL_0248 |
| DLD-1/5FU cells | Colon | Homo sapiens (Human) | CVCL_0248 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Trypan blue dye exclusion assay | |||
| Mechanism Description | The ectopic expression of miR-34a in the 5-FU-resistant cells inhibited growth, as in the parental cells, and attenuated the resistance to 5-FU through the down-regulation of Sirt1 and E2F3. | |||
| Key Molecule: NAD-dependent protein deacetylase sirtuin-1 (SIRT1) | [5] | |||
| Resistant Disease | Colon cancer [ICD-11: 2B90.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Colon cancer [ICD-11: 2B90] | |||
| The Specified Disease | Colon cancer | |||
| The Studied Tissue | Colon tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 2.34E-02 Fold-change: 1.46E-02 Z-score: 2.28E+00 |
|||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | PI3K/AKT signaling pathway | Activation | hsa04151 | |
| In Vitro Model | DLD1 cells | Colon | Homo sapiens (Human) | CVCL_0248 |
| DLD-1/5FU cells | Colon | Homo sapiens (Human) | CVCL_0248 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Trypan blue dye exclusion assay | |||
| Mechanism Description | The ectopic expression of miR-34a in the 5-FU-resistant cells inhibited growth, as in the parental cells, and attenuated the resistance to 5-FU through the down-regulation of Sirt1 and E2F3. | |||
| Key Molecule: Wee1-like protein kinase (WEE1) | [49] | |||
| Resistant Disease | Colon cancer [ICD-11: 2B90.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Colon cancer [ICD-11: 2B90] | |||
| The Specified Disease | Colon cancer | |||
| The Studied Tissue | Colon tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 4.92E-41 Fold-change: 1.05E-01 Z-score: 1.63E+01 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | HCT116 cells | Colon | Homo sapiens (Human) | CVCL_0291 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay; Flow cytometric analysis | |||
| Mechanism Description | Inhibition of miR195 sensitized resistant cells to 5-FU by downregulating WEE1 and CHk1. | |||
| Key Molecule: DNA-binding factor KBF1 (p105) (NFKB1) | [42] | |||
| Resistant Disease | Colon cancer [ICD-11: 2B90.1] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Colon cancer [ICD-11: 2B90] | |||
| The Specified Disease | Colon cancer | |||
| The Studied Tissue | Colon tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 3.70E-46 Fold-change: -7.40E-02 Z-score: -1.70E+01 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| NF-kappaB signaling pathway | Inhibition | hsa04064 | ||
| In Vitro Model | DLD1 cells | Colon | Homo sapiens (Human) | CVCL_0248 |
| SW620 cells | Colon | Homo sapiens (Human) | CVCL_0547 | |
| HCT116 cells | Colon | Homo sapiens (Human) | CVCL_0291 | |
| NCM460 cells | Colon | Homo sapiens (Human) | CVCL_0460 | |
| SW1116 cells | Colon | Homo sapiens (Human) | CVCL_0544 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis; Dual-Luciferase Reporter Assay | |||
| Experiment for Drug Resistance |
CellTiter-Glo Luminescent Cell Viability Assay; CCK8 assay; Flow cytometric analysis | |||
| Mechanism Description | miR15b-5p resensitizes colon cancer cells to 5-fluorouracil by promoting apoptosis via the NF-kB/XIAP axis. miR15b-5p results in significant reductions in the levels of NF-kB1 and Ikk-alpha, two key modulators in inflammation and cell apoptosis. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Urothelial cancer associated 1 (UCA1) | [46] | |||
| Resistant Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Gastric cancer [ICD-11: 2B72] | |||
| The Specified Disease | Stomach adenocarcinoma | |||
| The Studied Tissue | Stomach | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 7.51E-08 Fold-change: 5.46E+00 Z-score: 5.48E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 |
| SGC-7901/DDP cells | Gastric | Homo sapiens (Human) | CVCL_0520 | |
| SGC-7901/FU cells | Gastric | Homo sapiens (Human) | CVCL_0520 | |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
MTT assay; Annexin V-FITC Apoptosis assay | |||
| Mechanism Description | LncRNA urothelial carcinoma associated 1 (UCA1) increases multi-drug resistance of gastric cancer via downregulating miR27b. | |||
| Key Molecule: Pvt1 oncogene (PVT1) | [82] | |||
| Resistant Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| In Vitro Model | SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 |
| In Vivo Model | Nod/SCID mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
RT-PCR | |||
| Experiment for Drug Resistance |
CCK8 assay; Flow cytometry assay | |||
| Mechanism Description | LncRNA PVT1 can inhibit apoptosis and enhance the 5-Fu resistance via Increasing Bcl2 expression in Gastric Cancer. | |||
| Key Molecule: Long non-protein coding RNA (XLOC_006753) | [83] | |||
| Resistant Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell migration | Activation | hsa04670 | ||
| Cell proliferation | Activation | hsa05200 | ||
| PI3K/AKT/mTOR signaling pathway | Activation | hsa04151 | ||
| In Vitro Model | SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
CCK8 assay; Flow cytometry assay | |||
| Mechanism Description | Long non-coding RNA XLOC_006753 promotes the development of multidrug resistance in gastric cancer cells through the PI3k/Akt/mTOR signaling pathway. | |||
| Key Molecule: Metastasis associated lung adenocarcinoma transcript 1 (MALAT1) | [9] | |||
| Resistant Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 |
| BGC823 cells | Gastric | Homo sapiens (Human) | CVCL_3360 | |
| Experiment for Molecule Alteration |
RT-PCR; Luciferase reporter assay; Pull down assay | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | MALAT1 acts as a competing endogenous RNA for miR23b-3p and attenuates the inhibitory effect of miR23b-3p on ATG12, leading to chemo-induced autophagy and chemoresistance in GC cells. | |||
| Key Molecule: hsa-miR-23b-3p | [9] | |||
| Resistant Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 |
| BGC823 cells | Gastric | Homo sapiens (Human) | CVCL_3360 | |
| Experiment for Molecule Alteration |
RT-PCR; Luciferase reporter assay; Pull down assay | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | MALAT1 acts as a competing endogenous RNA for miR23b-3p and attenuates the inhibitory effect of miR23b-3p on ATG12, leading to chemo-induced autophagy and chemoresistance in GC cells. MALAT1 promotes autophagy-associated chemoresistance of GC cells via sequestration of miR23b-3p. | |||
| Key Molecule: hsa-mir-145 | [53] | |||
| Resistant Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | MGC-803 cells | Gastric | Homo sapiens (Human) | CVCL_5334 |
| Experiment for Molecule Alteration |
RT-PCR; qRT-PCR | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | microRNA-145 exerts tumor-suppressive and chemo-resistance lowering effects by targeting CD44 in gastric cancer. | |||
| Key Molecule: hsa-mir-27b | [46] | |||
| Resistant Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 |
| SGC-7901/DDP cells | Gastric | Homo sapiens (Human) | CVCL_0520 | |
| SGC-7901/FU cells | Gastric | Homo sapiens (Human) | CVCL_0520 | |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
MTT assay; Annexin V-FITC Apoptosis assay | |||
| Mechanism Description | LncRNA urothelial carcinoma associated 1 (UCA1) increases multi-drug resistance of gastric cancer via downregulating miR27b. | |||
| Key Molecule: hsa-mir-363 | [84] | |||
| Resistant Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell proliferation | Activation | hsa05200 | |
| In Vitro Model | BGC-823 cells | Gastric | Homo sapiens (Human) | CVCL_3360 |
| MGC-803 cells | Gastric | Homo sapiens (Human) | CVCL_5334 | |
| SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 | |
| HGC27 cells | Gastric | Homo sapiens (Human) | CVCL_1279 | |
| Experiment for Molecule Alteration |
RT-PCR | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | miR-363 promotes gastric cancer cells proliferation by inhibiting FBW7 expression and was associated with chemo-resistance of gastric cancer cells. Silencing FBW7 largely phenocopied miR-363-induced resistance to chemotherapy agents and promoted proliferation in gastric cancer cells. In addition, an inverse correlation between miR-363 and FBW7 mRNA expression was observed in gastric cancer tissues. | |||
| Key Molecule: hsa-mir-19a | [20] | |||
| Resistant Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| PTEN/AKT signaling pathway | Inhibition | hsa05235 | ||
| In Vitro Model | SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 |
| SGC7901/VCR cells | Gastric | Homo sapiens (Human) | CVCL_VU58 | |
| SGC7901/ADR cells | Gastric | Homo sapiens (Human) | CVCL_VU57 | |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miR-19a/b are upregulated in multidrug-resistant gastric cancer cell line, miR-19a/b suppress the sensitivity of gastric cancer cells to anticancer drugs, miR-19a/b accelerate the efflux of ADR through P-gp upregulation. | |||
| Key Molecule: hsa-mir-19b | [20] | |||
| Resistant Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| PTEN/AKT signaling pathway | Inhibition | hsa05235 | ||
| In Vitro Model | SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 |
| SGC7901/VCR cells | Gastric | Homo sapiens (Human) | CVCL_VU58 | |
| SGC7901/ADR cells | Gastric | Homo sapiens (Human) | CVCL_VU57 | |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miR-19a/b are upregulated in multidrug-resistant gastric cancer cell line, miR-19a/b suppress the sensitivity of gastric cancer cells to anticancer drugs, miR-19a/b accelerate the efflux of ADR through P-gp upregulation. | |||
|
|
||||
| Key Molecule: Transforming growth factor beta 1 (TGFB1) | [50] | |||
| Resistant Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Gastric cancer [ICD-11: 2B72] | |||
| The Specified Disease | Gastric cancer | |||
| The Studied Tissue | Gastric tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 5.13E-01 Fold-change: 2.49E-02 Z-score: 7.76E-01 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | FAO signaling pathway | Activation | hsa04550 | |
| In Vitro Model | AGS cells | Gastric | Homo sapiens (Human) | CVCL_0139 |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Enzyme-linked immunosorbent assay | |||
| Experiment for Drug Resistance |
MTT assay; Colony formation assays | |||
| Mechanism Description | Transforming growth factor beta1 (TGF-beta1) secretion by MSCs activated SMAD2/3 through TGF-beta receptors and induced long non-coding RNA (LncRNA) MACC1-AS1 expression in GC cells, which promoted FAO-dependent stemness and chemoresistance through antagonizing miR-145-5p. | |||
| Key Molecule: hsa-miR-145-5p | [50] | |||
| Resistant Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | FAO signaling pathway | Activation | hsa04550 | |
| In Vitro Model | AGS cells | Gastric | Homo sapiens (Human) | CVCL_0139 |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
MTT assay; Colony formation assays | |||
| Mechanism Description | Transforming growth factor beta1 (TGF-beta1) secretion by MSCs activated SMAD2/3 through TGF-beta receptors and induced long non-coding RNA (LncRNA) MACC1-AS1 expression in GC cells, which promoted FAO-dependent stemness and chemoresistance through antagonizing miR-145-5p. | |||
| Key Molecule: MACC1 antisense RNA 1 (MACC1-AS1) | [50] | |||
| Resistant Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | FAO signaling pathway | Activation | hsa04550 | |
| In Vitro Model | AGS cells | Gastric | Homo sapiens (Human) | CVCL_0139 |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
MTT assay; Colony formation assays | |||
| Mechanism Description | Transforming growth factor beta1 (TGF-beta1) secretion by MSCs activated SMAD2/3 through TGF-beta receptors and induced long non-coding RNA (LncRNA) MACC1-AS1 expression in GC cells, which promoted FAO-dependent stemness and chemoresistance through antagonizing miR-145-5p. | |||
|
|
||||
| Key Molecule: Extracellular matrix receptor III (CD44) | [53] | |||
| Resistant Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Gastric cancer [ICD-11: 2B72] | |||
| The Specified Disease | Gastric cancer | |||
| The Studied Tissue | Gastric tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 2.62E-01 Fold-change: 1.66E-01 Z-score: 1.54E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | MGC-803 cells | Gastric | Homo sapiens (Human) | CVCL_5334 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | microRNA-145 exerts tumor-suppressive and chemo-resistance lowering effects by targeting CD44 in gastric cancer. | |||
| Key Molecule: Apoptosis regulator Bcl-2 (BCL2) | [82] | |||
| Resistant Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| In Vitro Model | SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 |
| In Vivo Model | Nod/SCID mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis; RT-qPCR | |||
| Experiment for Drug Resistance |
CCK8 assay; Flow cytometry assay | |||
| Mechanism Description | LncRNA PVT1 can inhibit apoptosis and enhance the 5-Fu resistance via Increasing Bcl2 expression in Gastric Cancer. | |||
| Key Molecule: F-box/WD repeat-containing protein 7 (FBXW7) | [84] | |||
| Resistant Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell proliferation | Activation | hsa05200 | |
| In Vitro Model | BGC-823 cells | Gastric | Homo sapiens (Human) | CVCL_3360 |
| MGC-803 cells | Gastric | Homo sapiens (Human) | CVCL_5334 | |
| SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 | |
| HGC27 cells | Gastric | Homo sapiens (Human) | CVCL_1279 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | miR-363 promotes gastric cancer cells proliferation by inhibiting FBW7 expression and was associated with chemo-resistance of gastric cancer cells. Silencing FBW7 largely phenocopied miR-363-induced resistance to chemotherapy agents and promoted proliferation in gastric cancer cells. In addition, an inverse correlation between miR-363 and FBW7 mRNA expression was observed in gastric cancer tissues. | |||
| Key Molecule: Phosphatase and tensin homolog (PTEN) | [20] | |||
| Resistant Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| PTEN/AKT signaling pathway | Inhibition | hsa05235 | ||
| In Vitro Model | SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 |
| SGC7901/VCR cells | Gastric | Homo sapiens (Human) | CVCL_VU58 | |
| SGC7901/ADR cells | Gastric | Homo sapiens (Human) | CVCL_VU57 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miR-19a/b are upregulated in multidrug-resistant gastric cancer cell line, miR-19a/b suppress the sensitivity of gastric cancer cells to anticancer drugs, miR-19a/b accelerate the efflux of ADR through P-gp upregulation. | |||
| Key Molecule: Serine/threonine-protein kinase mTOR (mTOR) | [86] | |||
| Resistant Disease | Gastric adenocarcinoma [ICD-11: 2B72.0] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | PI3K/AKT/mTOR signaling pathway | Inhibition | hsa04151 | |
| In Vitro Model | MKN-45/R cells | Gastric | Homo sapiens (Human) | N.A. |
| MKN-74/R cells | Gastric | Homo sapiens (Human) | N.A. | |
| Experiment for Molecule Alteration |
Western blot assay; qRT-PCR | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | The PI3K/Akt/mTOR signaling pathway was activated in drug-resistant GC cells and tumor tissues of patients refractory to 5-FU chemotherapy, as evidenced by high PI3K, Akt, and mTOR levels in MKN-45/R, MKN-74/R, and GC tissues resistant to 5-FU. Silencing of the PI3K/Akt/mTOR signaling pathway suppressed the 5-FU resistance of GC cells. | |||
| Key Molecule: Serine/threonine-protein kinase mTOR (mTOR) | [86] | |||
| Resistant Disease | Gastric adenocarcinoma [ICD-11: 2B72.0] | |||
| Molecule Alteration | Phosphorylation | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | PI3K/AKT/mTOR signaling pathway | Inhibition | hsa04151 | |
| In Vitro Model | MKN-45/R cells | Gastric | Homo sapiens (Human) | N.A. |
| MKN-74/R cells | Gastric | Homo sapiens (Human) | N.A. | |
| Experiment for Molecule Alteration |
Western blot assay; qRT-PCR | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | The PI3K/Akt/mTOR signaling pathway was activated in drug-resistant GC cells and tumor tissues of patients refractory to 5-FU chemotherapy, as evidenced by high PI3K, Akt, and mTOR levels in MKN-45/R, MKN-74/R, and GC tissues resistant to 5-FU. Silencing of the PI3K/Akt/mTOR signaling pathway suppressed the 5-FU resistance of GC cells. | |||
| Key Molecule: Phosphatidylinositol 3-kinase (PI3K) | [86] | |||
| Resistant Disease | Gastric adenocarcinoma [ICD-11: 2B72.0] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | PI3K/AKT/mTOR signaling pathway | Inhibition | hsa04151 | |
| In Vitro Model | MKN-45/R cells | Gastric | Homo sapiens (Human) | N.A. |
| MKN-74/R cells | Gastric | Homo sapiens (Human) | N.A. | |
| Experiment for Molecule Alteration |
Western blot assay; qRT-PCR | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | The PI3K/Akt/mTOR signaling pathway was activated in drug-resistant GC cells and tumor tissues of patients refractory to 5-FU chemotherapy, as evidenced by high PI3K, Akt, and mTOR levels in MKN-45/R, MKN-74/R, and GC tissues resistant to 5-FU. Silencing of the PI3K/Akt/mTOR signaling pathway suppressed the 5-FU resistance of GC cells. | |||
| Key Molecule: Phosphatidylinositol 3-kinase (PI3K) | [86] | |||
| Resistant Disease | Gastric adenocarcinoma [ICD-11: 2B72.0] | |||
| Molecule Alteration | Phosphorylation | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | PI3K/AKT/mTOR signaling pathway | Inhibition | hsa04151 | |
| In Vitro Model | MKN-45/R cells | Gastric | Homo sapiens (Human) | N.A. |
| Experiment for Molecule Alteration |
Western blot assay; qRT-PCR | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | The PI3K/Akt/mTOR signaling pathway was activated in drug-resistant GC cells and tumor tissues of patients refractory to 5-FU chemotherapy, as evidenced by high PI3K, Akt, and mTOR levels in MKN-45/R, MKN-74/R, and GC tissues resistant to 5-FU. Silencing of the PI3K/Akt/mTOR signaling pathway suppressed the 5-FU resistance of GC cells. | |||
| Key Molecule: Phosphatidylinositol 3-kinase (PI3K) | [86] | |||
| Resistant Disease | Gastric adenocarcinoma [ICD-11: 2B72.0] | |||
| Molecule Alteration | Phosphorylation | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | PI3K/AKT/mTOR signaling pathway | Inhibition | hsa04151 | |
| In Vitro Model | MKN-74/R cells | Gastric | Homo sapiens (Human) | N.A. |
| Experiment for Molecule Alteration |
Western blot assay; qRT-PCR | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | The PI3K/Akt/mTOR signaling pathway was activated in drug-resistant GC cells and tumor tissues of patients refractory to 5-FU chemotherapy, as evidenced by high PI3K, Akt, and mTOR levels in MKN-45/R, MKN-74/R, and GC tissues resistant to 5-FU. Silencing of the PI3K/Akt/mTOR signaling pathway suppressed the 5-FU resistance of GC cells. | |||
| Key Molecule: AKT serine/threonine kinase (AKT) | [86] | |||
| Resistant Disease | Gastric adenocarcinoma [ICD-11: 2B72.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | PI3K/AKT/mTOR signaling pathway | Inhibition | hsa04151 | |
| In Vitro Model | MKN-45/R cells | Gastric | Homo sapiens (Human) | N.A. |
| MKN-74/R cells | Gastric | Homo sapiens (Human) | N.A. | |
| Experiment for Molecule Alteration |
Western blot assay; qRT-PCR | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | The PI3K/Akt/mTOR signaling pathway was activated in drug-resistant GC cells and tumor tissues of patients refractory to 5-FU chemotherapy, as evidenced by high PI3K, Akt, and mTOR levels in MKN-45/R, MKN-74/R, and GC tissues resistant to 5-FU. Silencing of the PI3K/Akt/mTOR signaling pathway suppressed the 5-FU resistance of GC cells. | |||
| Key Molecule: AKT serine/threonine kinase (AKT) | [86] | |||
| Resistant Disease | Gastric adenocarcinoma [ICD-11: 2B72.0] | |||
| Molecule Alteration | Phosphorylation | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | PI3K/AKT/mTOR signaling pathway | Inhibition | hsa04151 | |
| In Vitro Model | MKN-45/R cells | Gastric | Homo sapiens (Human) | N.A. |
| MKN-74/R cells | Gastric | Homo sapiens (Human) | N.A. | |
| Experiment for Molecule Alteration |
Western blot assay; qRT-PCR | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | The PI3K/Akt/mTOR signaling pathway was activated in drug-resistant GC cells and tumor tissues of patients refractory to 5-FU chemotherapy, as evidenced by high PI3K, Akt, and mTOR levels in MKN-45/R, MKN-74/R, and GC tissues resistant to 5-FU. Silencing of the PI3K/Akt/mTOR signaling pathway suppressed the 5-FU resistance of GC cells. | |||
|
|
||||
| Key Molecule: Dihydroorotate dehydrogenase (DHODH) | [85] | |||
| Metabolic Type | Nucleic acid metabolism | |||
| Resistant Disease | Gastric adenocarcinoma [ICD-11: 2B72.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | AGS cells | Gastric | Homo sapiens (Human) | CVCL_0139 |
| HGC27 cells | Gastric | Homo sapiens (Human) | CVCL_1279 | |
| Experiment for Molecule Alteration |
qRT-PCR; Western blot analysis | |||
| Experiment for Drug Resistance |
IC50 assay | |||
| Mechanism Description | Mechanistically, pyrimidine biosynthesis augmented Notch signaling and transcriptionally increased c-Myc expression, leading to up-regulation of critical glycolytic enzymes. Further studies revealed that pyrimidine synthesis could stabilize gamma-secretase subunit Nicastrin at post-translational N-linked glycosylation level, thereby inducing the cleavage and activation of Notch. Besides, we found that up-regulation of the key enzymes for de novo pyrimidine synthesis CAD and DHODH conferred the chemotherapeutic resistance of gastric cancer via accelerating glycolysis, and pharmacologic inhibition of pyrimidine biosynthetic pathway sensitized cancer cells to chemotherapy in vitro and in vivo. | |||
| Key Molecule: Carbamoyl-phosphate synthetase 2, aspartate transcarbamylase, and dihydroorotase (CAD) | [85] | |||
| Metabolic Type | Nucleic acid metabolism | |||
| Resistant Disease | Gastric adenocarcinoma [ICD-11: 2B72.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | AGS cells | Gastric | Homo sapiens (Human) | CVCL_0139 |
| HGC27 cells | Gastric | Homo sapiens (Human) | CVCL_1279 | |
| Experiment for Molecule Alteration |
qRT-PCR; Western blot analysis | |||
| Experiment for Drug Resistance |
IC50 assay | |||
| Mechanism Description | Mechanistically, pyrimidine biosynthesis augmented Notch signaling and transcriptionally increased c-Myc expression, leading to up-regulation of critical glycolytic enzymes. Further studies revealed that pyrimidine synthesis could stabilize gamma-secretase subunit Nicastrin at post-translational N-linked glycosylation level, thereby inducing the cleavage and activation of Notch. Besides, we found that up-regulation of the key enzymes for de novo pyrimidine synthesis CAD and DHODH conferred the chemotherapeutic resistance of gastric cancer via accelerating glycolysis, and pharmacologic inhibition of pyrimidine biosynthetic pathway sensitized cancer cells to chemotherapy in vitro and in vivo. | |||
| Key Molecule: Pyruvate kinase muscle isozyme 1 (PKM1) | [17] | |||
| Metabolic Type | Mitochondrial metabolism | |||
| Resistant Disease | Gastric adenocarcinoma [ICD-11: 2B72.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | MKN45 cells | Liver | Homo sapiens (Human) | CVCL_0434 |
| MKN-45/F2R cells | Stomach | Homo sapiens (Human) | CVCL_0434 | |
| NUGC3 cells | Gastric | Homo sapiens (Human) | CVCL_1612 | |
| NUGC-3/5-FUR cells | Stomach | Homo sapiens (Human) | CVCL_1612 | |
| Experiment for Molecule Alteration |
Expression profiles | |||
| Experiment for Drug Resistance |
Cell viability assay | |||
| Mechanism Description | The overexpression of PKM1 resulted in resistance of the parental cells to 5-FU and oxaliplatin. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Protein lin-28 homolog A (CSDD1) | [54] | |||
| Sensitive Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Gastric cancer [ICD-11: 2B72] | |||
| The Specified Disease | Gastric cancer | |||
| The Studied Tissue | Gastric tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 2.28E-02 Fold-change: 1.60E-01 Z-score: 4.12E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell proliferation | Inhibition | hsa05200 | ||
| Lin28/miR107 pathway | Regulation | N.A. | ||
| In Vitro Model | MkN-45 cells | Gastric | Homo sapiens (Human) | CVCL_0434 |
| MkN28 cells | Gastric | Homo sapiens (Human) | CVCL_1416 | |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
MTT assay; Flow cytometry assay | |||
| Mechanism Description | Lin28 could inhibit the expression of miR-107, thereby up-regulating C-myc, P-gp and down-regulating Cyclin D1, subsequently result in chemo-resistance of gastric cancer cells. The Lin28/miR-107 pathway might be served as one of many signaling pathways that is associated with gastric cancer chemo-resistance. | |||
| Key Molecule: HOX transcript antisense RNA (HOTAIR) | [87] | |||
| Sensitive Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell invasion | Inhibition | hsa05200 | ||
| Cell migration | Inhibition | hsa04670 | ||
| Cell proliferation | Inhibition | hsa05200 | ||
| In Vitro Model | BGC-823 cells | Gastric | Homo sapiens (Human) | CVCL_3360 |
| MkN-45 cells | Gastric | Homo sapiens (Human) | CVCL_0434 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
Bromodeoxyuridine incorporation assay; Flow cytometry assay; Transwell assay | |||
| Mechanism Description | Down-regulation of HOTAIR could promote chemosensitivity, induce apoptosis of GC cells, and significantly inhibit GC cell proliferation, invasion, and metastasis in vivo and in vitro. | |||
| Key Molecule: hsa-mir-147 | [88] | |||
| Sensitive Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell invasion | Inhibition | hsa05200 | ||
| Cell proliferation | Inhibition | hsa05200 | ||
| PI3K/AKT signaling pathway | Inhibition | hsa04151 | ||
| In Vitro Model | BGC-823 cells | Gastric | Homo sapiens (Human) | CVCL_3360 |
| MGC-803 cells | Gastric | Homo sapiens (Human) | CVCL_5334 | |
| SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 | |
| AGS cells | Gastric | Homo sapiens (Human) | CVCL_0139 | |
| MkN-45 cells | Gastric | Homo sapiens (Human) | CVCL_0434 | |
| Experiment for Molecule Alteration |
RT-qPCR | |||
| Experiment for Drug Resistance |
MTT assay; Flow cytometric analysis | |||
| Mechanism Description | miR147 suppressed the proliferation and enhanced the chemosensitivity of gastric cancer cells to 5-FU by promoting cell apoptosis through directly targeting PTEN and regulating the PI3k/AkT signaling pathway. knockdown of pten reverses the effects of miR147 downregulation on gastric cancer cells. | |||
| Key Molecule: hsa-mir-31 | [66] | |||
| Sensitive Disease | Gastric adenocarcinoma [ICD-11: 2B72.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell invasion | Inhibition | hsa05200 | |
| Cell migration | Inhibition | hsa04670 | ||
| Cell proliferation | Inhibition | hsa05200 | ||
| In Vitro Model | 293T cells | Breast | Homo sapiens (Human) | CVCL_0063 |
| MkN-45 cells | Gastric | Homo sapiens (Human) | CVCL_0434 | |
| Experiment for Molecule Alteration |
RT-PCR | |||
| Experiment for Drug Resistance |
MTT assay; Flow cytometric analysis | |||
| Mechanism Description | Induction of miR31 in MkN-45 followed by suppression of RhoA expression resulted in increased sensitivity to 5-fluorouracil, inhibition of cell proliferation, and invasion compared to the control groups. | |||
| Key Molecule: Hepatocellular carcinoma up-regulated long non-coding RNA (HULC) | [89] | |||
| Sensitive Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| In Vitro Model | MGC-803 cells | Gastric | Homo sapiens (Human) | CVCL_5334 |
| GES-1 cells | Gastric | Homo sapiens (Human) | CVCL_EQ22 | |
| MkN-45 cells | Gastric | Homo sapiens (Human) | CVCL_0434 | |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
CCK8 assay; Flow cytometric analysis | |||
| Mechanism Description | Silencing LncRNA HULC could enhance chemotherapy induced apoptosis in GC cells. | |||
| Key Molecule: hsa-mir-939 | [90] | |||
| Sensitive Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell invasion | Inhibition | hsa05200 | ||
| Cell migration | Inhibition | hsa04670 | ||
| RAF/MEK/ERK signaling pathway | Inhibition | hsa04010 | ||
| In Vitro Model | BGC-823 cells | Gastric | Homo sapiens (Human) | CVCL_3360 |
| MGC-803 cells | Gastric | Homo sapiens (Human) | CVCL_5334 | |
| SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 | |
| AGS cells | Gastric | Homo sapiens (Human) | CVCL_0139 | |
| GES-1 cells | Gastric | Homo sapiens (Human) | CVCL_EQ22 | |
| HGC27 cells | Gastric | Homo sapiens (Human) | CVCL_1279 | |
| MkN-45 cells | Gastric | Homo sapiens (Human) | CVCL_0434 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
MTT assay; Colony formation assay; Flow cytometric analysis; Wound-healing, migration and invasion assay | |||
| Mechanism Description | Decreased expression of miR939 contributes to chemoresistance and metastasis of gastric cancer via dysregulation of SLC34A2 and Raf/MEk/ERk pathway. miR939 exerted its function mainly through inhibiting SLC34A2/Raf/MEk/ERk pathway, which is activated in GC. | |||
| Key Molecule: hsa-mir-31 | [91] | |||
| Sensitive Disease | Gastric adenocarcinoma [ICD-11: 2B72.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell invasion | Inhibition | hsa05200 | ||
| Cell migration | Inhibition | hsa04670 | ||
| Cell proliferation | Inhibition | hsa05200 | ||
| Cell viability | Inhibition | hsa05200 | ||
| In Vitro Model | AGS cells | Gastric | Homo sapiens (Human) | CVCL_0139 |
| MkN-45 cells | Gastric | Homo sapiens (Human) | CVCL_0434 | |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
MTT assay; Flow cytometry assay | |||
| Mechanism Description | MkN-45-miR-31 showed an increased sensitivity to 5-FU, decreased migration and cell invasion compared to the control groups and induction of miR-31 expression in MkN-45 caused a significant reduction of E2F6 and SMUG1 genes. | |||
| Key Molecule: hsa-mir-31 | [92] | |||
| Sensitive Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell colony | Inhibition | hsa05200 | ||
| Cell invasion | Inhibition | hsa05200 | ||
| Cell migration | Inhibition | hsa04670 | ||
| Cell viability | Inhibition | hsa05200 | ||
| In Vitro Model | AGS cells | Gastric | Homo sapiens (Human) | CVCL_0139 |
| SNU-1 cells | Gastric | Homo sapiens (Human) | CVCL_0099 | |
| SNU-5 cells | Gastric | Homo sapiens (Human) | CVCL_0078 | |
| SNU-16 cells | Gastric | Homo sapiens (Human) | CVCL_0076 | |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | microRNA-31 triggers G 2/M cell cycle arrest, enhances the chemosensitivity and inhibits migration and invasion of human gastric cancer cells by downregulating the expression of zeste homolog 2 (ZH2). | |||
| Key Molecule: hsa-mir-495 | [93] | |||
| Sensitive Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell proliferation | Inhibition | hsa05200 | ||
| mTOR signaling pathway | Inhibition | hsa04150 | ||
| In Vitro Model | SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 |
| Experiment for Molecule Alteration |
RT-qPCR | |||
| Experiment for Drug Resistance |
MTT assay; Flow cytometry assay | |||
| Mechanism Description | The miR-495 exerts promotive effects on GC chemosensitivity via inactivation of the mTOR signaling pathway by suppressing ERBB2. | |||
| Key Molecule: hsa-miR-195-5p | [94] | |||
| Sensitive Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell viability | Inhibition | hsa05200 | |
| In Vitro Model | MkN28 cells | Gastric | Homo sapiens (Human) | CVCL_1416 |
| Experiment for Molecule Alteration |
RT-qPCR | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Overexpression of miR 195 5p inhibit multi drug resistance of gastric cancer cells via downregulating ZNF139. | |||
| Key Molecule: hsa-miR-623 | [95] | |||
| Sensitive Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell viability | Inhibition | hsa05200 | ||
| In Vitro Model | BGC-823 cells | Gastric | Homo sapiens (Human) | CVCL_3360 |
| MGC-803 cells | Gastric | Homo sapiens (Human) | CVCL_5334 | |
| SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 | |
| MkN-45 cells | Gastric | Homo sapiens (Human) | CVCL_0434 | |
| Experiment for Molecule Alteration |
RT-qPCR | |||
| Experiment for Drug Resistance |
CCK8 assay; Flow cytometry assay | |||
| Mechanism Description | The restored miR-623 expression could inhibit the proliferation of GC cells and enhance their chemosensitivity to 5-FU via the cell apoptosis pathway and the recovered CCND1 expression counteracted the effects of miR-623 on GC cell proliferation, chemosensitivity, and 5-FU-induced apoptosis. | |||
| Key Molecule: Protein lin-28 homolog B (CSDD2) | [54] | |||
| Sensitive Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell proliferation | Inhibition | hsa05200 | ||
| Lin28/miR107 pathway | Regulation | N.A. | ||
| In Vitro Model | MkN-45 cells | Gastric | Homo sapiens (Human) | CVCL_0434 |
| MkN28 cells | Gastric | Homo sapiens (Human) | CVCL_1416 | |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
MTT assay; Flow cytometry assay | |||
| Mechanism Description | Lin28 could inhibit the expression of miR-107, thereby up-regulating C-myc, P-gp and down-regulating Cyclin D1, subsequently result in chemo-resistance of gastric cancer cells. The Lin28/miR-107 pathway might be served as one of many signaling pathways that is associated with gastric cancer chemo-resistance. | |||
| Key Molecule: hsa-miR-107 | [54] | |||
| Sensitive Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell proliferation | Inhibition | hsa05200 | ||
| Lin28/miR107 pathway | Regulation | N.A. | ||
| In Vitro Model | MkN-45 cells | Gastric | Homo sapiens (Human) | CVCL_0434 |
| MkN28 cells | Gastric | Homo sapiens (Human) | CVCL_1416 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay; Flow cytometry assay | |||
| Mechanism Description | Lin28 could inhibit the expression of miR-107, thereby up-regulating C-myc, P-gp and down-regulating Cyclin D1, subsequently result in chemo-resistance of gastric cancer cells. The Lin28/miR-107 pathway might be served as one of many signaling pathways that is associated with gastric cancer chemo-resistance. | |||
| Key Molecule: hsa-mir-218 | [55] | |||
| Sensitive Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| In Vitro Model | SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miR-218 may inhibit efflux of ADM and oxaliplatin by down-regulating P-gp expression. | |||
| Key Molecule: hsa-mir-197 | [96] | |||
| Sensitive Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | MAPK signaling pathway | Inhibition | hsa04010 | |
| In Vitro Model | SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
RT-qPCR | |||
| Experiment for Drug Resistance |
MTT assay; Flow cytometry assay | |||
| Mechanism Description | When miR-197 was overexpressed in SGC7901 cells, the protein levels of MAPk1 were downregulated. Furthermore, MAPk1 knockdown significantly increased the growth inhibition rate of the SGC7901/5-FU cells compared with those in the control group. These results indicated that miR-197 may influence the sensitivity of 5-FU treatment in a gastric cancer cell line by targeting MAPk1. | |||
| Key Molecule: hsa-miR-23b-3p | [97] | |||
| Sensitive Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | miR23b-3p/ATG12/HMGB2/autophagy regulatory loop signaling pathway | Regulation | N.A. | |
| In Vitro Model | SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 |
| BGC823 cells | Gastric | Homo sapiens (Human) | CVCL_3360 | |
| AGS cells | Gastric | Homo sapiens (Human) | CVCL_0139 | |
| In Vivo Model | SCID-SHO mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | ATG12 and HMGB2 were the direct targets of miR-23b-3p. Meanwhile, ATG12 and HMGB2 were positively associated with the occurrence of autophagy. Reducing the expression of these target genes by siRNA or inhibition of autophagy both sensitized GC cells to chemotherapy. These findings suggest that a miR-23b-3p/ATG12/HMGB2/autophagy-regulatory loop has a critical role in MDR in GC. In addition, miR-23b-3p could be used as a prognostic factor for overall survival in GC. miR-23b-3p inhibited autophagy mediated by ATG12 and HMGB2 and sensitized GC cells to chemotherapy, and suggested the potential application of miR-23b-3p in drug resistance prediction and treatment. | |||
| Key Molecule: hsa-miR-508-5p | [98] | |||
| Sensitive Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| In Vitro Model | SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 |
| SGC7901/VCR cells | Gastric | Homo sapiens (Human) | CVCL_VU58 | |
| SGC7901/ADR cells | Gastric | Homo sapiens (Human) | CVCL_VU57 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | The overexpression of miR-508-5p was sufficient to reverse cancer cell resistance to multiple chemotherapeutics in vitro and sensitize tumours to chemotherapy in vivo. Further studies showed that miR-508-5p could directly target the 3'-untranslated regions of ABCB1 and Zinc ribbon domain-containing 1 (ZNRD1), and suppress their expression at the mRNA and protein levels. Meanwhile, the suppression of ZNRD1 led to a decrease in ABCB1. | |||
| Key Molecule: hsa-mir-204 | [99] | |||
| Sensitive Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| In Vitro Model | NCI-N87 cells | Gastric | Homo sapiens (Human) | CVCL_1603 |
| GTL-16 cells | Gastric | Homo sapiens (Human) | CVCL_7668 | |
| In Vivo Model | CD1 nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
RT-PCR | |||
| Experiment for Drug Resistance |
Flow cytometry assay | |||
| Mechanism Description | miR-204 targeted Bcl-2 messenger RNA and increased responsiveness of GC cells to 5-fluorouracil and oxaliplatin treatment. Ectopic expression of Bcl-2 protein counteracted miR-204 pro-apoptotic activity in response to 5-fluorouracil. | |||
| Key Molecule: hsa-mir-27a | [100] | |||
| Sensitive Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell proliferation | Inhibition | hsa05200 | |
| Tumorigenesis | Inhibition | hsa05200 | ||
| In Vitro Model | MkN-45 cells | Gastric | Homo sapiens (Human) | CVCL_0434 |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
RT-PCR | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Down-regulation of miR-27a could also confer sensitivity of drugs on gastric cancer cells, and might increase accumulation and decrease releasing amount of adriamycin in gastric cancer cells. Down-regulation of miR-27a could significantly decrease the expression of P-glycoprotein and the transcriptional activity of cyclin D1, and up-regulate the expression of p21. | |||
| Key Molecule: hsa-mir-181 | [101] | |||
| Sensitive Disease | Gastric adenocarcinoma [ICD-11: 2B72.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| In Vitro Model | SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 |
| SGC7901/VCR cells | Gastric | Homo sapiens (Human) | CVCL_VU58 | |
| Experiment for Molecule Alteration |
RT-PCR | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | The antiapoptotic protein BCL2 is upregulated, whereas miR-181b is downregulated in both SGC7901/VCR and A549/CDDP cells, compared with SGC7901 and A549 cells, respectively. Enforced miR-181b expression reduced BCL2 protein level and sensitized SGC7901/VCR and A549/CDDP cells to VCR-induced and CDDP-induced apoptosis, respectively. | |||
|
|
||||
| Key Molecule: ATP-binding cassette sub-family B5 (ABCB5) | [55] | |||
| Sensitive Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Gastric cancer [ICD-11: 2B72] | |||
| The Specified Disease | Gastric cancer | |||
| The Studied Tissue | Gastric tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 9.75E-01 Fold-change: -5.59E-04 Z-score: -3.48E-02 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| In Vitro Model | SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miR-218 may inhibit efflux of ADM and oxaliplatin by down-regulating P-gp expression. | |||
| Key Molecule: Solute carrier family 34 member 2 (SLC34A2) | [90] | |||
| Sensitive Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell invasion | Inhibition | hsa05200 | |
| Cell migration | Inhibition | hsa04670 | ||
| RAF/MEK/ERK signaling pathway | Inhibition | hsa04010 | ||
| In Vitro Model | BGC-823 cells | Gastric | Homo sapiens (Human) | CVCL_3360 |
| MGC-803 cells | Gastric | Homo sapiens (Human) | CVCL_5334 | |
| SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 | |
| AGS cells | Gastric | Homo sapiens (Human) | CVCL_0139 | |
| GES-1 cells | Gastric | Homo sapiens (Human) | CVCL_EQ22 | |
| HGC27 cells | Gastric | Homo sapiens (Human) | CVCL_1279 | |
| MkN-45 cells | Gastric | Homo sapiens (Human) | CVCL_0434 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay; Colony formation assay; Flow cytometric analysis; Wound-healing, migration and invasion assay | |||
| Mechanism Description | Decreased expression of miR939 contributes to chemoresistance and metastasis of gastric cancer via dysregulation of SLC34A2 and Raf/MEk/ERk pathway. miR939 exerted its function mainly through inhibiting SLC34A2/Raf/MEk/ERk pathway, which is activated in GC. | |||
| Key Molecule: Multidrug resistance protein 1 (ABCB1) | [98] | |||
| Sensitive Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| In Vitro Model | SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 |
| SGC7901/VCR cells | Gastric | Homo sapiens (Human) | CVCL_VU58 | |
| SGC7901/ADR cells | Gastric | Homo sapiens (Human) | CVCL_VU57 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | The overexpression of miR-508-5p was sufficient to reverse cancer cell resistance to multiple chemotherapeutics in vitro and sensitize tumours to chemotherapy in vivo. Further studies showed that miR-508-5p could directly target the 3'-untranslated regions of ABCB1 and Zinc ribbon domain-containing 1 (ZNRD1), and suppress their expression at the mRNA and protein levels. Meanwhile, the suppression of ZNRD1 led to a decrease in ABCB1. | |||
|
|
||||
| Key Molecule: TGF-beta receptor type II (TGFBR2) | [56] | |||
| Sensitive Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Gastric cancer [ICD-11: 2B72] | |||
| The Specified Disease | Gastric cancer | |||
| The Studied Tissue | Gastric tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 9.24E-01 Fold-change: -4.81E-03 Z-score: -1.07E-01 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell invasion | Inhibition | hsa05200 | ||
| Cell proliferation | Inhibition | hsa05200 | ||
| Cell viability | Inhibition | hsa05200 | ||
| TGF-beta signaling pathway | Inhibition | hsa04350 | ||
| In Vitro Model | SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 |
| AGS cells | Gastric | Homo sapiens (Human) | CVCL_0139 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay; Caspase 3 assay kit | |||
| Mechanism Description | Sensitization of Gastric Cancer Cells to 5-FU by microRNA-204 Through Targeting the TGFBR2-Mediated Epithelial to Mesenchymal Transition. | |||
| Key Molecule: hsa-mir-30a | [102] | |||
| Sensitive Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 |
| SGC-7901/DDP cells | Gastric | Homo sapiens (Human) | CVCL_0520 | |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
MTT assay; Flow cytometry assay | |||
| Mechanism Description | miR30a can decrease multidrug resistance (MDR) of gastric cancer cells, miR30a overexpression decreased the expression of P-gp, a MDR-related protein. It is also an important miRNA modulating EMT of the cancer cells. | |||
| Key Molecule: Death effector domain-containing protein (DEDD) | [103] | |||
| Sensitive Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell viability | Inhibition | hsa05200 | ||
| In Vitro Model | SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 |
| BGC823 cells | Gastric | Homo sapiens (Human) | CVCL_3360 | |
| AGS cells | Gastric | Homo sapiens (Human) | CVCL_0139 | |
| HGC27 cells | Gastric | Homo sapiens (Human) | CVCL_1279 | |
| MkN-45 cells | Gastric | Homo sapiens (Human) | CVCL_0434 | |
| Experiment for Molecule Alteration |
Western blot analysis; RIP assay; Luciferase reporter assay | |||
| Experiment for Drug Resistance |
CCK8 assay; Flow cytometry assay | |||
| Mechanism Description | The inhibition of miR-17 may have tumor suppressive effects on GC and enhance its chemosensitivity by promoting DEDD, impairing EMT in GC cells. | |||
| Key Molecule: hsa-mir-17 | [103] | |||
| Sensitive Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell viability | Inhibition | hsa05200 | ||
| In Vitro Model | SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 |
| BGC823 cells | Gastric | Homo sapiens (Human) | CVCL_3360 | |
| AGS cells | Gastric | Homo sapiens (Human) | CVCL_0139 | |
| HGC27 cells | Gastric | Homo sapiens (Human) | CVCL_1279 | |
| MkN-45 cells | Gastric | Homo sapiens (Human) | CVCL_0434 | |
| Experiment for Molecule Alteration |
RT-qPCR | |||
| Experiment for Drug Resistance |
CCK8 assay; Flow cytometry assay | |||
| Mechanism Description | The inhibition of miR-17 may have tumor suppressive effects on GC and enhance its chemosensitivity by promoting DEDD, impairing EMT in GC cells. | |||
| Key Molecule: hsa-mir-204 | [56] | |||
| Sensitive Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell proliferation | Inhibition | hsa05200 | ||
| Cell viability | Inhibition | hsa05200 | ||
| TGF-beta signaling pathway | Inhibition | hsa04350 | ||
| In Vitro Model | SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 |
| AGS cells | Gastric | Homo sapiens (Human) | CVCL_0139 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
RT-PCR | |||
| Experiment for Drug Resistance |
MTT assay; Caspase 3 assay kit | |||
| Mechanism Description | Sensitization of Gastric Cancer Cells to 5-FU by microRNA-204 Through Targeting the TGFBR2-Mediated Epithelial to Mesenchymal Transition. | |||
| Key Molecule: Long non-protein coding RNA (LEIGC) | [104] | |||
| Sensitive Disease | Gastric carcinoma [ICD-11: 2B72.Z] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell migration | Inhibition | hsa04670 | |
| Cell proliferation | Inhibition | hsa05200 | ||
| In Vitro Model | MGC-803 cells | Gastric | Homo sapiens (Human) | CVCL_5334 |
| SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 | |
| AGS cells | Gastric | Homo sapiens (Human) | CVCL_0139 | |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
MTS assay | |||
| Mechanism Description | Overexpression of LEIGC suppressed tumor growth and cell proliferation, and (+) the sensitivity of gastric cancer cells to 5-fluorouracil (5-FU), whereas knockdown of LEIGC showed the opposite effect. We further demonstrated LEIGC functions by inhibiting the epithelial-to-mesenchymal transition (EMT) in gastric cancer. | |||
|
|
||||
| Key Molecule: Transforming protein RhoA (RHOA) | [66] | |||
| Sensitive Disease | Gastric adenocarcinoma [ICD-11: 2B72.0] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Gastric cancer [ICD-11: 2B72] | |||
| The Specified Disease | Gastric cancer | |||
| The Studied Tissue | Gastric tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 2.78E-02 Fold-change: -4.29E-02 Z-score: -4.37E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell invasion | Inhibition | hsa05200 | |
| Cell migration | Inhibition | hsa04670 | ||
| Cell proliferation | Inhibition | hsa05200 | ||
| In Vitro Model | 293T cells | Breast | Homo sapiens (Human) | CVCL_0063 |
| MkN-45 cells | Gastric | Homo sapiens (Human) | CVCL_0434 | |
| Experiment for Molecule Alteration |
qRT-PCR; Western blot analysis; Immunohistochemical assay | |||
| Experiment for Drug Resistance |
MTT assay; Flow cytometric analysis | |||
| Mechanism Description | Induction of miR31 in MkN-45 followed by suppression of RhoA expression resulted in increased sensitivity to 5-fluorouracil, inhibition of cell proliferation, and invasion compared to the control groups. | |||
| Key Molecule: Smoothened homolog (SMO) | [55] | |||
| Sensitive Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Gastric cancer [ICD-11: 2B72] | |||
| The Specified Disease | Gastric cancer | |||
| The Studied Tissue | Gastric tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 3.86E-01 Fold-change: -1.09E-01 Z-score: -1.10E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| In Vitro Model | SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Higher miR-218 levels increased the level of Bax and reduced the level of Bcl-2 and miR-218 inhibits multidrug resistance (MDR) of gastric cancer cells by targeting Hedgehog/smoothened. | |||
| Key Molecule: Phosphatase and tensin homolog (PTEN) | [88] | |||
| Sensitive Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell proliferation | Inhibition | hsa05200 | |
| PI3K/AKT signaling pathway | Inhibition | hsa04151 | ||
| In Vitro Model | BGC-823 cells | Gastric | Homo sapiens (Human) | CVCL_3360 |
| MGC-803 cells | Gastric | Homo sapiens (Human) | CVCL_5334 | |
| SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 | |
| AGS cells | Gastric | Homo sapiens (Human) | CVCL_0139 | |
| MkN-45 cells | Gastric | Homo sapiens (Human) | CVCL_0434 | |
| Experiment for Molecule Alteration |
Luciferase reporter assay; Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay; Flow cytometric analysis | |||
| Mechanism Description | miR147 suppressed the proliferation and enhanced the chemosensitivity of gastric cancer cells to 5-FU by promoting cell apoptosis through directly targeting PTEN and regulating the PI3k/AkT signaling pathway. knockdown of pten reverses the effects of miR147 downregulation on gastric cancer cells. | |||
| Key Molecule: Transcription factor E2F6 (E2F6) | [91] | |||
| Sensitive Disease | Gastric adenocarcinoma [ICD-11: 2B72.0] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell invasion | Inhibition | hsa05200 | ||
| Cell proliferation | Inhibition | hsa05200 | ||
| Cell viability | Inhibition | hsa05200 | ||
| In Vitro Model | AGS cells | Gastric | Homo sapiens (Human) | CVCL_0139 |
| MkN-45 cells | Gastric | Homo sapiens (Human) | CVCL_0434 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay; Flow cytometry assay | |||
| Mechanism Description | MkN-45-miR-31 showed an increased sensitivity to 5-FU, decreased migration and cell invasion compared to the control groups and induction of miR-31 expression in MkN-45 caused a significant reduction of E2F6 and SMUG1 genes. | |||
| Key Molecule: Single-strand selective monofunctional uracil DNA glycosylase (SMUG1) | [91] | |||
| Sensitive Disease | Gastric adenocarcinoma [ICD-11: 2B72.0] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell invasion | Inhibition | hsa05200 | ||
| Cell proliferation | Inhibition | hsa05200 | ||
| Cell viability | Inhibition | hsa05200 | ||
| In Vitro Model | AGS cells | Gastric | Homo sapiens (Human) | CVCL_0139 |
| MkN-45 cells | Gastric | Homo sapiens (Human) | CVCL_0434 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay; Flow cytometry assay | |||
| Mechanism Description | MkN-45-miR-31 showed an increased sensitivity to 5-FU, decreased migration and cell invasion compared to the control groups and induction of miR-31 expression in MkN-45 caused a significant reduction of E2F6 and SMUG1 genes. | |||
| Key Molecule: Zeste homolog 2 (ZH2) | [92] | |||
| Sensitive Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell colony | Inhibition | hsa05200 | ||
| Cell invasion | Inhibition | hsa05200 | ||
| Cell migration | Inhibition | hsa04670 | ||
| Cell viability | Inhibition | hsa05200 | ||
| In Vitro Model | AGS cells | Gastric | Homo sapiens (Human) | CVCL_0139 |
| SNU-1 cells | Gastric | Homo sapiens (Human) | CVCL_0099 | |
| SNU-5 cells | Gastric | Homo sapiens (Human) | CVCL_0078 | |
| SNU-16 cells | Gastric | Homo sapiens (Human) | CVCL_0076 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | microRNA-31 triggers G 2/M cell cycle arrest, enhances the chemosensitivity and inhibits migration and invasion of human gastric cancer cells by downregulating the expression of zeste homolog 2 (ZH2). | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Long non-protein coding RNA 261 (LINC00261) | [48] | |||
| Sensitive Disease | Esophageal cancer [ICD-11: 2B70.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Esophageal cancer [ICD-11: 2B70] | |||
| The Specified Disease | Esophageal carcinoma | |||
| The Studied Tissue | Esophagus | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 9.38E-04 Fold-change: 2.73E+00 Z-score: 3.38E+00 |
|||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell colony | Inhibition | hsa05200 | ||
| Cell viability | Inhibition | hsa05200 | ||
| In Vitro Model | ECA-109 cells | Esophagus | Homo sapiens (Human) | CVCL_6898 |
| TE-1 cells | Esophagus | Homo sapiens (Human) | CVCL_1759 | |
| KYSE150 cells | Esophagus | Homo sapiens (Human) | CVCL_1348 | |
| TE-5 cells | Esophageal | Homo sapiens (Human) | CVCL_1764 | |
| In Vivo Model | BALB/c nude mouse xenograft mode | Mus musculus | ||
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
WST-1 assay; Flow cytometry assay | |||
| Mechanism Description | Long noncoding RNA LINC00261 induces chemosensitization to 5-fluorouracil by mediating methylation-dependent repression of DPYD in human esophageal cancer. | |||
| Key Molecule: Tumor suppressor candidate 7 (TUSC7) | [51] | |||
| Sensitive Disease | Esophageal squamous cell carcinoma [ICD-11: 2B70.3] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Esophageal cancer [ICD-11: 2B70] | |||
| The Specified Disease | Esophageal carcinoma | |||
| The Studied Tissue | Esophagus | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 2.61E-01 Fold-change: 2.29E+00 Z-score: 1.16E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell viability | Inhibition | hsa05200 | |
| DESC1/EGFR/AKT signaling pathway | Regulation | N.A. | ||
| In Vitro Model | KYSE30 cells | Esophagus | Homo sapiens (Human) | CVCL_1351 |
| EC9706 cells | Esophagus | Homo sapiens (Human) | CVCL_E307 | |
| KYSE140 cells | Esophagus | Homo sapiens (Human) | CVCL_1347 | |
| TE13 cells | Esophageal | Homo sapiens (Human) | CVCL_4463 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | TUSC7 suppressed the proliferation and chemotherapy resistance of ESCC cells by increasing DESC1 expression via inhibiting miR-224. | |||
| Key Molecule: hsa-miR-130a-3p | [77] | |||
| Sensitive Disease | Esophageal squamous cell carcinoma [ICD-11: 2B70.3] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell migration | Inhibition | hsa04670 | ||
| p53 signaling pathway | Activation | hsa04115 | ||
| In Vitro Model | KYSE-270 cells | Esophagus | Homo sapiens (Human) | CVCL_1350 |
| KYSE-410 cells | Esophagus | Homo sapiens (Human) | CVCL_1352 | |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
Flow cytometry assay | |||
| Mechanism Description | The effect of miR-130a-3p downregulation on enhancement of protein levels was more pronounced for Bcl-2 compared to XIAP, whereas the increase of miR-130a-3p resulted in a more pronounced increase of protein levels of XIAP compared to Bcl-2. Both, up- and downregulation of miR-130a-3p and miR-148a-3p increased sensitivity towards chemotherapy in ESCC and complex role of miR-130a-3p and miR-148a-3p balance on drug resistance and tumor biology in esophageal squamous cell carcinoma. | |||
| Key Molecule: hsa-miR-130a-3p | [77] | |||
| Sensitive Disease | Esophageal squamous cell carcinoma [ICD-11: 2B70.3] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | p53 signaling pathway | Activation | hsa04115 | |
| In Vitro Model | KYSE-270 cells | Esophagus | Homo sapiens (Human) | CVCL_1350 |
| KYSE-410 cells | Esophagus | Homo sapiens (Human) | CVCL_1352 | |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
Flow cytometry assay | |||
| Mechanism Description | The effect of miR-130a-3p upregulation on suppression of protein levels was more pronounced for Bcl-2 compared to XIAP, whereas the inhibition of miR-130a-3p resulted in a more pronounced increase of protein levels of XIAP compared to Bcl-2. Both, up- and downregulation of miR-130a-3p and miR-148a-3p increased sensitivity towards chemotherapy in ESCC and complex role of miR-130a-3p and miR-148a-3p balance on drug resistance and tumor biology in esophageal squamous cell carcinoma. | |||
| Key Molecule: hsa-miR-148a-3p | [77] | |||
| Sensitive Disease | Esophageal squamous cell carcinoma [ICD-11: 2B70.3] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell migration | Inhibition | hsa04670 | ||
| p53 signaling pathway | Activation | hsa04115 | ||
| In Vitro Model | KYSE-270 cells | Esophagus | Homo sapiens (Human) | CVCL_1350 |
| KYSE-410 cells | Esophagus | Homo sapiens (Human) | CVCL_1352 | |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
Flow cytometry assay | |||
| Mechanism Description | The effect of miR-148a-3p downregulation on enhancement of protein levels was more pronounced for Bcl-2 compared to XIAP, whereas the increase of miR-130a-3p resulted in a more pronounced increase of protein levels of XIAP compared to Bcl-2. Both, up- and downregulation of miR-130a-3p and miR-148a-3p increased sensitivity towards chemotherapy in ESCC and complex role of miR-130a-3p and miR-148a-3p balance on drug resistance and tumor biology in esophageal squamous cell carcinoma. | |||
| Key Molecule: hsa-miR-148a-3p | [77] | |||
| Sensitive Disease | Esophageal squamous cell carcinoma [ICD-11: 2B70.3] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | p53 signaling pathway | Activation | hsa04115 | |
| In Vitro Model | KYSE-270 cells | Esophagus | Homo sapiens (Human) | CVCL_1350 |
| KYSE-410 cells | Esophagus | Homo sapiens (Human) | CVCL_1352 | |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
Flow cytometry assay | |||
| Mechanism Description | The effect of miR-148a-3p upregulation on suppression of protein levels was more pronounced for Bcl-2 compared to XIAP, whereas the inhibition of miR-130a-3p resulted in a more pronounced increase of protein levels of XIAP compared to Bcl-2. Both, up- and downregulation of miR-130a-3p and miR-148a-3p increased sensitivity towards chemotherapy in ESCC and complex role of miR-130a-3p and miR-148a-3p balance on drug resistance and tumor biology in esophageal squamous cell carcinoma. | |||
| Key Molecule: hsa-mir-224 | [51] | |||
| Sensitive Disease | Esophageal squamous cell carcinoma [ICD-11: 2B70.3] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell viability | Inhibition | hsa05200 | |
| DESC1/EGFR/AKT signaling pathway | Regulation | N.A. | ||
| In Vitro Model | KYSE30 cells | Esophagus | Homo sapiens (Human) | CVCL_1351 |
| EC9706 cells | Esophagus | Homo sapiens (Human) | CVCL_E307 | |
| KYSE140 cells | Esophagus | Homo sapiens (Human) | CVCL_1347 | |
| TE13 cells | Esophageal | Homo sapiens (Human) | CVCL_4463 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | TUSC7 suppressed the proliferation and chemotherapy resistance of ESCC cells by increasing DESC1 expression via inhibiting miR-224. | |||
| Key Molecule: hsa-mir-200c | [78] | |||
| Sensitive Disease | Esophageal cancer [ICD-11: 2B70.1] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
Endoscopy; Computed tomography assay; Positron emission tomography assay | |||
| Mechanism Description | Serum miR-200c levels are useful for predicting the response to chemotherapy (cisplatin, 5-fluorouracil, and Adriamycin (ACF) or cisplatin, 5-fluorouracil, and docetaxel (DCF) ) in patients with esophageal cancer who underwent preoperative chemotherapy followed by surgery. | |||
| Key Molecule: hsa-mir-148a | [79] | |||
| Sensitive Disease | Esophageal adenocarcinoma [ICD-11: 2B70.2] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | kYSE410 cells | Esophagus | Homo sapiens (Human) | CVCL_1352 |
| Experiment for Molecule Alteration |
RT-PCR | |||
| Experiment for Drug Resistance |
MTS assay | |||
| Mechanism Description | miR-148a sensitized chemotherapy-sensitive oesophageal cancer cell lines to cisplatin and, to a lesser extent, to 5-flurouracil and attenuated resistance in chemotherapy-resistant variants. | |||
| Key Molecule: hsa-mir-148a | [79] | |||
| Sensitive Disease | Esophageal squamous cell carcinoma [ICD-11: 2B70.3] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | kYSE410 cells | Esophagus | Homo sapiens (Human) | CVCL_1352 |
| Experiment for Molecule Alteration |
RT-PCR | |||
| Experiment for Drug Resistance |
MTS assay | |||
| Mechanism Description | miR-148a sensitized chemotherapy-sensitive oesophageal cancer cell lines to cisplatin and, to a lesser extent, to 5-flurouracil and attenuated resistance in chemotherapy-resistant variants. | |||
| Key Molecule: hsa-mir-296 | [80] | |||
| Sensitive Disease | Esophageal squamous cell carcinoma [ICD-11: 2B70.3] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell growth | Inhibition | hsa05200 | ||
| In Vitro Model | ECA-109 cells | Esophagus | Homo sapiens (Human) | CVCL_6898 |
| Experiment for Molecule Alteration |
RT-PCR; Northern blotting analysis | |||
| Experiment for Drug Resistance |
WST-1 assay | |||
| Mechanism Description | Down-regulation of miR-296 could confer sensitivity of both P-glycoprotein-related and P-glycoprotein-nonrelated drugs on esophageal cancer cells, and might promote ADR-induced apoptosis, accompanied by increased accumulation and decreased releasing amount of ADR. Down-regulation of miR-296 could significantly decrease the expression of P-glycoprotein, Bcl-2, and the transcription of MDR1, but up-regulate the expression of Bax. | |||
| Key Molecule: hsa-mir-27a | [81] | |||
| Sensitive Disease | Esophageal squamous cell carcinoma [ICD-11: 2B70.3] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | ECA-109 cells | Esophagus | Homo sapiens (Human) | CVCL_6898 |
| TE13 cells | Esophageal | Homo sapiens (Human) | CVCL_4463 | |
| Experiment for Molecule Alteration |
qRT-PCR; Northern blotting analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Down-regulation of miR-27a significantly decreased expression of MDR1, but did not alter the expression of MRP, miR-27a could possibly mediate drug resistance, at least in part through regulation of MDR1 and apoptosis. | |||
|
|
||||
| Key Molecule: Multidrug resistance protein 1 (ABCB1) | [80], [81] | |||
| Sensitive Disease | Esophageal squamous cell carcinoma [ICD-11: 2B70.3] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell growth | Inhibition | hsa05200 | ||
| In Vitro Model | ECA-109 cells | Esophagus | Homo sapiens (Human) | CVCL_6898 |
| TE13 cells | Esophageal | Homo sapiens (Human) | CVCL_4463 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
WST-1 assay | |||
| Mechanism Description | Down-regulation of miR-296 could confer sensitivity of both P-glycoprotein-related and P-glycoprotein-nonrelated drugs on esophageal cancer cells, and might promote ADR-induced apoptosis, accompanied by increased accumulation and decreased releasing amount of ADR. Down-regulation of miR-296 could significantly decrease the expression of P-glycoprotein, Bcl-2, and the transcription of MDR1, but up-regulate the expression of Bax. And down-regulation of miR-27a significantly decreased expression of MDR1, but did not alter the expression of MRP, miR-27a could possibly mediate drug resistance, at least in part through regulation of MDR1 and apoptosis. | |||
|
|
||||
| Key Molecule: Dihydropyrimidine dehydrogenase [NADP(+)] | [48] | |||
| Sensitive Disease | Esophageal cancer [ICD-11: 2B70.1] | |||
| Molecule Alteration | Methylation | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell colony | Inhibition | hsa05200 | ||
| Cell viability | Inhibition | hsa05200 | ||
| In Vitro Model | ECA-109 cells | Esophagus | Homo sapiens (Human) | CVCL_6898 |
| TE-1 cells | Esophagus | Homo sapiens (Human) | CVCL_1759 | |
| KYSE150 cells | Esophagus | Homo sapiens (Human) | CVCL_1348 | |
| TE-5 cells | Esophageal | Homo sapiens (Human) | CVCL_1764 | |
| In Vivo Model | BALB/c nude mouse xenograft mode | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
WST-1 assay; Flow cytometry assay | |||
| Mechanism Description | Long noncoding RNA LINC00261 induces chemosensitization to 5-fluorouracil by mediating methylation-dependent repression of DPYD in human esophageal cancer. | |||
| Key Molecule: Transmembrane protease serine 11E (TM11E) | [51] | |||
| Sensitive Disease | Esophageal squamous cell carcinoma [ICD-11: 2B70.3] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell viability | Inhibition | hsa05200 | |
| DESC1/EGFR/AKT signaling pathway | Regulation | N.A. | ||
| In Vitro Model | KYSE30 cells | Esophagus | Homo sapiens (Human) | CVCL_1351 |
| EC9706 cells | Esophagus | Homo sapiens (Human) | CVCL_E307 | |
| KYSE140 cells | Esophagus | Homo sapiens (Human) | CVCL_1347 | |
| TE13 cells | Esophageal | Homo sapiens (Human) | CVCL_4463 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | TUSC7 suppressed the proliferation and chemotherapy resistance of ESCC cells by increasing DESC1 expression via inhibiting miR-224. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: hsa-miR-141-3p | [76] | |||
| Resistant Disease | Esophageal cancer [ICD-11: 2B70.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | TE-1 cells | Esophagus | Homo sapiens (Human) | CVCL_1759 |
| EC9706 cells | Esophagus | Homo sapiens (Human) | CVCL_E307 | |
| KYSE150 cells | Esophagus | Homo sapiens (Human) | CVCL_1348 | |
| EC109 cells | Esophagus | Homo sapiens (Human) | CVCL_6898 | |
| EC9706-R cells | Esophagus | Homo sapiens (Human) | CVCL_E307 | |
| Het-1A cells | Esophagus | Homo sapiens (Human) | CVCL_3702 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
RT-PCR | |||
| Experiment for Drug Resistance |
MTT assay; Annexin V-FITC Apoptosis Detection assay | |||
| Mechanism Description | Involvement of microRNA-141-3p in 5-fluorouracil and oxaliplatin chemo-resistance in esophageal cancer cells via down-regulation of PTEN. | |||
| Key Molecule: hsa-mir-221 | [8] | |||
| Resistant Disease | Esophageal adenocarcinoma [ICD-11: 2B70.2] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell proliferation | Activation | hsa05200 | ||
| Wnt/Beta-catenin/EMT signaling pathway | Activation | hsa04310 | ||
| In Vitro Model | OE19 cells | Esophagus | Homo sapiens (Human) | CVCL_1622 |
| OE33 cellss | Esophagus | Homo sapiens (Human) | CVCL_0471 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
Annexin V-fluorescein isothiocyanate (FITC) and propidium iodide (PI) staining assay | |||
| Mechanism Description | miR-221 mediates chemoresistance of esophageal adenocarcinoma by direct targeting and reducing of Dkk2 expression. | |||
| Key Molecule: hsa-miR-193a-3p | [19] | |||
| Resistant Disease | Esophageal cancer [ICD-11: 2B70.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell proliferation | Activation | hsa05200 | ||
| In Vitro Model | KYSE150 cells | Esophagus | Homo sapiens (Human) | CVCL_1348 |
| KYSE510 cells | Esophagus | Homo sapiens (Human) | CVCL_1354 | |
| kYSE410 cells | Esophagus | Homo sapiens (Human) | CVCL_1352 | |
| kYSE450 cells | Esophagus | Homo sapiens (Human) | CVCL_1353 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Over-expression of miR-193a-3p increased the radioresistance and chemoresistance of oesophageal squamous cell carcinoma (ESCC) cells. In contrast, the down-regulation of miR-193a-3p decreased the radioresistance and chemoresistance of ESCC cells. In addition, miR-193a-3p inducing DNA damage has also been demonstrated through measuring the level of gamma-H2AX associated with miR-193a-3p. Moreover, a small interfering RNA(siRNA)-induced repression of the PSEN1 gene had an effect similar to that of miR-193a-3p up-regulation. The above processes also inhibited oesophageal cancer cells apoptosis. These findings suggest that miR-193a-3p contributes to the radiation and chemotherapy resistance of oesophageal carcinoma by down-regulating PSEN1. | |||
| Key Molecule: hsa-miR-193a-3p | [19] | |||
| Resistant Disease | Esophageal cancer [ICD-11: 2B70.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | KYSE150 cells | Esophagus | Homo sapiens (Human) | CVCL_1348 |
| KYSE510 cells | Esophagus | Homo sapiens (Human) | CVCL_1354 | |
| kYSE410 cells | Esophagus | Homo sapiens (Human) | CVCL_1352 | |
| kYSE450 cells | Esophagus | Homo sapiens (Human) | CVCL_1353 | |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Over-expression of miR-193a-3p increased the radioresistance and chemoresistance of oesophageal squamous cell carcinoma (ESCC) cells. The regulation role of miR-193a-3p on multi-chemoresistance and radioresistance were mediated by PSEN1. | |||
| Key Molecule: hsa-miR-193a-3p | [19] | |||
| Resistant Disease | Esophageal cancer [ICD-11: 2B70.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | KYSE150 cells | Esophagus | Homo sapiens (Human) | CVCL_1348 |
| KYSE510 cells | Esophagus | Homo sapiens (Human) | CVCL_1354 | |
| kYSE410 cells | Esophagus | Homo sapiens (Human) | CVCL_1352 | |
| kYSE450 cells | Esophagus | Homo sapiens (Human) | CVCL_1353 | |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Over-expression of miR-193a-3p increased the radioresistance and chemoresistance of oesophageal squamous cell carcinoma (ESCC) cells. The regulation role of miR-193a-3p on multi-chemoresistance and radioresistance were mediated by PSEN1. | |||
|
|
||||
| Key Molecule: Phosphatase and tensin homolog (PTEN) | [76] | |||
| Resistant Disease | Esophageal cancer [ICD-11: 2B70.1] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | TE-1 cells | Esophagus | Homo sapiens (Human) | CVCL_1759 |
| EC9706 cells | Esophagus | Homo sapiens (Human) | CVCL_E307 | |
| KYSE150 cells | Esophagus | Homo sapiens (Human) | CVCL_1348 | |
| EC109 cells | Esophagus | Homo sapiens (Human) | CVCL_6898 | |
| EC9706-R cells | Esophagus | Homo sapiens (Human) | CVCL_E307 | |
| Het-1A cells | Esophagus | Homo sapiens (Human) | CVCL_3702 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay; Annexin V-FITC Apoptosis Detection assay | |||
| Mechanism Description | Involvement of microRNA-141-3p in 5-fluorouracil and oxaliplatin chemo-resistance in esophageal cancer cells via down-regulation of PTEN. | |||
| Key Molecule: Dickkopf-related protein 2 (DKK2) | [8] | |||
| Resistant Disease | Esophageal adenocarcinoma [ICD-11: 2B70.2] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell colony | Activation | hsa05200 | ||
| Cell proliferation | Activation | hsa05200 | ||
| Wnt/Beta-catenin/EMT signaling pathway | Activation | hsa04310 | ||
| In Vitro Model | OE19 cells | Esophagus | Homo sapiens (Human) | CVCL_1622 |
| OE33 cellss | Esophagus | Homo sapiens (Human) | CVCL_0471 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Annexin V-fluorescein isothiocyanate (FITC) and propidium iodide (PI) staining assay | |||
| Mechanism Description | miR-221 mediates chemoresistance of esophageal adenocarcinoma by direct targeting and reducing of Dkk2 expression. | |||
| Key Molecule: Presenilin-1 (PSEN1) | [19] | |||
| Resistant Disease | Esophageal cancer [ICD-11: 2B70.1] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell proliferation | Activation | hsa05200 | ||
| In Vitro Model | KYSE150 cells | Esophagus | Homo sapiens (Human) | CVCL_1348 |
| KYSE510 cells | Esophagus | Homo sapiens (Human) | CVCL_1354 | |
| kYSE410 cells | Esophagus | Homo sapiens (Human) | CVCL_1352 | |
| kYSE450 cells | Esophagus | Homo sapiens (Human) | CVCL_1353 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Over-expression of miR-193a-3p increased the radioresistance and chemoresistance of oesophageal squamous cell carcinoma (ESCC) cells. In contrast, the down-regulation of miR-193a-3p decreased the radioresistance and chemoresistance of ESCC cells. In addition, miR-193a-3p inducing DNA damage has also been demonstrated through measuring the level of gamma-H2AX associated with miR-193a-3p. Moreover, a small interfering RNA(siRNA)-induced repression of the PSEN1 gene had an effect similar to that of miR-193a-3p up-regulation. The above processes also inhibited oesophageal cancer cells apoptosis. These findings suggest that miR-193a-3p contributes to the radiation and chemotherapy resistance of oesophageal carcinoma by down-regulating PSEN1. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Caspase-9 (CASP9) | [57] | |||
| Resistant Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Breast cancer [ICD-11: 2C60] | |||
| The Specified Disease | Breast cancer | |||
| The Studied Tissue | Breast tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.78E-02 Fold-change: -1.19E-02 Z-score: -2.38E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell invasion | Activation | hsa05200 | ||
| Cell migration | Activation | hsa04670 | ||
| Epithelial mesenchymal transition signaling pathway | Activation | hsa01521 | ||
| In Vitro Model | MCF-7 cells | Breast | Homo sapiens (Human) | CVCL_0031 |
| MDA-MB-231 cells | Breast | Homo sapiens (Human) | CVCL_0062 | |
| MDA-MB-468 cells | Breast | Homo sapiens (Human) | CVCL_0419 | |
| Experiment for Molecule Alteration |
Western blot analysis; TUNEL assay | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Long noncoding RNA LINP1 acts as an oncogene and promotes chemoresistance against 5-fluoroutacil and doxorubicin by inhibiting chemotherapeutics-induced apoptosis (apoptosis-related proteins such as caspase-8, caspase-9 and Bax proteins) in breast cancer. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Nuclear factor of activated T-cells 3 (NFATC3) | [60] | |||
| Sensitive Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Breast cancer [ICD-11: 2C60] | |||
| The Specified Disease | Breast cancer | |||
| The Studied Tissue | Breast tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.18E-08 Fold-change: -2.53E-02 Z-score: -5.81E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell proliferation | Inhibition | hsa05200 | |
| TRPC5 signaling pathway | Regulation | N.A. | ||
| In Vitro Model | MCF-7/ADM cells | Breast | Homo sapiens (Human) | CVCL_0031 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | The overexpression of miR-320a, which downregulated TRPC5 and NFATC3, colud inreduce chemoresistance. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: ETS homologous factor (EHF) | [69] | |||
| Sensitive Disease | Oral squamous cell carcinoma [ICD-11: 2B6E.0] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Oral squamous cell carcinoma [ICD-11: 2B6E] | |||
| The Specified Disease | Oral cancer | |||
| The Studied Tissue | Oral tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.86E-05 Fold-change: -1.81E-01 Z-score: -4.92E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Beta5-integrin/c-Met signaling pathway | Inhibition | hsa01521 | |
| Cell apoptosis | Activation | hsa04210 | ||
| Cell invasion | Inhibition | hsa05200 | ||
| Cell migration | Inhibition | hsa04670 | ||
| Cell viability | Inhibition | hsa05200 | ||
| In Vitro Model | C9-IV3 cells | Oral | Homo sapiens (Human) | N.A. |
| CGHNC9 cells | Oral | Homo sapiens (Human) | N.A. | |
| OC-3 cells | Oral | Homo sapiens (Human) | CVCL_WL09 | |
| In Vivo Model | CB17-SCID mice xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay; Flow cytometry assay | |||
| Mechanism Description | miR-365-3p targets EHF to inhibit OSCC migration, invasion, and metastasis through kRT16. | |||
|
|
||||
| Key Molecule: hsa-miR-365a-3p | [69] | |||
| Sensitive Disease | Oral squamous cell carcinoma [ICD-11: 2B6E.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Beta5-integrin/c-Met signaling pathway | Inhibition | hsa01521 | |
| Cell viability | Activation | hsa05200 | ||
| In Vitro Model | C9-IV3 cells | Oral | Homo sapiens (Human) | N.A. |
| CGHNC9 cells | Oral | Homo sapiens (Human) | N.A. | |
| OC-3 cells | Oral | Homo sapiens (Human) | CVCL_WL09 | |
| In Vivo Model | CB17-SCID mice xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
RT-PCR | |||
| Experiment for Drug Resistance |
MTT assay; Flow cytometry assay | |||
| Mechanism Description | miR-365-3p targets EHF to inhibit OSCC migration, invasion, and metastasis through kRT16. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: hsa-miR-654-5p | [23] | |||
| Resistant Disease | Oral squamous cell carcinoma [ICD-11: 2B6E.0] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | MAPK/RAS signaling pathway | Regulation | N.A. | |
| In Vitro Model | Tca8113 cells | Tongue | Homo sapiens (Human) | CVCL_6851 |
| CAL-27 cells | Tongue | Homo sapiens (Human) | CVCL_1107 | |
| Experiment for Molecule Alteration |
RT-PCR | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miR654-5p targets GRAP to promote proliferation, metastasis, and chemoresistance of oral squamous cell carcinoma through Ras/MAPk signaling. | |||
|
|
||||
| Key Molecule: GRB2-related adapter protein (GRAP) | [23] | |||
| Resistant Disease | Oral squamous cell carcinoma [ICD-11: 2B6E.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | MAPK/RAS signaling pathway | Regulation | N.A. | |
| In Vitro Model | Tca8113 cells | Tongue | Homo sapiens (Human) | CVCL_6851 |
| CAL-27 cells | Tongue | Homo sapiens (Human) | CVCL_1107 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miR654-5p targets GRAP to promote proliferation, metastasis, and chemoresistance of oral squamous cell carcinoma through Ras/MAPk signaling. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Pyruvate kinase muscle isozyme 1 (PKM1) | [17] | |||
| Metabolic Type | Mitochondrial metabolism | |||
| Resistant Disease | Chronic myeloid leukemia [ICD-11: 2A20.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | DLD-1 cells | Colon | Homo sapiens (Human) | CVCL_0248 |
| K562 cells | Blood | Homo sapiens (Human) | CVCL_0004 | |
| Experiment for Molecule Alteration |
Expression profiles | |||
| Experiment for Drug Resistance |
Cell viability assay | |||
| Mechanism Description | The overexpression of PKM1 resulted in resistance of the parental cells to 5-FU and oxaliplatin. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Cysteine and glycine-rich protein 1 (CSRP1) | [73] | |||
| Sensitive Disease | Acute myeloid leukemia [ICD-11: 2A60.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Discovered Using In-vivo Testing Model | |||
| Cell Pathway Regulation | Rap1 signaling pathway | Activation | hsa04015 | |
| HIF-1 signaling pathway | Activation | hsa04066 | ||
| JAK-STAT signaling pathway | Activation | hsa04630 | ||
| In Vivo Model | Patient-derived advanced AML model | Homo sapiens | ||
| Experiment for Drug Resistance |
OncoPredict assay | |||
| Mechanism Description | Based on the findings, the high?CSRP1?groups of patients in the TCGA datasets showed higher sensitivity to 5-fluorouracil, gemcitabine, rapamycin, and cisplatin and lower sensitivity to fludarabine. CSRP1 may serve as a potential prognostic marker and a therapeutic target for AML in the future. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: hsa-mir-138 | [74] | |||
| Sensitive Disease | Leukemia [ICD-11: 2B33.6] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| In Vitro Model | HL60 cells | Peripheral blood | Homo sapiens (Human) | CVCL_0002 |
| Experiment for Molecule Alteration |
qRT-PCR; Northern blotting analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miR-138 was found up-regulated in the vincristine-induced multidrug resistance (MDR) leukemia cell line HL-60/VCR as compared with HL-60 cells. Up-regulation of miR-138 could reverse resistance of both P-glycoprotein-related and P-glycoprotein-non-related drugs on HL-60/VCR cells, and promote adriamycin-induced apoptosis, accompanied by increased accumulation and decreased releasing amount of adriamycin. | |||
|
|
||||
| Key Molecule: Multidrug resistance protein 1 (ABCB1) | [74] | |||
| Sensitive Disease | Leukemia [ICD-11: 2B33.6] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| In Vitro Model | HL60 cells | Peripheral blood | Homo sapiens (Human) | CVCL_0002 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miR-138 was found up-regulated in the vincristine-induced multidrug resistance (MDR) leukemia cell line HL-60/VCR as compared with HL-60 cells. Up-regulation of miR-138 could reverse resistance of both P-glycoprotein-related and P-glycoprotein-non-related drugs on HL-60/VCR cells, and promote adriamycin-induced apoptosis, accompanied by increased accumulation and decreased releasing amount of adriamycin. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: hsa-mir-140 | [35] | |||
| Sensitive Disease | Osteosarcoma [ICD-11: 2B51.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell proliferation | Inhibition | hsa05200 | |
| In Vitro Model | MG63 cells | Bone marrow | Homo sapiens (Human) | CVCL_0426 |
| U2OS cells | Bone | Homo sapiens (Human) | CVCL_0042 | |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
WST-1 assay | |||
| Mechanism Description | miR-140 is involved in the chemoresistance by reduced cell proliferation via G1 and G2 phase arrest mediated in part. | |||
|
|
||||
| Key Molecule: Histone deacetylase 4 (HDAC4) | [35] | |||
| Sensitive Disease | Osteosarcoma [ICD-11: 2B51.0] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell proliferation | Inhibition | hsa05200 | |
| In Vitro Model | MG63 cells | Bone marrow | Homo sapiens (Human) | CVCL_0426 |
| U2OS cells | Bone | Homo sapiens (Human) | CVCL_0042 | |
| Experiment for Molecule Alteration |
Western blot analysis; Immunofluorescence analysis | |||
| Experiment for Drug Resistance |
WST-1 assay | |||
| Mechanism Description | miR-140 is involved in the chemoresistance by reduced cell proliferation via G1 and G2 phase arrest mediated in part. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: hsa-miR-3188 | [75] | |||
| Sensitive Disease | Nasopharyngeal carcinoma [ICD-11: 2B6B.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell proliferation | Inhibition | hsa05200 | |
| PI3K/AKT/mTOR signaling pathway | Inhibition | hsa04151 | ||
| In Vitro Model | HNE1 cells | Nasopharynx | Homo sapiens (Human) | CVCL_0308 |
| 5-8F cells | Nasopharynx | Homo sapiens (Human) | CVCL_C528 | |
| CNE2 cells | Nasopharynx | Homo sapiens (Human) | CVCL_6889 | |
| C666-1 cells | Throat | Homo sapiens (Human) | CVCL_7949 | |
| CNE1 cells | Throat | Homo sapiens (Human) | CVCL_6888 | |
| HONE1 cells | Throat | Homo sapiens (Human) | CVCL_8706 | |
| 6-10B cells | Nasopharynx | Homo sapiens (Human) | CVCL_C529 | |
| SUNE-1 cells | Nasopharynx | Homo sapiens (Human) | CVCL_6946 | |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miR-3188 regulates nasopharyngeal carcinoma proliferation and chemosensitivity through a FOXO1-modulated positive feedback loop with mTOR-p-PI3k/AkT-c-JUN. | |||
|
|
||||
| Key Molecule: Serine/threonine-protein kinase mTOR (mTOR) | [75] | |||
| Sensitive Disease | Nasopharyngeal carcinoma [ICD-11: 2B6B.0] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell proliferation | Inhibition | hsa05200 | |
| PI3K/AKT/mTOR signaling pathway | Inhibition | hsa04151 | ||
| In Vitro Model | HNE1 cells | Nasopharynx | Homo sapiens (Human) | CVCL_0308 |
| 5-8F cells | Nasopharynx | Homo sapiens (Human) | CVCL_C528 | |
| CNE2 cells | Nasopharynx | Homo sapiens (Human) | CVCL_6889 | |
| C666-1 cells | Throat | Homo sapiens (Human) | CVCL_7949 | |
| CNE1 cells | Throat | Homo sapiens (Human) | CVCL_6888 | |
| HONE1 cells | Throat | Homo sapiens (Human) | CVCL_8706 | |
| 6-10B cells | Nasopharynx | Homo sapiens (Human) | CVCL_C529 | |
| SUNE-1 cells | Nasopharynx | Homo sapiens (Human) | CVCL_6946 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miR-3188 regulates nasopharyngeal carcinoma proliferation and chemosensitivity through a FOXO1-modulated positive feedback loop with mTOR-p-PI3k/AkT-c-JUN. | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
