Drug Information
Drug (ID: DG00148) and It's Reported Resistant Information
| Name |
Etoposide
|
||||
|---|---|---|---|---|---|
| Synonyms |
Etoposide; 33419-42-0; VePesid; Toposar; trans-Etoposide; Lastet; (-)-Etoposide; Zuyeyidal; Etoposidum; Etoposido; Vepesid J; Eposin; Etoposidum [INN-Latin]; Etoposide (VP16); VP 16-213; VP 16 (pharmaceutical); Etoposido [INN-Spanish]; Etopophos (phosphate salt); VP-16-213; 4-Demethylepipodophyllotoxin beta-D-ethylideneglucoside; VP 16213; UNII-6PLQ3CP4P3; NK 171; NSC 141540; CCRIS 2392; HSDB 6517; 4'-Demethylepipodophyllotoxin 9-(4,6-O-(R)-ethylidene-beta-D-glucopyranoside); EINECS 251-509-1; NSC-141540; Eposide; Etopol; Etosid; Vepeside; Demethyl EpipodophyllotoxinEthylidine Glucoside; E0675; Demethyl-epiodophyllotoxin ethylidene glucoside; Epipodophyllotoxin VP-16213; Eposin (TN); Etopophos (TN); Trans-Etoposide; VePESID (TN); Vepesid (TN); DEMETHY-EPIPODOPHYLLOTOXIN, ETHYLIDENE GLUCOSIDE; VP-16 (TN); Demethylepipodophyllotoxin-beta-D-ethylideneglucoside; Etoposide (JP15/USP/INN); Etoposide [USAN:INN:BAN:JAN]; Eposin, Vepesid, VP-16, Toposar, Etoposide; Epipodophyllotoxin, 4'-demethyl-, 4,6-O-ethylidene-beta-D-glucopyranoside; Epipodophyllotoxin, 4'-demethyl-, 4,6-O-ethylidene-beta-D-glucopyranoside (8CI); Epipodophyllotoxin, 4'-demethyl-, 9-(4,6-O-ethylidene-beta-D-glucopyranoside); 4'-Demethyl-epipodophyllotoxin 9-[4,6-O-(R)-ethylidene-beta-D-glucopyranoside; 4'-Demethylepipodophyllotoxin 9-(4,6-O-ethylidene-beta-D-glucopyranoside); 4'-Demethylepipodophyllotoxin ethylidene-beta-D-glucoside; 4'-O-Demethyl-1-O-(4,6-O-ethylidene-beta-D-glucopyranosyl)epipodophyllotoxin; 4-Demethylepipodophyllotoxin-beta-D-ethylideneglucoside
Click to Show/Hide
|
||||
| Indication |
In total 1 Indication(s)
|
||||
| Structure |
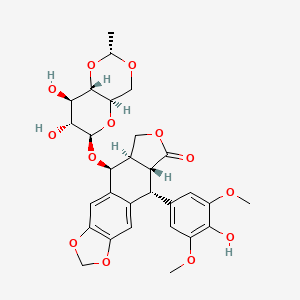
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Clinically Reported Resistance for This Drug
(10 diseases)
[2]
[3]
[2]
[4]
[5]
[6]
[7]
[8]
[9]
[10]
Disease(s) with Resistance Information Discovered by Cell Line Test for This Drug
(8 diseases)
[11]
[12]
[13]
[14]
[15]
[16]
[13]
[17]
|
||||
| Target | DNA topoisomerase II (TOP2) |
TOP2A_HUMAN
; TOP2B_HUMAN |
[1] | ||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
C29H32O13
|
||||
| IsoSMILES |
C[C@@H]1OC[C@@H]2[C@@H](O1)[C@@H]([C@H]([C@@H](O2)O[C@H]3[C@H]4COC(=O)[C@@H]4[C@@H](C5=CC6=C(C=C35)OCO6)C7=CC(=C(C(=C7)OC)O)OC)O)O
|
||||
| InChI |
1S/C29H32O13/c1-11-36-9-20-27(40-11)24(31)25(32)29(41-20)42-26-14-7-17-16(38-10-39-17)6-13(14)21(22-15(26)8-37-28(22)33)12-4-18(34-2)23(30)19(5-12)35-3/h4-7,11,15,20-22,24-27,29-32H,8-10H2,1-3H3/t11-,15+,20-,21-,22+,24-,25-,26-,27-,29+/m1/s1
|
||||
| InChIKey |
VJJPUSNTGOMMGY-MRVIYFEKSA-N
|
||||
| PubChem CID | |||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
| VARIDT ID | |||||
| INTEDE ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-02: Benign/in-situ/malignant neoplasm
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Geranylgeranyl diphosphate synthase 1 (GGPS1) | [18] | |||
| Metabolic Type | Lipid metabolism | |||
| Resistant Disease | Small cell lung carcinoma [ICD-11: 2C25.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Lung cancer [ICD-11: 2C25] | |||
| The Specified Disease | Small cell lung carcinoma | |||
| The Studied Tissue | Lung tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 3.06E-02 Fold-change: 8.03E-02 Z-score: 2.18E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Biosynthesis of terpenoids and steroids | Activation | hsa01062 | |
| Indole diterpene alkaloid biosynthesis | Activation | hsa00403 | ||
| In Vitro Model | DMS-114 cells | Lung | Homo sapiens (Human) | CVCL_1174 |
| H146 cells | Lung | Homo sapiens (Human) | CVCL_1473 | |
| H209 cells | Lung | Homo sapiens (Human) | CVCL_9V41 | |
| H446 cells | Lung | Homo sapiens (Human) | CVCL_1562 | |
| H526 cells | Lung | Homo sapiens (Human) | CVCL_1569 | |
| H82 cells | Lung | Homo sapiens (Human) | CVCL_1591 | |
| Experiment for Molecule Alteration |
qRT-PCR; Western blot analysis | |||
| Experiment for Drug Resistance |
Cell viability assay | |||
| Mechanism Description | Mechanistically, statins induce oxidative stress accumulation and apoptosis through the GGPP synthase?1 (GGPS1)-RAB7A-autophagy axis. Statin treatment overcomes both intrinsic and acquired SCLC chemoresistance in vivo across different SCLC PDX models bearing high GGPS1 levels. Moreover, we show that GGPS1 expression is negatively associated with survival in patients with SCLC | |||
| Key Molecule: Glutathione peroxidase 4 (GPX4) | [19] | |||
| Metabolic Type | Redox metabolism | |||
| Resistant Disease | Non-small cell lung carcinoma [ICD-11: 2C25.Y] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Lung cancer [ICD-11: 2C25] | |||
| The Specified Disease | Non-small cell lung carcinoma | |||
| The Studied Tissue | Lung tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 3.38E-05 Fold-change: 1.64E-01 Z-score: 4.26E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | A549 cells | Lung | Homo sapiens (Human) | CVCL_0023 |
| H1299 cells | Lung | Homo sapiens (Human) | CVCL_0060 | |
| H1688 cells | Lung | Homo sapiens (Human) | CVCL_1487 | |
| H446 cells | Lung | Homo sapiens (Human) | CVCL_1562 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Cell viability assay | |||
| Mechanism Description | Furthermore, we identified E3-ubiquitin ligase NEDD4L as a major regulator of GPX4 stability. Mechanistically, Lactate increases mitochondrial ROS generation and drives activation of the p38-SGK1 pathway, which attenuates the interaction of NEDD4L with GPX4 and subsequent ubiquitination and degradation of GPX4. | |||
| Key Molecule: Hexokinase 2 (HK2) | [20] | |||
| Metabolic Type | Glucose metabolism | |||
| Resistant Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Lung cancer [ICD-11: 2C25] | |||
| The Specified Disease | Lung adenocarcinoma | |||
| The Studied Tissue | Lung tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 2.61E-21 Fold-change: 5.58E-01 Z-score: 1.08E+01 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | A549 cells | Lung | Homo sapiens (Human) | CVCL_0023 |
| H1299 cells | Lung | Homo sapiens (Human) | CVCL_0060 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Here we showed that exposure to chemotherapeutic drug etoposide induces an exacerbation of ROS production which activates HIF-1-mediated the metabolic reprogramming toward increased glycolysis and lactate production in non-small cell lung cancer. | |||
| Key Molecule: Lactate dehydrogenase A (LDHA) | [20] | |||
| Metabolic Type | Glucose metabolism | |||
| Resistant Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Lung cancer [ICD-11: 2C25] | |||
| The Specified Disease | Lung adenocarcinoma | |||
| The Studied Tissue | Lung tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 6.61E-40 Fold-change: 6.78E-01 Z-score: 1.79E+01 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | A549 cells | Lung | Homo sapiens (Human) | CVCL_0023 |
| H1299 cells | Lung | Homo sapiens (Human) | CVCL_0060 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Here we showed that exposure to chemotherapeutic drug etoposide induces an exacerbation of ROS production which activates HIF-1-mediated the metabolic reprogramming toward increased glycolysis and lactate production in non-small cell lung cancer. | |||
| Key Molecule: Hypoxia-inducible factor 1-alpha (HIF-1alpha) | [20] | |||
| Metabolic Type | Redox metabolism | |||
| Resistant Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | |||
| Molecule Alteration | Activity | activation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | A549 cells | Lung | Homo sapiens (Human) | CVCL_0023 |
| H1299 cells | Lung | Homo sapiens (Human) | CVCL_0060 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Here we showed that exposure to chemotherapeutic drug etoposide induces an exacerbation of ROS production which activates HIF-1-mediated the metabolic reprogramming toward increased glycolysis and lactate production in non-small cell lung cancer. | |||
| Key Molecule: Monocarboxylate transporter 4 (MCT4) | [20] | |||
| Metabolic Type | Glucose metabolism | |||
| Resistant Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | A549 cells | Lung | Homo sapiens (Human) | CVCL_0023 |
| H1299 cells | Lung | Homo sapiens (Human) | CVCL_0060 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Here we showed that exposure to chemotherapeutic drug etoposide induces an exacerbation of ROS production which activates HIF-1-mediated the metabolic reprogramming toward increased glycolysis and lactate production in non-small cell lung cancer. | |||
|
|
||||
| Key Molecule: H19, imprinted maternally expressed transcript (H19) | [24] | |||
| Resistant Disease | Lung small cell carcinoma [ICD-11: 2C25.2] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Lung cancer [ICD-11: 2C25] | |||
| The Specified Disease | Lung adenocarcinoma | |||
| The Studied Tissue | Lung | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 3.58E-05 Fold-change: 2.58E+00 Z-score: 4.17E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | MCF-7 cells | Breast | Homo sapiens (Human) | CVCL_0031 |
| MCF-7/AdrVp cells | Breast | Homo sapiens (Human) | CVCL_4Y46 | |
| Experiment for Molecule Alteration |
RT-PCR; Northern blotting analysis | |||
| Experiment for Drug Resistance |
Clonogenic assay | |||
| Mechanism Description | The mRNA of the H19 gene is overexpressed in MCF-7/AdrVp cells relative toparental MCF-7 cells or drug-sensitive MCF-7/AdrVp revertant cells. H19is an imprinted gene with an important role in fetal differentiation, as well as a postulated function as a tumor suppressor gene. Another p95-over-expressing multidrug-resistant cell line, human lung carcinoma NCI-H1688, also displays high levels of 1119 mRNA. | |||
| Key Molecule: HOX transcript antisense RNA (HOTAIR) | [1] | |||
| Resistant Disease | Lung small cell carcinoma [ICD-11: 2C25.2] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| In Vitro Model | NCI-H446 cells | Lung | Homo sapiens (Human) | CVCL_1562 |
| NCI-H69 cells | Lung | Homo sapiens (Human) | CVCL_1579 | |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
CCK8 assay; Flow cytometry assay | |||
| Mechanism Description | H3kH3k27me3 induces multidrug resistance in small cell lung cancer by affecting HOXA1 DNA methylation via regulation of the LncRNA HOTAIR. | |||
| Key Molecule: hsa-mir-216a | [26] | |||
| Resistant Disease | Lung small cell carcinoma [ICD-11: 2C25.2] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell proliferation | Inhibition | hsa05200 | ||
| In Vitro Model | 16HBE cells | Lung | Homo sapiens (Human) | CVCL_0112 |
| H446 cells | Lung | Homo sapiens (Human) | CVCL_1562 | |
| H69 cells | Lung | Homo sapiens (Human) | CVCL_8121 | |
| H69AR cells | Lung | Homo sapiens (Human) | CVCL_3513 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
RT-qPCR | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | Long non-coding RNA HOTTIP promotes BCL-2 expression and induces chemoresistance in small cell lung cancer by sponging miR216a. | |||
| Key Molecule: hsa-mir-100 | [6] | |||
| Resistant Disease | Lung small cell carcinoma [ICD-11: 2C25.2] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell proliferation | Activation | hsa05200 | ||
| In Vitro Model | NCI-H69 cells | Lung | Homo sapiens (Human) | CVCL_1579 |
| NCI-H69AR cells | Lung | Homo sapiens (Human) | CVCL_3513 | |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | Forced expression of HOXA1 in immortalised human mammary epithelial cells results in oncogenic transformation and tumour formation in vivo. HOXA1 expression was inversely correlated with miR-100. HOXA1-mediated SCLC chemoresistance is under the regulation of miR-100. HOXA1 may be a prognostic predictor and potential therapeutic target in human SCLC. | |||
|
|
||||
| Key Molecule: Homeobox protein Hox-A13 (HOXA13) | [26] | |||
| Resistant Disease | Lung small cell carcinoma [ICD-11: 2C25.2] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Lung cancer [ICD-11: 2C25] | |||
| The Specified Disease | Lung cancer | |||
| The Studied Tissue | Lung tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 9.89E-43 Fold-change: 1.22E-01 Z-score: 1.43E+01 |
|||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell proliferation | Activation | hsa05200 | ||
| In Vitro Model | 16HBE cells | Lung | Homo sapiens (Human) | CVCL_0112 |
| H446 cells | Lung | Homo sapiens (Human) | CVCL_1562 | |
| H69 cells | Lung | Homo sapiens (Human) | CVCL_8121 | |
| H69AR cells | Lung | Homo sapiens (Human) | CVCL_3513 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis; RT-qPCR | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | HOTTIP acts at least partly by controlling HOXA13 in SCLC poor prognostic and chemoresistance progression. | |||
| Key Molecule: H3 lysine 27 trimethylation (H3K27) | [1] | |||
| Resistant Disease | Lung small cell carcinoma [ICD-11: 2C25.2] | |||
| Molecule Alteration | Methylation | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| In Vitro Model | NCI-H446 cells | Lung | Homo sapiens (Human) | CVCL_1562 |
| NCI-H69 cells | Lung | Homo sapiens (Human) | CVCL_1579 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay; Flow cytometry assay | |||
| Mechanism Description | H3kH3k27me3 induces multidrug resistance in small cell lung cancer by affecting HOXA1 DNA methylation via regulation of the LncRNA HOTAIR. | |||
| Key Molecule: Homeobox protein Hox-A1 (HOXA1) | [6] | |||
| Resistant Disease | Lung small cell carcinoma [ICD-11: 2C25.2] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell proliferation | Activation | hsa05200 | ||
| In Vitro Model | NCI-H69 cells | Lung | Homo sapiens (Human) | CVCL_1579 |
| NCI-H69AR cells | Lung | Homo sapiens (Human) | CVCL_3513 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | Forced expression of HOXA1 in immortalised human mammary epithelial cells results in oncogenic transformation and tumour formation in vivo. HOXA1 expression was inversely correlated with miR-100. HOXA1-mediated SCLC chemoresistance is under the regulation of miR-100. HOXA1 may be a prognostic predictor and potential therapeutic target in human SCLC. | |||
|
|
||||
| Key Molecule: Ephrin type-A receptor 3 (EPHA3) | [15] | |||
| Resistant Disease | Lung small cell carcinoma [ICD-11: 2C25.2] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Lung cancer [ICD-11: 2C25] | |||
| The Specified Disease | Lung cancer | |||
| The Studied Tissue | Lung tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.64E-12 Fold-change: -9.02E-02 Z-score: -7.41E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | NCI-H446 cells | Lung | Homo sapiens (Human) | CVCL_1562 |
| NCI-H69 cells | Lung | Homo sapiens (Human) | CVCL_1579 | |
| H69/AR cells | Lung | Homo sapiens (Human) | CVCL_3513 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Immunohistochemical staining; Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay; Cell scratch-wound healing assay; Flow cytometry assay | |||
| Mechanism Description | miR495 promotes the chemoresistance of SCLC through the epithelial-mesenchymal transition via Etk/BMX. Ectopic expression of Etk/BMX obviously rescued the miR495 elevation elevation-induced inhibition of drug resistance. | |||
| Key Molecule: Cytoplasmic tyrosine-protein kinase BMX (BMX) | [15] | |||
| Resistant Disease | Lung small cell carcinoma [ICD-11: 2C25.2] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Lung cancer [ICD-11: 2C25] | |||
| The Specified Disease | Lung cancer | |||
| The Studied Tissue | Lung tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 8.00E-33 Fold-change: -2.20E-01 Z-score: -1.36E+01 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | NCI-H446 cells | Lung | Homo sapiens (Human) | CVCL_1562 |
| NCI-H69 cells | Lung | Homo sapiens (Human) | CVCL_1579 | |
| H69/AR cells | Lung | Homo sapiens (Human) | CVCL_3513 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Immunohistochemical staining; Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay; Cell scratch-wound healing assay; Flow cytometry assay | |||
| Mechanism Description | miR495 promotes the chemoresistance of SCLC through the epithelial-mesenchymal transition via Etk/BMX. Ectopic expression of Etk/BMX obviously rescued the miR495 elevation elevation-induced inhibition of drug resistance. | |||
| Key Molecule: HOXA distal transcript antisense RNA (HOTTIP) | [26] | |||
| Resistant Disease | Lung small cell carcinoma [ICD-11: 2C25.2] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell proliferation | Inhibition | hsa05200 | ||
| In Vitro Model | 16HBE cells | Lung | Homo sapiens (Human) | CVCL_0112 |
| H446 cells | Lung | Homo sapiens (Human) | CVCL_1562 | |
| H69 cells | Lung | Homo sapiens (Human) | CVCL_8121 | |
| H69AR cells | Lung | Homo sapiens (Human) | CVCL_3513 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
RT-qPCR | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | HOTTIP acts as sponge of miR216a and enhanced the expression of its another target gene, anti-apoptotic gene BCL-2. | |||
| Key Molecule: hsa-mir-495 | [15] | |||
| Resistant Disease | Lung small cell carcinoma [ICD-11: 2C25.2] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | NCI-H446 cells | Lung | Homo sapiens (Human) | CVCL_1562 |
| NCI-H69 cells | Lung | Homo sapiens (Human) | CVCL_1579 | |
| H69/AR cells | Lung | Homo sapiens (Human) | CVCL_3513 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
CCK8 assay; Cell scratch-wound healing assay; Flow cytometry assay | |||
| Mechanism Description | miR495 promotes the chemoresistance of SCLC through the epithelial-mesenchymal transition via Etk/BMX. Ectopic expression of Etk/BMX obviously rescued the miR495 elevation elevation-induced inhibition of drug resistance. | |||
| Key Molecule: Cellular tumor antigen p53 (TP53) | [46] | |||
| Resistant Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | A549 cells | Lung | Homo sapiens (Human) | CVCL_0023 |
| Experiment for Molecule Alteration |
Western blot assay | |||
| Experiment for Drug Resistance |
Cell viability assay | |||
| Mechanism Description | M. arginini?and?M. salivarium, promoted the initiation of EMT and simultaneous suppression of the p53 tumor suppressor in A549 lung cancer cells. This led to an increase of cancer cell motility, resistance to the antitumor drug etoposide concomitantly with decreased autophagy. | |||
| Key Molecule: Zinc finger E-box-binding homeobox 1 (ZEB1) | [46] | |||
| Resistant Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | A549 cells | Lung | Homo sapiens (Human) | CVCL_0023 |
| Experiment for Molecule Alteration |
Western blot assay | |||
| Experiment for Drug Resistance |
Cell viability assay | |||
| Mechanism Description | M. arginini?and?M. salivarium, promoted the initiation of EMT and simultaneous suppression of the p53 tumor suppressor in A549 lung cancer cells. This led to an increase of cancer cell motility, resistance to the antitumor drug etoposide concomitantly with decreased autophagy. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Secretagogin (SCGN) | [22] | |||
| Sensitive Disease | Lung small cell carcinoma [ICD-11: 2C25.2] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Lung cancer [ICD-11: 2C25] | |||
| The Specified Disease | Lung small cell carcinoma | |||
| The Studied Tissue | Lung tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 6.88E-01 Fold-change: -1.07E-01 Z-score: -4.08E-01 |
|||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | BCL2 signaling pathway | Activation | hsa04210 | |
| Cell apoptosis | Activation | hsa04210 | ||
| Cell proliferation | Inhibition | hsa05200 | ||
| In Vitro Model | H69 cells | Lung | Homo sapiens (Human) | CVCL_8121 |
| H446 cells | Lung | Homo sapiens (Human) | CVCL_1562 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay; Flow cytometry assay | |||
| Mechanism Description | Knockdown of SCGN led to significantly increasing of chemosensitivity, which is similar to those induced by miR-494 mimics, and ectopic expression of SCGN could rescue the suppressive effect of miR-494. | |||
| Key Molecule: Beclin-1 (BECN1) | [23] | |||
| Sensitive Disease | Lung small cell carcinoma [ICD-11: 2C25.2] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Lung cancer [ICD-11: 2C25] | |||
| The Specified Disease | Lung small cell carcinoma | |||
| The Studied Tissue | Lung tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 7.03E-04 Fold-change: -6.88E-02 Z-score: -3.44E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | H446 cells | Lung | Homo sapiens (Human) | CVCL_1562 |
| Letp cells | Lung | Homo sapiens (Human) | N.A. | |
| Experiment for Molecule Alteration |
Luciferase reporter assay; Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay; WB assay; Colony formation assay; Fow cytometric analysis | |||
| Mechanism Description | Beclin-1-dependent autophagy in SCLC was directly regulated by miR30a-5p. miR30a-5p contributed to chemoresistance of SCLC cells partially in an Beclin-1-dependent manneRNA. | |||
| Key Molecule: Tetraspanin-12 (TSN12) | [28] | |||
| Sensitive Disease | Lung small cell carcinoma [ICD-11: 2C25.2] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Lung cancer [ICD-11: 2C25] | |||
| The Specified Disease | Lung cancer | |||
| The Studied Tissue | Lung tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 4.99E-31 Fold-change: -1.58E-01 Z-score: -1.28E+01 |
|||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | NCI-H446 cells | Lung | Homo sapiens (Human) | CVCL_1562 |
| NCI-H69 cells | Lung | Homo sapiens (Human) | CVCL_1579 | |
| NCI-H69AR cells | Lung | Homo sapiens (Human) | CVCL_3513 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay; Annexin V/propidium iodide detection assay; Scratch healing test | |||
| Mechanism Description | TSPAN12 promotes chemoresistance and proliferation of SCLC under the regulation of miR495, and TSPAN12 is negatively regulated by miR495. | |||
| Key Molecule: Poly[ADP-ribose] synthase 1 (PARP1) | [47] | |||
| Sensitive Disease | Lung small cell carcinoma [ICD-11: 2C25.2] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | NF-kappaB signaling pathway | Inhibition | hsa04064 | |
| In Vitro Model | H69 cells | Lung | Homo sapiens (Human) | CVCL_8121 |
| H69AR cells | Lung | Homo sapiens (Human) | CVCL_3513 | |
| H446 cells | Lung | Homo sapiens (Human) | CVCL_1562 | |
| H446/DDP cells | Lung | Homo sapiens (Human) | CVCL_RT21 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
qRT-PCR; Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay; Annexin V-PE Apoptosis assay; Flow cytometry assay; Wound healing assay; Colony formation assay | |||
| Mechanism Description | Overexpression of miR335 sensitized human SCLC cells to chemotherapy and radiotherapy, promoted cell apoptosis and inhibited cell migration ability of human SCLC in vitro, and inhibited tumor growth in vivo. Overexpression of miR335 decreased the expression of PARP-1 mRNA and protein, and NF-kB protein levels were correspondingly downregulated, thus regulating the chemo-radiosensitivity of SCLC. | |||
| Key Molecule: DNA repair protein RAD51 homolog 1 (RAD51) | [39] | |||
| Sensitive Disease | Lung cancer [ICD-11: 2C25.5] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell proliferation | Inhibition | hsa05200 | ||
| In Vitro Model | H1299 cells | Lung | Homo sapiens (Human) | CVCL_0060 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Survival assay/crystal violet staining assay | |||
| Mechanism Description | miR-103 and miR-107 reduced homology-directed repair and sensitized cells to various DNA damaging agents, including cisplatin and a PARP inhibitor. Mechanistic analyses revealed that both miR-103 and miR-107 directly target and regulate RAD51 and RAD51D, which is critical for miR-103/107-mediated chemosensitization. | |||
| Key Molecule: DNA repair protein RAD51 homolog 4 (RAD51D) | [39] | |||
| Sensitive Disease | Lung cancer [ICD-11: 2C25.5] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell proliferation | Inhibition | hsa05200 | ||
| In Vitro Model | H1299 cells | Lung | Homo sapiens (Human) | CVCL_0060 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Survival assay/crystal violet staining assay | |||
| Mechanism Description | miR-103 and miR-107 reduced homology-directed repair and sensitized cells to various DNA damaging agents, including cisplatin and a PARP inhibitor. Mechanistic analyses revealed that both miR-103 and miR-107 directly target and regulate RAD51 and RAD51D, which is critical for miR-103/107-mediated chemosensitization. | |||
| Key Molecule: Apoptosis regulator Bcl-2 (BCL2) | [41] | |||
| Sensitive Disease | Lung cancer [ICD-11: 2C25.5] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| In Vitro Model | A549 cells | Lung | Homo sapiens (Human) | CVCL_0023 |
| A549/CDDP cells | Lung | Homo sapiens (Human) | CVCL_0023 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | The antiapoptotic protein BCL2 is upregulated, whereas miR-181b is downregulated in both SGC7901/VCR and A549/CDDP cells, compared with SGC7901 and A549 cells, respectively. Enforced miR-181b expression reduced BCL2 protein level and sensitized SGC7901/VCR and A549/CDDP cells to VCR-induced and CDDP-induced apoptosis, respectively. | |||
|
|
||||
| Key Molecule: Apoptosis regulator Bcl-2 (BCL2) | [26] | |||
| Sensitive Disease | Lung small cell carcinoma [ICD-11: 2C25.2] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell proliferation | Inhibition | hsa05200 | ||
| In Vitro Model | 16HBE cells | Lung | Homo sapiens (Human) | CVCL_0112 |
| H446 cells | Lung | Homo sapiens (Human) | CVCL_1562 | |
| H69 cells | Lung | Homo sapiens (Human) | CVCL_8121 | |
| H69AR cells | Lung | Homo sapiens (Human) | CVCL_3513 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Luciferase reporter assay; RT-qPCR | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | Long non-coding RNA HOTTIP promotes BCL-2 expression and induces chemoresistance in small cell lung cancer by sponging miR216a. | |||
| Key Molecule: hsa-miR-30a-5p | [23] | |||
| Sensitive Disease | Lung small cell carcinoma [ICD-11: 2C25.2] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | H446 cells | Lung | Homo sapiens (Human) | CVCL_1562 |
| Letp cells | Lung | Homo sapiens (Human) | N.A. | |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
MTT assay; WB assay; Colony formation assay; Fow cytometric analysis | |||
| Mechanism Description | Beclin-1-dependent autophagy in SCLC was directly regulated by miR30a-5p. miR30a-5p contributed to chemoresistance of SCLC cells partially in an Beclin-1-dependent manneRNA. | |||
| Key Molecule: hsa-mir-335 | [47] | |||
| Sensitive Disease | Lung small cell carcinoma [ICD-11: 2C25.2] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | NF-kappaB signaling pathway | Inhibition | hsa04064 | |
| In Vitro Model | H69 cells | Lung | Homo sapiens (Human) | CVCL_8121 |
| H69AR cells | Lung | Homo sapiens (Human) | CVCL_3513 | |
| H446 cells | Lung | Homo sapiens (Human) | CVCL_1562 | |
| H446/DDP cells | Lung | Homo sapiens (Human) | CVCL_RT21 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
CCK8 assay; Annexin V-PE Apoptosis assay; Flow cytometry assay; Wound healing assay; Colony formation assay | |||
| Mechanism Description | Overexpression of miR335 sensitized human SCLC cells to chemotherapy and radiotherapy, promoted cell apoptosis and inhibited cell migration ability of human SCLC in vitro, and inhibited tumor growth in vivo. Overexpression of miR335 decreased the expression of PARP-1 mRNA and protein, and NF-kB protein levels were correspondingly downregulated, thus regulating the chemo-radiosensitivity of SCLC. | |||
| Key Molecule: hsa-miR-662 | [48] | |||
| Sensitive Disease | Lung squamous cell carcinoma [ICD-11: 2C25.3] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | H1703 cells | Lung | Homo sapiens (Human) | CVCL_1490 |
| NCI-H520 cells | Lung | Homo sapiens (Human) | CVCL_1566 | |
| Experiment for Molecule Alteration |
RT-qPCR | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miR-192 and miR-662 enhance chemoresistance and invasiveness of squamous cell lung carcinoma. | |||
| Key Molecule: hsa-mir-494 | [22] | |||
| Sensitive Disease | Lung small cell carcinoma [ICD-11: 2C25.2] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | BCL2 signaling pathway | Activation | hsa04210 | |
| Cell apoptosis | Activation | hsa04210 | ||
| Cell proliferation | Inhibition | hsa05200 | ||
| In Vitro Model | H69 cells | Lung | Homo sapiens (Human) | CVCL_8121 |
| H446 cells | Lung | Homo sapiens (Human) | CVCL_1562 | |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
CCK8 assay; Flow cytometry assay | |||
| Mechanism Description | Knockdown of SCGN led to significantly increasing of chemosensitivity, which is similar to those induced by miR-494 mimics, and ectopic expression of SCGN could rescue the suppressive effect of miR-494. | |||
| Key Molecule: hsa-mir-103 | [39] | |||
| Sensitive Disease | Lung cancer [ICD-11: 2C25.5] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell proliferation | Inhibition | hsa05200 | ||
| In Vitro Model | H1299 cells | Lung | Homo sapiens (Human) | CVCL_0060 |
| Experiment for Molecule Alteration |
RT-PCR | |||
| Experiment for Drug Resistance |
Survival assay/crystal violet staining assay | |||
| Mechanism Description | miR-103 and miR-107 reduced homology-directed repair and sensitized cells to various DNA damaging agents, including cisplatin and a PARP inhibitor. Mechanistic analyses revealed that both miR-103 and miR-107 directly target and regulate RAD51 and RAD51D, which is critical for miR-103/107-mediated chemosensitization. | |||
| Key Molecule: hsa-miR-107 | [39] | |||
| Sensitive Disease | Lung cancer [ICD-11: 2C25.5] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell proliferation | Inhibition | hsa05200 | ||
| In Vitro Model | H1299 cells | Lung | Homo sapiens (Human) | CVCL_0060 |
| Experiment for Molecule Alteration |
RT-PCR | |||
| Experiment for Drug Resistance |
Survival assay/crystal violet staining assay | |||
| Mechanism Description | miR-103 and miR-107 reduced homology-directed repair and sensitized cells to various DNA damaging agents, including cisplatin and a PARP inhibitor. Mechanistic analyses revealed that both miR-103 and miR-107 directly target and regulate RAD51 and RAD51D, which is critical for miR-103/107-mediated chemosensitization. | |||
| Key Molecule: hsa-mir-181 | [41] | |||
| Sensitive Disease | Lung cancer [ICD-11: 2C25.5] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| In Vitro Model | A549 cells | Lung | Homo sapiens (Human) | CVCL_0023 |
| A549/CDDP cells | Lung | Homo sapiens (Human) | CVCL_0023 | |
| Experiment for Molecule Alteration |
RT-PCR | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | The antiapoptotic protein BCL2 is upregulated, whereas miR-181b is downregulated in both SGC7901/VCR and A549/CDDP cells, compared with SGC7901 and A549 cells, respectively. Enforced miR-181b expression reduced BCL2 protein level and sensitized SGC7901/VCR and A549/CDDP cells to VCR-induced and CDDP-induced apoptosis, respectively. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Multidrug resistance protein 1 (ABCB1) | [11] | |||
| Sensitive Disease | Ependymoma [ICD-11: 2A00.05] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Brain cancer [ICD-11: 2A00] | |||
| The Specified Disease | Ependymoma | |||
| The Studied Tissue | Nervous tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 2.79E-03 Fold-change: -3.77E-01 Z-score: -3.50E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell migration | Activation | hsa04670 | |
| Cell invasion | Activation | hsa05200 | ||
| In Vitro Model | BXD-1425EPN cells | Embryo | Homo sapiens (Human) | CVCL_Y105 |
| EPN1 cells | Embryo | Homo sapiens (Human) | N.A. | |
| EPN7 cells | Embryo | Homo sapiens (Human) | N.A. | |
| EPN7R cells | Embryo | Homo sapiens (Human) | N.A. | |
| DKFZ-EP1 cells | Embryo | Homo sapiens (Human) | N.A. | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | ABCB1 gene expression was observed in 4 out of 5 paediatric ependymoma cell lines and increased in stem cell enriched neurospheres. Functional inhibition of ABCB1 using vardenafil or verapamil significantly (p < 0.05-0.001) potentiated the response to three chemotherapeutic drugs (vincristine, etoposide and methotrexate). Both inhibitors were also able to significantly reduce migration (p < 0.001) and invasion (p < 0.001). | |||
| Key Molecule: Multidrug resistance protein 1 (ABCB1) | [11] | |||
| Sensitive Disease | Ependymoma [ICD-11: 2A00.05] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Brain cancer [ICD-11: 2A00] | |||
| The Specified Disease | Ependymoma | |||
| The Studied Tissue | Nervous tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 2.79E-03 Fold-change: -3.77E-01 Z-score: -3.50E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell migration | Activation | hsa04670 | |
| Cell invasion | Activation | hsa05200 | ||
| In Vitro Model | BXD-1425EPN cells | Embryo | Homo sapiens (Human) | CVCL_Y105 |
| EPN1 cells | Embryo | Homo sapiens (Human) | N.A. | |
| EPN7 cells | Embryo | Homo sapiens (Human) | N.A. | |
| EPN7R cells | Embryo | Homo sapiens (Human) | N.A. | |
| DKFZ-EP1 cells | Embryo | Homo sapiens (Human) | N.A. | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | ABCB1 gene expression was observed in 4 out of 5 paediatric ependymoma cell lines and increased in stem cell enriched neurospheres. Functional inhibition of ABCB1 using vardenafil or verapamil significantly (p < 0.05-0.001) potentiated the response to three chemotherapeutic drugs (vincristine, etoposide and methotrexate). Both inhibitors were also able to significantly reduce migration (p < 0.001) and invasion (p < 0.001). | |||
|
|
||||
| Key Molecule: BDNF/NT-3 growth factors receptor (NTRK2) | [21] | |||
| Sensitive Disease | Neuroblastoma [ICD-11: 2A00.11] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Brain cancer [ICD-11: 2A00] | |||
| The Specified Disease | Neuroblastoma | |||
| The Studied Tissue | Nervous tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 9.60E-12 Fold-change: -2.22E+00 Z-score: -1.89E+01 |
|||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| PI3K/AKT signaling pathway | Inhibition | hsa04151 | ||
| In Vitro Model | Kelly cells | Adrenal | Homo sapiens (Human) | CVCL_2092 |
| Sk-N-AS cells | Adrenal | Homo sapiens (Human) | CVCL_1700 | |
| SH-SY5Y cells | Abdomen | Homo sapiens (Human) | CVCL_0019 | |
| In Vivo Model | Orthotopic xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTS assay | |||
| Mechanism Description | miR-204 direct targeting of the 3' UTR of BCL2 and NTRk2 (TrkB). BCL2 has a critical role in ensuring the survival of early developing cell types, NTRk2 is also a well-established pro-survival oncogene in neuroblastoma, signalling the activation of the PI3k/AkT pathway, a significant mechanism of drug resistance in neuroblastoma. Ectopic miR-204 expression significantly increased sensitivity to cisplatin and etoposide in vitro. | |||
| Key Molecule: Potassium voltage-gated channel subfamily H member 1 (KCNH1) | [29] | |||
| Sensitive Disease | Glioblastoma [ICD-11: 2A00.02] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Brain cancer [ICD-11: 2A00] | |||
| The Specified Disease | Brain cancer | |||
| The Studied Tissue | Nervous tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 8.48E-65 Fold-change: -1.66E-01 Z-score: -2.01E+01 |
|||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell invasion | Inhibition | hsa05200 | |
| Cell proliferation | Inhibition | hsa05200 | ||
| In Vitro Model | U251 cells | Brain | Homo sapiens (Human) | CVCL_0021 |
| U251AR cells | Brain | Homo sapiens (Human) | CVCL_1G29 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | EAG1 channel might be involved in cell-cycle progression of tumour cells because a significant reduction in the proliferation of tumour cell lines could be achieved by inhibiting EAG1 expression using antisense oligonucleotides. Ectopic expression of miR-296-3p reduced EAG1 expression and suppressed cell proliferation drug resistance. | |||
| Key Molecule: Apoptosis regulator Bcl-2 (BCL2) | [21] | |||
| Sensitive Disease | Neuroblastoma [ICD-11: 2A00.11] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| PI3K/AKT signaling pathway | Inhibition | hsa04151 | ||
| In Vitro Model | Kelly cells | Adrenal | Homo sapiens (Human) | CVCL_2092 |
| Sk-N-AS cells | Adrenal | Homo sapiens (Human) | CVCL_1700 | |
| SH-SY5Y cells | Abdomen | Homo sapiens (Human) | CVCL_0019 | |
| In Vivo Model | Orthotopic xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTS assay | |||
| Mechanism Description | miR-204 direct targeting of the 3' UTR of BCL2 and NTRk2 (TrkB). BCL2 has a critical role in ensuring the survival of early developing cell types, NTRk2 is also a well-established pro-survival oncogene in neuroblastoma, signalling the activation of the PI3k/AkT pathway, a significant mechanism of drug resistance in neuroblastoma. Ectopic miR-204 expression significantly increased sensitivity to cisplatin and etoposide in vitro. | |||
|
|
||||
| Key Molecule: hsa-miR-296-3p | [29] | |||
| Sensitive Disease | Glioblastoma [ICD-11: 2A00.02] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell invasion | Inhibition | hsa05200 | |
| Cell proliferation | Inhibition | hsa05200 | ||
| In Vitro Model | U251 cells | Brain | Homo sapiens (Human) | CVCL_0021 |
| U251AR cells | Brain | Homo sapiens (Human) | CVCL_1G29 | |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | EAG1 channel might be involved in cell-cycle progression of tumour cells because a significant reduction in the proliferation of tumour cell lines could be achieved by inhibiting EAG1 expression using antisense oligonucleotides. Ectopic expression of miR-296-3p reduced EAG1 expression and suppressed cell proliferation drug resistance. | |||
| Key Molecule: hsa-mir-204 | [21] | |||
| Sensitive Disease | Neuroblastoma [ICD-11: 2A00.11] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| PI3K/AKT signaling pathway | Inhibition | hsa04151 | ||
| In Vitro Model | Kelly cells | Adrenal | Homo sapiens (Human) | CVCL_2092 |
| Sk-N-AS cells | Adrenal | Homo sapiens (Human) | CVCL_1700 | |
| SH-SY5Y cells | Abdomen | Homo sapiens (Human) | CVCL_0019 | |
| In Vivo Model | Orthotopic xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
MTS assay | |||
| Mechanism Description | miR-204 direct targeting of the 3' UTR of BCL2 and NTRk2 (TrkB). BCL2 has a critical role in ensuring the survival of early developing cell types, NTRk2 is also a well-established pro-survival oncogene in neuroblastoma, signalling the activation of the PI3k/AkT pathway, a significant mechanism of drug resistance in neuroblastoma. Ectopic miR-204 expression significantly increased sensitivity to cisplatin and etoposide in vitro. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: hsa-mir-125b | [2] | |||
| Resistant Disease | Primitive neuroectodermal tumor [ICD-11: 2A00.08] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| miR125b-p53/BAKT signaling pathway | Activation | hsa05206 | ||
| In Vitro Model | RD-ES cells | Bones | Homo sapiens (Human) | CVCL_2169 |
| Sk-ES cells | Bones | Homo sapiens (Human) | CVCL_0627 | |
| Sk-N-MC cells | Bones | Homo sapiens (Human) | CVCL_0530 | |
| TC-71 cells | Bones | Homo sapiens (Human) | CVCL_2213 | |
| VH-64 cells | Bones | Homo sapiens (Human) | CVCL_9672 | |
| WE-68 cells | Bones | Homo sapiens (Human) | CVCL_9717 | |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
Celltiter-glo luminescent cell viability assay | |||
| Mechanism Description | miR-125b led to the development of chemoresistance by suppressing the expression of p53 and Bak, and repression of miR-125b sensitized EWS cells to apoptosis induced by treatment with various cytotoxic drugs. | |||
|
|
||||
| Key Molecule: Multidrug resistance protein 1 (ABCB1) | [11] | |||
| Resistant Disease | Ependymoma [ICD-11: 2A00.05] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell migration | Activation | hsa04670 | |
| Cell invasion | Activation | hsa05200 | ||
| In Vitro Model | BXD-1425EPN cells | Embryo | Homo sapiens (Human) | CVCL_Y105 |
| EPN1 cells | Embryo | Homo sapiens (Human) | N.A. | |
| EPN7 cells | Embryo | Homo sapiens (Human) | N.A. | |
| EPN7R cells | Embryo | Homo sapiens (Human) | N.A. | |
| DKFZ-EP1 cells | Embryo | Homo sapiens (Human) | N.A. | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | ABCB1 gene expression was observed in 4 out of 5 paediatric ependymoma cell lines and increased in stem cell enriched neurospheres. Functional inhibition of ABCB1 using vardenafil or verapamil significantly (p < 0.05-0.001) potentiated the response to three chemotherapeutic drugs (vincristine, etoposide and methotrexate). Both inhibitors were also able to significantly reduce migration (p < 0.001) and invasion (p < 0.001). | |||
| Key Molecule: Multidrug resistance protein 1 (ABCB1) | [30] | |||
| Resistant Disease | Anaplastic astrocytoma [ICD-11: 2A00.04] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Malignant gliomas tissue | N.A. | ||
| Experiment for Molecule Alteration |
Immunohistochemistry assay | |||
| Experiment for Drug Resistance |
Oncotech EDR assay | |||
| Mechanism Description | Cisplatin and etoposide are both substrates for membrane-bound efflux pumps, such as MRP and MDR1, which prevent their entry into the extracellular space of the central nervous system. The low levels of in vitro drug resistance noted for cisplatin and etoposide may be explained in part by the absence of such a barrier in our in vitro assay system. | |||
|
|
||||
| Key Molecule: Pyruvate carboxylase (PC) | [31] | |||
| Metabolic Type | Glucose metabolism | |||
| Resistant Disease | Glioblastoma [ICD-11: 2A00.02] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Cell viability assay | |||
| Mechanism Description | Further analysis revealed that GSC relies on pyruvate carboxylase (PC) activity for survival and self-renewal capacity. Interestingly, inhibition of PC led to GSC death, particularly when the glutamine pool was low, and increased differentiation. Finally, while GSC displayed resistance to the chemotherapy drug etoposide, genetic or pharmacological inhibition of PC restored etoposide sensitivity in GSC, both in vitro and in orthotopic murine models. | |||
|
|
||||
| Key Molecule: Bcl-2 homologous antagonist/killer (BAK1) | [2] | |||
| Resistant Disease | Primitive neuroectodermal tumor [ICD-11: 2A00.08] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| miR125b-p53/BAKT signaling pathway | Activation | hsa05206 | ||
| In Vitro Model | RD-ES cells | Bones | Homo sapiens (Human) | CVCL_2169 |
| Sk-ES cells | Bones | Homo sapiens (Human) | CVCL_0627 | |
| Sk-N-MC cells | Bones | Homo sapiens (Human) | CVCL_0530 | |
| TC-71 cells | Bones | Homo sapiens (Human) | CVCL_2213 | |
| VH-64 cells | Bones | Homo sapiens (Human) | CVCL_9672 | |
| WE-68 cells | Bones | Homo sapiens (Human) | CVCL_9717 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Celltiter-glo luminescent cell viability assay | |||
| Mechanism Description | miR-125b led to the development of chemoresistance by suppressing the expression of p53 and Bak, and repression of miR-125b sensitized EWS cells to apoptosis induced by treatment with various cytotoxic drugs. | |||
| Key Molecule: Beclin-1 (BECN1) | [32] | |||
| Resistant Disease | Glioma [ICD-11: 2A00.1] | |||
| Molecule Alteration | Expression | . |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell autophagy | Inhibition | hsa04140 | |
| In Vitro Model | SH-SY5Y cells | Abdomen | Homo sapiens (Human) | CVCL_0019 |
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | The overexpression of BECN1 and TXNDC17 reduced NB sensitivity to cisplatin (DDP), etoposide (VP16), and cyclophosphamide (CTX). Autophagy mediated by BECN1 was regulated by TXNDC17, and this process was involved in the resistance to DDP, VP16, and CTX in NB. Suberoylanilide hydroxamic acid (SAHA) can enhance the sensitivity and apoptosis of NB cells to chemotherapeutics by inhibiting TXNDC17, ultimately decreasing autophagy-mediated chemoresistance. | |||
| Key Molecule: Thioredoxin domain-containing protein 17 (TXNDC17) | [32] | |||
| Resistant Disease | Glioma [ICD-11: 2A00.1] | |||
| Molecule Alteration | Expression | . |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell autophagy | Inhibition | hsa04140 | |
| In Vitro Model | SH-SY5Y cells | Abdomen | Homo sapiens (Human) | CVCL_0019 |
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | The overexpression of BECN1 and TXNDC17 reduced NB sensitivity to cisplatin (DDP), etoposide (VP16), and cyclophosphamide (CTX). Autophagy mediated by BECN1 was regulated by TXNDC17, and this process was involved in the resistance to DDP, VP16, and CTX in NB. Suberoylanilide hydroxamic acid (SAHA) can enhance the sensitivity and apoptosis of NB cells to chemotherapeutics by inhibiting TXNDC17, ultimately decreasing autophagy-mediated chemoresistance. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Insulin-like growth factor 2 mRNA-binding protein 1 (IGF2BP1) | [25] | |||
| Sensitive Disease | Hepatocellular carcinoma [ICD-11: 2C12.2] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Liver cancer [ICD-11: 2C12] | |||
| The Specified Disease | Liver cancer | |||
| The Studied Tissue | Liver tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 6.39E-14 Fold-change: 1.27E-01 Z-score: 8.66E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell proliferation | Inhibition | hsa05200 | ||
| c-Myc signaling pathway | Activation | hsa05230 | ||
| In Vitro Model | HepG2 cells | Liver | Homo sapiens (Human) | CVCL_0027 |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
RT-PCR; Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miR-196b overexpression decreased IGF2BP1 RNA expression and protein level. The IGF2BP1 down-regulation by either miR-196b or IGF2BP1 siRNA led to an increase in apoptosis and a decrease in cell viability and proliferation in normal culture conditions. However, IGF2BP1 silencing did not modify the chemoresistance induced by hypoxia, probably because it is not the only target of miR-196b involved in the regulation of apoptosis. | |||
| Key Molecule: Cyclin-G1 (CCNG1) | [43] | |||
| Sensitive Disease | Liver cancer [ICD-11: 2C12.6] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| miR27b/CCNG1/p53 signaling pathway | Regulation | N.A. | ||
| In Vitro Model | HepG2 cells | Liver | Homo sapiens (Human) | CVCL_0027 |
| SNU182 cells | Liver | Homo sapiens (Human) | CVCL_0090 | |
| SNU-739 cells | Liver | Homo sapiens (Human) | CVCL_5088 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CellTiter-Glo luminescent cell viability assay | |||
| Mechanism Description | miR-27b synergizes with anticancer drugs througth enhancing anticancer drug-induced cell death which due to p53 activation and CYP1B1 suppression. | |||
| Key Molecule: DNA topoisomerase 1 (TOP1) | [44] | |||
| Sensitive Disease | Hepatocellular carcinoma [ICD-11: 2C12.2] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell proliferation | Inhibition | hsa05200 | ||
| In Vitro Model | HepG2 cells | Liver | Homo sapiens (Human) | CVCL_0027 |
| MHCC97-L cells | Liver | Homo sapiens (Human) | CVCL_4973 | |
| In Vivo Model | BALB/c nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Luciferase assay | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Overexpression of miR-23a could significantly potentiate the in vitro and in vivo anti-tumor effect of etoposide; miR-23a could directly bind to 3'untranslated region of TOP1 mRNA, and suppress the corresponding protein expression and inhibition of miR-23a further arguments the expression of TOP1. Suppression of TOP1 expression by miR-23a results in reduction of overall intracellular topoisomerase activity when the cells are exposed to etoposide, which in consequence enhances drug response of HCC cells. | |||
|
|
||||
| Key Molecule: Cytochrome P450 family 1 subfamily B member1 (CYP1B1) | [43] | |||
| Sensitive Disease | Liver cancer [ICD-11: 2C12.6] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | miR27b/CCNG1/p53 signaling pathway | Regulation | N.A. | |
| In Vitro Model | HepG2 cells | Liver | Homo sapiens (Human) | CVCL_0027 |
| SNU182 cells | Liver | Homo sapiens (Human) | CVCL_0090 | |
| SNU-739 cells | Liver | Homo sapiens (Human) | CVCL_5088 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CellTiter-Glo luminescent cell viability assay | |||
| Mechanism Description | miR-27b synergizes with anticancer drugs througth enhancing anticancer drug-induced cell death which due to p53 activation and CYP1B1 suppression. | |||
|
|
||||
| Key Molecule: hsa-mir-196b | [25] | |||
| Sensitive Disease | Hepatocellular carcinoma [ICD-11: 2C12.2] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell proliferation | Inhibition | hsa05200 | ||
| c-Myc signaling pathway | Activation | hsa05230 | ||
| In Vitro Model | HepG2 cells | Liver | Homo sapiens (Human) | CVCL_0027 |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
RT-qPCR | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miR-196b overexpression decreased IGF2BP1 RNA expression and protein level. The IGF2BP1 down-regulation by either miR-196b or IGF2BP1 siRNA led to an increase in apoptosis and a decrease in cell viability and proliferation in normal culture conditions. However, IGF2BP1 silencing did not modify the chemoresistance induced by hypoxia, probably because it is not the only target of miR-196b involved in the regulation of apoptosis. | |||
| Key Molecule: hsa-mir-27b | [43] | |||
| Sensitive Disease | Liver cancer [ICD-11: 2C12.6] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | miR27b/CCNG1/p53 signaling pathway | Regulation | N.A. | |
| In Vitro Model | HepG2 cells | Liver | Homo sapiens (Human) | CVCL_0027 |
| SNU182 cells | Liver | Homo sapiens (Human) | CVCL_0090 | |
| SNU-739 cells | Liver | Homo sapiens (Human) | CVCL_5088 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
qPCR | |||
| Experiment for Drug Resistance |
CellTiter-Glo luminescent cell viability assay | |||
| Mechanism Description | miR-27b synergizes with anticancer drugs througth enhancing anticancer drug-induced cell death which due to p53 activation and CYP1B1 suppression. | |||
| Key Molecule: hsa-mir-23a | [44] | |||
| Sensitive Disease | Hepatocellular carcinoma [ICD-11: 2C12.2] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell proliferation | Inhibition | hsa05200 | ||
| In Vitro Model | HepG2 cells | Liver | Homo sapiens (Human) | CVCL_0027 |
| MHCC97-L cells | Liver | Homo sapiens (Human) | CVCL_4973 | |
| In Vivo Model | BALB/c nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Overexpression of miR-23a could significantly potentiate the in vitro and in vivo anti-tumor effect of etoposide; miR-23a could directly bind to 3'untranslated region of TOP1 mRNA, and suppress the corresponding protein expression and inhibition of miR-23a further arguments the expression of TOP1. Suppression of TOP1 expression by miR-23a results in reduction of overall intracellular topoisomerase activity when the cells are exposed to etoposide, which in consequence enhances drug response of HCC cells. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Programmed cell death protein 4 (PDCD4) | [13] | |||
| Resistant Disease | Melanoma [ICD-11: 2C30.0] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Melanoma [ICD-11: 2C30] | |||
| The Specified Disease | Melanoma | |||
| The Studied Tissue | Skin | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 6.26E-02 Fold-change: -1.06E-01 Z-score: -1.95E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell proliferation | Activation | hsa05200 | ||
| In Vitro Model | HCT116 cells | Colon | Homo sapiens (Human) | CVCL_0291 |
| A375 cells | Skin | Homo sapiens (Human) | CVCL_0132 | |
| U251 cells | Brain | Homo sapiens (Human) | CVCL_0021 | |
| HEK293T cells | Kidney | Homo sapiens (Human) | CVCL_0063 | |
| PARP cells | Skin | Homo sapiens (Human) | N.A. | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | Hypoxia induces miR-424 expression and that miR-424 in turn suppresses the level of PDCD4 protein, a tumor suppressor that is involved in apoptosis, by targeting its 3' untranslated region. Functionally, miR-424 overexpression decreases the sensitivity of cancer cells (HCT116 and A375) to doxorubicin (Dox) and etoposide. In contrast, the inhibition of miR-424 (+) apoptosis and increased the sensitivity of cancer cells to Dox. In a xenograft tumor model, miR-424 overexpression promoted tumor growth following Dox treatment, suggesting that miR-424 promotes tumor cell resistance to Dox. Furthermore, miR-424 levels are inversely correlated with PDCD4 expression in clinical breast cancer samples. | |||
|
|
||||
| Key Molecule: hsa-mir-424 | [13] | |||
| Resistant Disease | Melanoma [ICD-11: 2C30.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell proliferation | Activation | hsa05200 | ||
| In Vitro Model | HCT116 cells | Colon | Homo sapiens (Human) | CVCL_0291 |
| A375 cells | Skin | Homo sapiens (Human) | CVCL_0132 | |
| U251 cells | Brain | Homo sapiens (Human) | CVCL_0021 | |
| HEK293T cells | Kidney | Homo sapiens (Human) | CVCL_0063 | |
| PARP cells | Skin | Homo sapiens (Human) | N.A. | |
| Experiment for Molecule Alteration |
RT-PCR | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | Hypoxia induces miR-424 expression and that miR-424 in turn suppresses the level of PDCD4 protein, a tumor suppressor that is involved in apoptosis, by targeting its 3' untranslated region. Functionally, miR-424 overexpression decreases the sensitivity of cancer cells (HCT116 and A375) to doxorubicin (Dox) and etoposide. In contrast, the inhibition of miR-424 (+) apoptosis and increased the sensitivity of cancer cells to Dox. In a xenograft tumor model, miR-424 overexpression promoted tumor growth following Dox treatment, suggesting that miR-424 promotes tumor cell resistance to Dox. Furthermore, miR-424 levels are inversely correlated with PDCD4 expression in clinical breast cancer samples. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: ATP-binding cassette sub-family G2 (ABCG2) | [27] | |||
| Sensitive Disease | Retinoblastoma [ICD-11: 2D02.2] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Retina cancer [ICD-11: 2D02] | |||
| The Specified Disease | Retinoblastoma tumor | |||
| The Studied Tissue | Uvea | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.43E-02 Fold-change: -1.35E-01 Z-score: -2.66E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell proliferation | Inhibition | hsa05200 | ||
| In Vitro Model | WERI-Rb-1 cells | Retina | Homo sapiens (Human) | CVCL_1792 |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | Silencing of ABCG2 by MicroRNA-3163 inhibits multidrug resistance in retinoblastoma cancer stem cells. | |||
|
|
||||
| Key Molecule: hsa-mir-34 | [52] | |||
| Sensitive Disease | Retinoblastoma [ICD-11: 2D02.2] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| MAGE-A/p53 signaling pathway | Regulation | N.A. | ||
| In Vitro Model | HXO-Rb44 cells | Retina | Homo sapiens (Human) | CVCL_D542 |
| SO-Rb50 cells | Retina | Homo sapiens (Human) | CVCL_D543 | |
| WERI-Rb-1 cells | Retina | Homo sapiens (Human) | CVCL_1792 | |
| Y79 cells | Retina | Homo sapiens (Human) | CVCL_1893 | |
| Experiment for Molecule Alteration |
RT-qPCR | |||
| Experiment for Drug Resistance |
Freedom Evolyzer-2200 Enzyme-Linked Immunometric meter; Flow cytometry assay | |||
| Mechanism Description | miR-34a may function as a tumor suppressor for RB by targeting MAGE-A and upregulating p53 expression to enhance cell apoptosis and chemosensitivity (Carboplatin; Etoposide; Adriamycin; vincristine). | |||
| Key Molecule: hsa-miR-3163 | [27] | |||
| Sensitive Disease | Retinoblastoma [ICD-11: 2D02.2] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell proliferation | Inhibition | hsa05200 | ||
| In Vitro Model | WERI-Rb-1 cells | Retina | Homo sapiens (Human) | CVCL_1792 |
| Experiment for Molecule Alteration |
RT-PCR | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | Silencing of ABCG2 by MicroRNA-3163 inhibits multidrug resistance in retinoblastoma cancer stem cells. | |||
|
|
||||
| Key Molecule: Melanoma antigen A 4 (MAGE4) | [52] | |||
| Sensitive Disease | Retinoblastoma [ICD-11: 2D02.2] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| MAGE-A/p53 signaling pathway | Regulation | N.A. | ||
| In Vitro Model | HXO-Rb44 cells | Retina | Homo sapiens (Human) | CVCL_D542 |
| SO-Rb50 cells | Retina | Homo sapiens (Human) | CVCL_D543 | |
| WERI-Rb-1 cells | Retina | Homo sapiens (Human) | CVCL_1792 | |
| Y79 cells | Retina | Homo sapiens (Human) | CVCL_1893 | |
| Experiment for Molecule Alteration |
Western blot analysis; RT-qPCR | |||
| Experiment for Drug Resistance |
Freedom Evolyzer-2200 Enzyme-Linked Immunometric meter; Flow cytometry assay | |||
| Mechanism Description | miR-34a may function as a tumor suppressor for RB by targeting MAGE-A and upregulating p53 expression to enhance cell apoptosis and chemosensitivity (Carboplatin; Etoposide; Adriamycin; vincristine). | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Programmed cell death protein 4 (PDCD4) | [13] | |||
| Resistant Disease | Colon cancer [ICD-11: 2B90.1] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Colon cancer [ICD-11: 2B90] | |||
| The Specified Disease | Colon cancer | |||
| The Studied Tissue | Colon tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 5.93E-12 Fold-change: -1.62E-01 Z-score: -7.40E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell proliferation | Activation | hsa05200 | ||
| In Vitro Model | HCT116 cells | Colon | Homo sapiens (Human) | CVCL_0291 |
| A375 cells | Skin | Homo sapiens (Human) | CVCL_0132 | |
| U251 cells | Brain | Homo sapiens (Human) | CVCL_0021 | |
| HEK293T cells | Kidney | Homo sapiens (Human) | CVCL_0063 | |
| PARP cells | Skin | Homo sapiens (Human) | N.A. | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | Hypoxia induces miR-424 expression and that miR-424 in turn suppresses the level of PDCD4 protein, a tumor suppressor that is involved in apoptosis, by targeting its 3' untranslated region. Functionally, miR-424 overexpression decreases the sensitivity of cancer cells (HCT116 and A375) to doxorubicin (Dox) and etoposide. In contrast, the inhibition of miR-424 (+) apoptosis and increased the sensitivity of cancer cells to Dox. In a xenograft tumor model, miR-424 overexpression promoted tumor growth following Dox treatment, suggesting that miR-424 promotes tumor cell resistance to Dox. Furthermore, miR-424 levels are inversely correlated with PDCD4 expression in clinical breast cancer samples. | |||
|
|
||||
| Key Molecule: hsa-mir-424 | [13] | |||
| Resistant Disease | Colon cancer [ICD-11: 2B90.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell proliferation | Activation | hsa05200 | ||
| In Vitro Model | HCT116 cells | Colon | Homo sapiens (Human) | CVCL_0291 |
| A375 cells | Skin | Homo sapiens (Human) | CVCL_0132 | |
| U251 cells | Brain | Homo sapiens (Human) | CVCL_0021 | |
| HEK293T cells | Kidney | Homo sapiens (Human) | CVCL_0063 | |
| PARP cells | Skin | Homo sapiens (Human) | N.A. | |
| Experiment for Molecule Alteration |
RT-PCR | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | Hypoxia induces miR-424 expression and that miR-424 in turn suppresses the level of PDCD4 protein, a tumor suppressor that is involved in apoptosis, by targeting its 3' untranslated region. Functionally, miR-424 overexpression decreases the sensitivity of cancer cells (HCT116 and A375) to doxorubicin (Dox) and etoposide. In contrast, the inhibition of miR-424 (+) apoptosis and increased the sensitivity of cancer cells to Dox. In a xenograft tumor model, miR-424 overexpression promoted tumor growth following Dox treatment, suggesting that miR-424 promotes tumor cell resistance to Dox. Furthermore, miR-424 levels are inversely correlated with PDCD4 expression in clinical breast cancer samples. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: hsa-miR-17-92 | [16] | |||
| Resistant Disease | Mantle cell lymphoma [ICD-11: 2A85.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell proliferation | Activation | hsa05200 | ||
| PI3K/AKT signaling pathway | Activation | hsa04151 | ||
| In Vitro Model | Jeko-1 cells | Blood | Homo sapiens (Human) | CVCL_1865 |
| Granta-519 cells | Blood | Homo sapiens (Human) | CVCL_1818 | |
| Z138c cells | Blood | Homo sapiens (Human) | CVCL_B077 | |
| In Vivo Model | CB-17/SCID nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
RT-PCR | |||
| Experiment for Drug Resistance |
Xenograft experiments assay | |||
| Mechanism Description | The protein phosphatase PHLPP2, an important negative regulator of the PI3k/AkT pathway, was a direct target of miR-17 92 miRNAs, miRNA-17 92 cluster mediates chemoresistance and enhances tumor growth in mantle cell lymphoma via PI3k/AkT pathway activation. | |||
|
|
||||
| Key Molecule: PH domain leucine-rich repeat-containing protein phosphatase 2 (PHLPP2) | [16] | |||
| Resistant Disease | Mantle cell lymphoma [ICD-11: 2A85.0] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell proliferation | Activation | hsa05200 | ||
| PI3K/AKT signaling pathway | Activation | hsa04151 | ||
| In Vitro Model | Jeko-1 cells | Blood | Homo sapiens (Human) | CVCL_1865 |
| Granta-519 cells | Blood | Homo sapiens (Human) | CVCL_1818 | |
| Z138c cells | Blood | Homo sapiens (Human) | CVCL_B077 | |
| In Vivo Model | CB-17/SCID nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Luciferase assay | |||
| Experiment for Drug Resistance |
Xenograft experiments assay | |||
| Mechanism Description | The protein phosphatase PHLPP2, an important negative regulator of the PI3k/AkT pathway, was a direct target of miR-17 92 miRNAs, miRNA-17 92 cluster mediates chemoresistance and enhances tumor growth in mantle cell lymphoma via PI3k/AkT pathway activation. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: CAMPATH-1 antigen (CD52) | [33] | |||
| Sensitive Disease | t-cell prolymphocytic leukemia [ICD-11: 2A90.0] | |||
| Molecule Alteration | Expressiom | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vivo Model | T-cell prolymphocytic leukemia patient | Homo sapiens | ||
| Experiment for Molecule Alteration |
Flow cytometry | |||
| Experiment for Drug Resistance |
Overall survival assay | |||
| Mechanism Description | MTX-HOPE is a combination of classical chemotherapy agents originally developed for palliative chemotherapy in frail patients with refractory lymphoma. MTX-HOPE has been reported to be effective against T-cell tumors. Severe nonhematologic adverse events are rarely reported; however, bone marrow suppression is commonly observed. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: hsa-miR-485-3p | [34] | |||
| Sensitive Disease | Lymphocytic leukemia [ICD-11: 2B33.2] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | CEM cells | Pleural effusion | Homo sapiens (Human) | N.A. |
| CEM/VM-1-5 cells | Lymph | Homo sapiens (Human) | CVCL_1B35 | |
| Experiment for Molecule Alteration |
RT-PCR | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miR-485-3p expression can mediate etoposide sensitivity indirectly by fine-tuning Top2alpha expression through the modification of NF-YB expression. Accordingly, miR-485-3p can be a putative therapeutic target to modulate etoposide resistance in tumor cells. | |||
|
|
||||
| Key Molecule: Nuclear transcription factor Y subunit beta (NFYB) | [34] | |||
| Sensitive Disease | Lymphocytic leukemia [ICD-11: 2B33.2] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | CEM cells | Pleural effusion | Homo sapiens (Human) | N.A. |
| CEM/VM-1-5 cells | Lymph | Homo sapiens (Human) | CVCL_1B35 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miR-485-3p expression can mediate etoposide sensitivity indirectly by fine-tuning Top2alpha expression through the modification of NF-YB expression. Accordingly, miR-485-3p can be a putative therapeutic target to modulate etoposide resistance in tumor cells. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: hsa-miR-34a-5p | [35], [36] | |||
| Resistant Disease | Osteosarcoma [ICD-11: 2B51.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | ATF2/ATF3/ATF4 signaling pathway | Inhibition | hsa04915 | |
| In Vitro Model | G-292 cells | Bone | Homo sapiens (Human) | CVCL_2909 |
| SJSA-1 cells | Bone | Homo sapiens (Human) | CVCL_1697 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
MTT assay; Annexin V-FITC/propidium iodide (PI) staining assay | |||
| Mechanism Description | The miR34a-5p promotes the multi-chemoresistance of osteosarcoma via repression of the AGTR1 gene. And miR34a-5p promotes multi-chemoresistance of osteosarcoma through down-regulation of the DLL1 gene. | |||
| Key Molecule: hsa-miR-20a-5p | [37] | |||
| Resistant Disease | Osteosarcoma [ICD-11: 2B51.0] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | G-292 cells | Bone | Homo sapiens (Human) | CVCL_2909 |
| SJSA-1 cells | Bone | Homo sapiens (Human) | CVCL_1697 | |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
CCK8 assay; Flow cytometry assay | |||
| Mechanism Description | miR20a-5p modulates multi-drug resistance by repressing SDC2 expression in OS cells. | |||
| Key Molecule: Delta-like protein 1 (DLL1) | [36] | |||
| Resistant Disease | Osteosarcoma [ICD-11: 2B51.0] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | ATF2/ATF3/ATF4 signaling pathway | Inhibition | hsa04915 | |
| In Vitro Model | G-292 cells | Bone | Homo sapiens (Human) | CVCL_2909 |
| SJSA-1 cells | Bone | Homo sapiens (Human) | CVCL_1697 | |
| MG63.2 cells | Bone | Homo sapiens (Human) | CVCL_R705 | |
| MNNG/HOS cells | Bone | Homo sapiens (Human) | CVCL_0439 | |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
IC50 assay; Flow cytometric analysis | |||
| Mechanism Description | miR34a-5p promotes multi-chemoresistance of osteosarcoma through down-regulation of the DLL1 gene. The activity of the ATF2/ATF3/ATF4 pathway was reduced in the miR34a-5p mimic-transfected G-292 cells but increased in the miR34a-5p antagomiRtransfected SJSA-1 cells, hence the ATF2/ATF3/ATF4 pathway was validated to be involved in the OS chemoresistance mediated by miR34a-5p. | |||
| Key Molecule: hsa-miR-34a-5p | [17] | |||
| Resistant Disease | Osteosarcoma [ICD-11: 2B51.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell invasion | Activation | hsa05200 | |
| Cell migration | Activation | hsa04670 | ||
| Cell proliferation | Activation | hsa05200 | ||
| MEF2 signaling pathway | Regulation | N.A. | ||
| In Vitro Model | MG63 cells | Bone marrow | Homo sapiens (Human) | CVCL_0426 |
| SAOS-2 cells | Bone marrow | Homo sapiens (Human) | CVCL_0548 | |
| U2OS cells | Bone | Homo sapiens (Human) | CVCL_0042 | |
| G-292 cells | Bone | Homo sapiens (Human) | CVCL_2909 | |
| SJSA-1 cells | Bone | Homo sapiens (Human) | CVCL_1697 | |
| MG63.2 cells | Bone | Homo sapiens (Human) | CVCL_R705 | |
| MNNG/HOS cells | Bone | Homo sapiens (Human) | CVCL_0439 | |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | The down-regulation of CD117 mediated by miR-34a-5p might be one of the reasons for OS drug resistance. CD117 may also regulate other processes, including cell adhesion, differentiation and migration, which are significant for cancer development and treatment. | |||
|
|
||||
| Key Molecule: Syndecan-2 (SDC2) | [37] | |||
| Resistant Disease | Osteosarcoma [ICD-11: 2B51.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | G-292 cells | Bone | Homo sapiens (Human) | CVCL_2909 |
| SJSA-1 cells | Bone | Homo sapiens (Human) | CVCL_1697 | |
| Experiment for Molecule Alteration |
Luciferase reporter assay; Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay; Flow cytometry assay | |||
| Mechanism Description | miR20a-5p modulates multi-drug resistance by repressing SDC2 expression in OS cells. | |||
| Key Molecule: Type-1 angiotensin II receptor (AGTR1) | [35] | |||
| Resistant Disease | Osteosarcoma [ICD-11: 2B51.0] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | G-292 cells | Bone | Homo sapiens (Human) | CVCL_2909 |
| SJSA-1 cells | Bone | Homo sapiens (Human) | CVCL_1697 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay; Annexin V-FITC/propidium iodide (PI) staining assay | |||
| Mechanism Description | The miR34a-5p promotes the multi-chemoresistance of osteosarcoma via repression of the AGTR1 gene. | |||
| Key Molecule: Mast/stem cell growth factor receptor Kit (KIT) | [17] | |||
| Resistant Disease | Osteosarcoma [ICD-11: 2B51.0] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell invasion | Activation | hsa05200 | |
| Cell migration | Activation | hsa04670 | ||
| Cell proliferation | Activation | hsa05200 | ||
| MEF2 signaling pathway | Regulation | N.A. | ||
| In Vitro Model | MG63 cells | Bone marrow | Homo sapiens (Human) | CVCL_0426 |
| SAOS-2 cells | Bone marrow | Homo sapiens (Human) | CVCL_0548 | |
| U2OS cells | Bone | Homo sapiens (Human) | CVCL_0042 | |
| G-292 cells | Bone | Homo sapiens (Human) | CVCL_2909 | |
| SJSA-1 cells | Bone | Homo sapiens (Human) | CVCL_1697 | |
| MG63.2 cells | Bone | Homo sapiens (Human) | CVCL_R705 | |
| MNNG/HOS cells | Bone | Homo sapiens (Human) | CVCL_0439 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | The down-regulation of CD117 mediated by miR-34a-5p might be one of the reasons for OS drug resistance. CD117 may also regulate other processes, including cell adhesion, differentiation and migration, which are significant for cancer development and treatment. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: hsa-mir-124 | [38] | |||
| Sensitive Disease | Osteosarcoma [ICD-11: 2B51.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell invasion | Inhibition | hsa05200 | ||
| Cell migration | Inhibition | hsa04670 | ||
| Cell proliferation | Inhibition | hsa05200 | ||
| PI3K/AKT signaling pathway | Inhibition | hsa04151 | ||
| In Vitro Model | MDA-MB-231 cells | Breast | Homo sapiens (Human) | CVCL_0062 |
| T47D cells | Breast | Homo sapiens (Human) | CVCL_0553 | |
| ZR75-1 cells | Breast | Homo sapiens (Human) | CVCL_0588 | |
| HCC1937 cells | Breast | Homo sapiens (Human) | CVCL_0290 | |
| Experiment for Molecule Alteration |
RT-PCR | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miR-124 may be involved in DNA repair by directly targeting ATMIN and PARP1, suggesting that multiple DNA repair pathways are affected by miR-124 and therefore manipulation of miR-124 level/activity may improve the efficacy of chemotherapies that induce DNA damage. repression of ATMIN (+) the HR repair defect induced by miR-124, and restoration of ATMIN reversed the effect of miR-124 overexpression in breast cancer cells. Therefore, it is intriguing to further speculate which of the multiple roles of ATMIN is specifically affected in breast carcinogenesis. On the other hand, PARP1-mediated processes play a role in oncogenesis, cancer progression, and therapeutic resistance. | |||
| Key Molecule: hsa-mir-103 | [39] | |||
| Sensitive Disease | Osteosarcoma [ICD-11: 2B51.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell proliferation | Inhibition | hsa05200 | ||
| In Vitro Model | U2OS cells | Bone | Homo sapiens (Human) | CVCL_0042 |
| Experiment for Molecule Alteration |
RT-PCR | |||
| Experiment for Drug Resistance |
Survival assay/crystal violet staining assay | |||
| Mechanism Description | miR-103 and miR-107 reduced homology-directed repair and sensitized cells to various DNA damaging agents, including cisplatin and a PARP inhibitor. Mechanistic analyses revealed that both miR-103 and miR-107 directly target and regulate RAD51 and RAD51D, which is critical for miR-103/107-mediated chemosensitization. | |||
| Key Molecule: hsa-miR-107 | [39] | |||
| Sensitive Disease | Osteosarcoma [ICD-11: 2B51.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell proliferation | Inhibition | hsa05200 | ||
| In Vitro Model | U2OS cells | Bone | Homo sapiens (Human) | CVCL_0042 |
| Experiment for Molecule Alteration |
RT-PCR | |||
| Experiment for Drug Resistance |
Survival assay/crystal violet staining assay | |||
| Mechanism Description | miR-103 and miR-107 reduced homology-directed repair and sensitized cells to various DNA damaging agents, including cisplatin and a PARP inhibitor. Mechanistic analyses revealed that both miR-103 and miR-107 directly target and regulate RAD51 and RAD51D, which is critical for miR-103/107-mediated chemosensitization. | |||
|
|
||||
| Key Molecule: ATM interactor (ATMIN) | [38] | |||
| Sensitive Disease | Osteosarcoma [ICD-11: 2B51.0] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell invasion | Inhibition | hsa05200 | ||
| Cell migration | Inhibition | hsa04670 | ||
| Cell proliferation | Inhibition | hsa05200 | ||
| PI3K/AKT signaling pathway | Inhibition | hsa04151 | ||
| In Vitro Model | MDA-MB-231 cells | Breast | Homo sapiens (Human) | CVCL_0062 |
| T47D cells | Breast | Homo sapiens (Human) | CVCL_0553 | |
| ZR75-1 cells | Breast | Homo sapiens (Human) | CVCL_0588 | |
| HCC1937 cells | Breast | Homo sapiens (Human) | CVCL_0290 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miR-124 may be involved in DNA repair by directly targeting ATMIN and PARP1, suggesting that multiple DNA repair pathways are affected by miR-124 and therefore manipulation of miR-124 level/activity may improve the efficacy of chemotherapies that induce DNA damage. repression of ATMIN (+) the HR repair defect induced by miR-124, and restoration of ATMIN reversed the effect of miR-124 overexpression in breast cancer cells. Therefore, it is intriguing to further speculate which of the multiple roles of ATMIN is specifically affected in breast carcinogenesis. On the other hand, PARP1-mediated processes play a role in oncogenesis, cancer progression, and therapeutic resistance. | |||
| Key Molecule: Poly[ADP-ribose] synthase 1 (PARP1) | [38] | |||
| Sensitive Disease | Osteosarcoma [ICD-11: 2B51.0] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell invasion | Inhibition | hsa05200 | ||
| Cell migration | Inhibition | hsa04670 | ||
| Cell proliferation | Inhibition | hsa05200 | ||
| PI3K/AKT signaling pathway | Inhibition | hsa04151 | ||
| In Vitro Model | MDA-MB-231 cells | Breast | Homo sapiens (Human) | CVCL_0062 |
| T47D cells | Breast | Homo sapiens (Human) | CVCL_0553 | |
| ZR75-1 cells | Breast | Homo sapiens (Human) | CVCL_0588 | |
| HCC1937 cells | Breast | Homo sapiens (Human) | CVCL_0290 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miR-124 may be involved in DNA repair by directly targeting ATMIN and PARP1, suggesting that multiple DNA repair pathways are affected by miR-124 and therefore manipulation of miR-124 level/activity may improve the efficacy of chemotherapies that induce DNA damage. repression of ATMIN (+) the HR repair defect induced by miR-124, and restoration of ATMIN reversed the effect of miR-124 overexpression in breast cancer cells. Therefore, it is intriguing to further speculate which of the multiple roles of ATMIN is specifically affected in breast carcinogenesis. On the other hand, PARP1-mediated processes play a role in oncogenesis, cancer progression, and therapeutic resistance. | |||
| Key Molecule: E2F1-regulated inhibitor of cell death (ERICD) | [40] | |||
| Sensitive Disease | Osteosarcoma [ICD-11: 2B51.0] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | SAOS-2 cells | Bone marrow | Homo sapiens (Human) | CVCL_0548 |
| U2OS cells | Bone | Homo sapiens (Human) | CVCL_0042 | |
| Experiment for Molecule Alteration |
RT-PCR | |||
| Experiment for Drug Resistance |
Fluorescence-activated cell sorting analysis | |||
| Mechanism Description | The long non-coding RNA ERIC is regulated by E2F and modulates the cellular response to DNA damage, ERIC levels were increased following DNA damage by the chemotherapeutic drug Etoposide, and inhibition of ERIC expression enhanced Etoposide -induced apoptosis. | |||
| Key Molecule: DNA repair protein RAD51 homolog 1 (RAD51) | [39] | |||
| Sensitive Disease | Osteosarcoma [ICD-11: 2B51.0] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell proliferation | Inhibition | hsa05200 | ||
| In Vitro Model | U2OS cells | Bone | Homo sapiens (Human) | CVCL_0042 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Survival assay/crystal violet staining assay | |||
| Mechanism Description | miR-103 and miR-107 reduced homology-directed repair and sensitized cells to various DNA damaging agents, including cisplatin and a PARP inhibitor. Mechanistic analyses revealed that both miR-103 and miR-107 directly target and regulate RAD51 and RAD51D, which is critical for miR-103/107-mediated chemosensitization. | |||
| Key Molecule: DNA repair protein RAD51 homolog 4 (RAD51D) | [39] | |||
| Sensitive Disease | Osteosarcoma [ICD-11: 2B51.0] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell proliferation | Inhibition | hsa05200 | ||
| In Vitro Model | U2OS cells | Bone | Homo sapiens (Human) | CVCL_0042 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Survival assay/crystal violet staining assay | |||
| Mechanism Description | miR-103 and miR-107 reduced homology-directed repair and sensitized cells to various DNA damaging agents, including cisplatin and a PARP inhibitor. Mechanistic analyses revealed that both miR-103 and miR-107 directly target and regulate RAD51 and RAD51D, which is critical for miR-103/107-mediated chemosensitization. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: hsa-mir-125b | [2] | |||
| Resistant Disease | Ewing sarcoma [ICD-11: 2B52.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| miR125b-p53/BAKT signaling pathway | Activation | hsa05206 | ||
| In Vitro Model | RD-ES cells | Bones | Homo sapiens (Human) | CVCL_2169 |
| Sk-ES cells | Bones | Homo sapiens (Human) | CVCL_0627 | |
| Sk-N-MC cells | Bones | Homo sapiens (Human) | CVCL_0530 | |
| TC-71 cells | Bones | Homo sapiens (Human) | CVCL_2213 | |
| VH-64 cells | Bones | Homo sapiens (Human) | CVCL_9672 | |
| WE-68 cells | Bones | Homo sapiens (Human) | CVCL_9717 | |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
Celltiter-glo luminescent cell viability assay | |||
| Mechanism Description | miR-125b led to the development of chemoresistance by suppressing the expression of p53 and Bak, and repression of miR-125b sensitized EWS cells to apoptosis induced by treatment with various cytotoxic drugs. | |||
|
|
||||
| Key Molecule: Cellular tumor antigen p53 (TP53) | [2] | |||
| Resistant Disease | Ewing sarcoma [ICD-11: 2B52.0] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| miR125b-p53/BAKT signaling pathway | Activation | hsa05206 | ||
| In Vitro Model | RD-ES cells | Bones | Homo sapiens (Human) | CVCL_2169 |
| Sk-ES cells | Bones | Homo sapiens (Human) | CVCL_0627 | |
| Sk-N-MC cells | Bones | Homo sapiens (Human) | CVCL_0530 | |
| TC-71 cells | Bones | Homo sapiens (Human) | CVCL_2213 | |
| VH-64 cells | Bones | Homo sapiens (Human) | CVCL_9672 | |
| WE-68 cells | Bones | Homo sapiens (Human) | CVCL_9717 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Celltiter-glo luminescent cell viability assay | |||
| Mechanism Description | miR-125b led to the development of chemoresistance by suppressing the expression of p53 and Bak, and repression of miR-125b sensitized EWS cells to apoptosis induced by treatment with various cytotoxic drugs. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Nijmegen breakage syndrome protein 1 (NBS1) | [14] | |||
| Metabolic Type | Glucose metabolism | |||
| Resistant Disease | Gastric adenocarcinoma [ICD-11: 2B72.0] | |||
| Molecule Alteration | Lactylation | K388 |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | AGS cells | Gastric | Homo sapiens (Human) | CVCL_0139 |
| Experiment for Molecule Alteration |
LC-MS/MS analysis | |||
| Mechanism Description | Lactylation of NBS1 at lysine 388 (K388) is essential for MRE11-RAD50-NBS1 (MRN) complex formation and the accumulation of HR repair proteins at the sites of DNA double-strand breaks.It promotes DNA-damaging treatment resistance via HR repair. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: hsa-mir-181 | [41] | |||
| Sensitive Disease | Gastric adenocarcinoma [ICD-11: 2B72.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| In Vitro Model | SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 |
| SGC7901/VCR cells | Gastric | Homo sapiens (Human) | CVCL_VU58 | |
| Experiment for Molecule Alteration |
RT-PCR | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | The antiapoptotic protein BCL2 is upregulated, whereas miR-181b is downregulated in both SGC7901/VCR and A549/CDDP cells, compared with SGC7901 and A549 cells, respectively. Enforced miR-181b expression reduced BCL2 protein level and sensitized SGC7901/VCR and A549/CDDP cells to VCR-induced and CDDP-induced apoptosis, respectively. | |||
| Key Molecule: hsa-mir-15b | [42] | |||
| Sensitive Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Mitochondrial signaling pathway | Activation | hsa04217 | ||
| In Vitro Model | SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 |
| SGC7901/VCR cells | Gastric | Homo sapiens (Human) | CVCL_VU58 | |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miR-15b and miR-16, among the downregulated miRNAs in SGC7901/VCR cells, were demonstrated to play a role in the development of MDR in gastric cancer cells by targeting the antiapoptotic gene BCL2. | |||
| Key Molecule: hsa-mir-16 | [42] | |||
| Sensitive Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Mitochondrial signaling pathway | Activation | hsa04217 | ||
| In Vitro Model | SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 |
| SGC7901/VCR cells | Gastric | Homo sapiens (Human) | CVCL_VU58 | |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miR-15b and miR-16, among the downregulated miRNAs in SGC7901/VCR cells, were demonstrated to play a role in the development of MDR in gastric cancer cells by targeting the antiapoptotic gene BCL2. | |||
|
|
||||
| Key Molecule: Apoptosis regulator Bcl-2 (BCL2) | [41] | |||
| Sensitive Disease | Gastric adenocarcinoma [ICD-11: 2B72.0] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| In Vitro Model | SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 |
| SGC7901/VCR cells | Gastric | Homo sapiens (Human) | CVCL_VU58 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | The antiapoptotic protein BCL2 is upregulated, whereas miR-181b is downregulated in both SGC7901/VCR and A549/CDDP cells, compared with SGC7901 and A549 cells, respectively. Enforced miR-181b expression reduced BCL2 protein level and sensitized SGC7901/VCR and A549/CDDP cells to VCR-induced and CDDP-induced apoptosis, respectively. | |||
| Key Molecule: Apoptosis regulator Bcl-2 (BCL2) | [42] | |||
| Sensitive Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Mitochondrial signaling pathway | Activation | hsa04217 | ||
| In Vitro Model | SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 |
| SGC7901/VCR cells | Gastric | Homo sapiens (Human) | CVCL_VU58 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miR-15b and miR-16, among the downregulated miRNAs in SGC7901/VCR cells, were demonstrated to play a role in the development of MDR in gastric cancer cells by targeting the antiapoptotic gene BCL2. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: hsa-mir-125a | [45] | |||
| Sensitive Disease | Laryngeal cancer [ICD-11: 2C23.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | HEp-2 cells | Skin | Homo sapiens (Human) | CVCL_1906 |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
RT-PCR | |||
| Experiment for Drug Resistance |
MTT assay; Annexin V-FITC apoptosis assay | |||
| Mechanism Description | Inhibition of HAX-1 by miR125a reverses cisplatin resistance in laryngeal cancer stem cells. Overexpression of miR125a increases the sensitivity of Hep-2-CSCs to cisplatin by inhibiting HAX-1. | |||
|
|
||||
| Key Molecule: HCLS1-associated protein X-1 (HAX1) | [45] | |||
| Sensitive Disease | Laryngeal cancer [ICD-11: 2C23.1] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | HEp-2 cells | Skin | Homo sapiens (Human) | CVCL_1906 |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis; Luciferase reporter assay | |||
| Experiment for Drug Resistance |
MTT assay; Annexin V-FITC apoptosis assay | |||
| Mechanism Description | Inhibition of HAX-1 by miR125a reverses cisplatin resistance in laryngeal cancer stem cells. Overexpression of miR125a increases the sensitivity of Hep-2-CSCs to cisplatin by inhibiting HAX-1. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: ATP-binding cassette sub-family B5 (ABCB5) | [7] | |||
| Resistant Disease | Merkel cell carcinoma [ICD-11: 2C34.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | MKL-2 cells | Peripheral blood | Homo sapiens (Human) | CVCL_D027 |
| WaGa cells | Ascites | Homo sapiens (Human) | CVCL_E998 | |
| MKL-1 cells | Liver | Homo sapiens (Human) | CVCL_2600 | |
| MS-1 cells | Lung | Homo sapiens (Human) | CVCL_IQ55 | |
| In Vivo Model | NSG mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
qRT-PCR; Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | These findings in patient specimens were consistent with the possibility that ABCB5+ MCC cells are preferentially resistant to treatment with the first-line chemotherapeutic agents, carboplatin and etoposide. | |||
| Key Molecule: ATP-binding cassette sub-family B5 (ABCB5) | [7] | |||
| Resistant Disease | Merkel cell carcinoma [ICD-11: 2C34.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | MKL-2 cells | Peripheral blood | Homo sapiens (Human) | CVCL_D027 |
| WaGa cells | Ascites | Homo sapiens (Human) | CVCL_E998 | |
| MKL-1 cells | Liver | Homo sapiens (Human) | CVCL_2600 | |
| MS-1 cells | Lung | Homo sapiens (Human) | CVCL_IQ55 | |
| In Vivo Model | NSG mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
qRT-PCR; Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | These findings in patient specimens were consistent with the possibility that ABCB5+ MCC cells are preferentially resistant to treatment with the first-line chemotherapeutic agents, carboplatin and etoposide. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: hsa-miR-106b~25 | [12] | |||
| Resistant Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| miR106b~25 cluster/EP300/E-cadherin signaling pathway | Regulation | N.A. | ||
| In Vitro Model | MTMECs cells | Breast | Homo sapiens (Human) | N.A. |
| Experiment for Molecule Alteration |
RT-PCR | |||
| Experiment for Drug Resistance |
Flow cytometry assay | |||
| Mechanism Description | miR-106b~25 cluster controls transporter-independent MDR by apoptosis evasion via downregulation of EP300. | |||
| Key Molecule: hsa-mir-128a | [49] | |||
| Resistant Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| In Vitro Model | MDA-MB-231 cells | Breast | Homo sapiens (Human) | CVCL_0062 |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | miR-128 regulates sensitivity to drugs and apoptosis in breast cancer cells, pro-apoptotic protein bax is negatively post-transcriptionally regulated by miR-128. Bax overexpression could lead to a generalised enhancement of the apoptotic response to death stimuli, miR-128 was significantly associated with a drug fast in breast cancer cells by resisting the activation of the apoptosis pathway. | |||
| Key Molecule: hsa-mir-155 | [3] | |||
| Resistant Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| TGF-beta/Smad signaling pathway | Regulation | N.A. | ||
| In Vitro Model | Breast cancer cell lines | Colon | Homo sapiens (Human) | N.A. |
| Experiment for Molecule Alteration |
qRT-PCR; Northern blotting analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Loss of FOXO3a is often linked to a decline in apoptotic activity and increased chemoresistance in cancer cells. miR-155 directly interacts with 3'-UTR of FOXO3a and blocks FOXO3a translation. knockdown of miR-155 renders cells to apoptosis and enhances chemosensitivity. | |||
|
|
||||
| Key Molecule: Histone acetyltransferase p300 (EP300) | [12] | |||
| Resistant Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| miR106b~25 cluster/EP300/E-cadherin signaling pathway | Regulation | N.A. | ||
| In Vitro Model | MTMECs cells | Breast | Homo sapiens (Human) | N.A. |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Flow cytometry assay | |||
| Mechanism Description | miR-106b~25 cluster controls transporter-independent MDR by apoptosis evasion via downregulation of EP300. | |||
| Key Molecule: Apoptosis regulator BAX (BAX) | [49] | |||
| Resistant Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| In Vitro Model | MDA-MB-231 cells | Breast | Homo sapiens (Human) | CVCL_0062 |
| Experiment for Molecule Alteration |
luciferase report assay | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | miR-128 regulates sensitivity to drugs and apoptosis in breast cancer cells, pro-apoptotic protein bax is negatively post-transcriptionally regulated by miR-128. Bax overexpression could lead to a generalised enhancement of the apoptotic response to death stimuli, miR-128 was significantly associated with a drug fast in breast cancer cells by resisting the activation of the apoptosis pathway. | |||
| Key Molecule: Forkhead box protein O3 (FOXO3) | [3] | |||
| Resistant Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| TGF-beta/Smad signaling pathway | Regulation | N.A. | ||
| In Vitro Model | Breast cancer cell lines | Colon | Homo sapiens (Human) | N.A. |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Loss of FOXO3a is often linked to a decline in apoptotic activity and increased chemoresistance in cancer cells. miR-155 directly interacts with 3'-UTR of FOXO3a and blocks FOXO3a translation. knockdown of miR-155 renders cells to apoptosis and enhances chemosensitivity. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: hsa-mir-124 | [38] | |||
| Sensitive Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell invasion | Inhibition | hsa05200 | ||
| Cell migration | Inhibition | hsa04670 | ||
| Cell proliferation | Inhibition | hsa05200 | ||
| PI3K/AKT signaling pathway | Inhibition | hsa04151 | ||
| In Vitro Model | MDA-MB-231 cells | Breast | Homo sapiens (Human) | CVCL_0062 |
| T47D cells | Breast | Homo sapiens (Human) | CVCL_0553 | |
| ZR75-1 cells | Breast | Homo sapiens (Human) | CVCL_0588 | |
| HCC1937 cells | Breast | Homo sapiens (Human) | CVCL_0290 | |
| Experiment for Molecule Alteration |
RT-PCR | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miR-124 may be involved in DNA repair by directly targeting ATMIN and PARP1, suggesting that multiple DNA repair pathways are affected by miR-124 and therefore manipulation of miR-124 level/activity may improve the efficacy of chemotherapies that induce DNA damage. repression of ATMIN (+) the HR repair defect induced by miR-124, and restoration of ATMIN reversed the effect of miR-124 overexpression in breast cancer cells. Therefore, it is intriguing to further speculate which of the multiple roles of ATMIN is specifically affected in breast carcinogenesis. On the other hand, PARP1-mediated processes play a role in oncogenesis, cancer progression, and therapeutic resistance. | |||
| Key Molecule: hsa-mir-103 | [39] | |||
| Sensitive Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell proliferation | Inhibition | hsa05200 | ||
| In Vitro Model | MDA-MB-231 cells | Breast | Homo sapiens (Human) | CVCL_0062 |
| Experiment for Molecule Alteration |
RT-PCR | |||
| Experiment for Drug Resistance |
Survival assay/crystal violet staining assay | |||
| Mechanism Description | miR-103 and miR-107 reduced homology-directed repair and sensitized cells to various DNA damaging agents, including cisplatin and a PARP inhibitor. Mechanistic analyses revealed that both miR-103 and miR-107 directly target and regulate RAD51 and RAD51D, which is critical for miR-103/107-mediated chemosensitization. | |||
| Key Molecule: hsa-miR-107 | [39] | |||
| Sensitive Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell proliferation | Inhibition | hsa05200 | ||
| In Vitro Model | MDA-MB-231 cells | Breast | Homo sapiens (Human) | CVCL_0062 |
| Experiment for Molecule Alteration |
RT-PCR | |||
| Experiment for Drug Resistance |
Survival assay/crystal violet staining assay | |||
| Mechanism Description | miR-103 and miR-107 reduced homology-directed repair and sensitized cells to various DNA damaging agents, including cisplatin and a PARP inhibitor. Mechanistic analyses revealed that both miR-103 and miR-107 directly target and regulate RAD51 and RAD51D, which is critical for miR-103/107-mediated chemosensitization. | |||
| Key Molecule: hsa-miR-326 | [50] | |||
| Sensitive Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell proliferation | Activation | hsa05200 | |
| In Vitro Model | MCF-7 cells | Breast | Homo sapiens (Human) | CVCL_0031 |
| Experiment for Molecule Alteration |
RT-PCR; qRT-PCR | |||
| Experiment for Drug Resistance |
MTS assay | |||
| Mechanism Description | The elevated levels of miR-326 in the mimics-transfected VP-16-resistant cell line, MCF-7/VP, downregulated MRP-1 expression and sensitized these cells to VP-16 and doxorubicin. | |||
|
|
||||
| Key Molecule: Multidrug resistance-associated protein 1 (MRP1) | [50] | |||
| Sensitive Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell proliferation | Activation | hsa05200 | |
| In Vitro Model | MCF-7 cells | Breast | Homo sapiens (Human) | CVCL_0031 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTS assay | |||
| Mechanism Description | The elevated levels of miR-326 in the mimics-transfected VP-16-resistant cell line, MCF-7/VP, downregulated MRP-1 expression and sensitized these cells to VP-16 and doxorubicin. | |||
|
|
||||
| Key Molecule: ATM interactor (ATMIN) | [38] | |||
| Sensitive Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell invasion | Inhibition | hsa05200 | ||
| Cell migration | Inhibition | hsa04670 | ||
| Cell proliferation | Inhibition | hsa05200 | ||
| PI3K/AKT signaling pathway | Inhibition | hsa04151 | ||
| In Vitro Model | MDA-MB-231 cells | Breast | Homo sapiens (Human) | CVCL_0062 |
| T47D cells | Breast | Homo sapiens (Human) | CVCL_0553 | |
| ZR75-1 cells | Breast | Homo sapiens (Human) | CVCL_0588 | |
| HCC1937 cells | Breast | Homo sapiens (Human) | CVCL_0290 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miR-124 may be involved in DNA repair by directly targeting ATMIN and PARP1, suggesting that multiple DNA repair pathways are affected by miR-124 and therefore manipulation of miR-124 level/activity may improve the efficacy of chemotherapies that induce DNA damage. repression of ATMIN (+) the HR repair defect induced by miR-124, and restoration of ATMIN reversed the effect of miR-124 overexpression in breast cancer cells. Therefore, it is intriguing to further speculate which of the multiple roles of ATMIN is specifically affected in breast carcinogenesis. On the other hand, PARP1-mediated processes play a role in oncogenesis, cancer progression, and therapeutic resistance. | |||
| Key Molecule: Poly[ADP-ribose] synthase 1 (PARP1) | [38] | |||
| Sensitive Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell invasion | Inhibition | hsa05200 | ||
| Cell migration | Inhibition | hsa04670 | ||
| Cell proliferation | Inhibition | hsa05200 | ||
| PI3K/AKT signaling pathway | Inhibition | hsa04151 | ||
| In Vitro Model | MDA-MB-231 cells | Breast | Homo sapiens (Human) | CVCL_0062 |
| T47D cells | Breast | Homo sapiens (Human) | CVCL_0553 | |
| ZR75-1 cells | Breast | Homo sapiens (Human) | CVCL_0588 | |
| HCC1937 cells | Breast | Homo sapiens (Human) | CVCL_0290 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miR-124 may be involved in DNA repair by directly targeting ATMIN and PARP1, suggesting that multiple DNA repair pathways are affected by miR-124 and therefore manipulation of miR-124 level/activity may improve the efficacy of chemotherapies that induce DNA damage. repression of ATMIN (+) the HR repair defect induced by miR-124, and restoration of ATMIN reversed the effect of miR-124 overexpression in breast cancer cells. Therefore, it is intriguing to further speculate which of the multiple roles of ATMIN is specifically affected in breast carcinogenesis. On the other hand, PARP1-mediated processes play a role in oncogenesis, cancer progression, and therapeutic resistance. | |||
| Key Molecule: DNA repair protein RAD51 homolog 1 (RAD51) | [39] | |||
| Sensitive Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell proliferation | Inhibition | hsa05200 | ||
| In Vitro Model | MDA-MB-231 cells | Breast | Homo sapiens (Human) | CVCL_0062 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Survival assay/crystal violet staining assay | |||
| Mechanism Description | miR-103 and miR-107 reduced homology-directed repair and sensitized cells to various DNA damaging agents, including cisplatin and a PARP inhibitor. Mechanistic analyses revealed that both miR-103 and miR-107 directly target and regulate RAD51 and RAD51D, which is critical for miR-103/107-mediated chemosensitization. | |||
| Key Molecule: DNA repair protein RAD51 homolog 4 (RAD51D) | [39] | |||
| Sensitive Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell proliferation | Inhibition | hsa05200 | ||
| In Vitro Model | MDA-MB-231 cells | Breast | Homo sapiens (Human) | CVCL_0062 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Survival assay/crystal violet staining assay | |||
| Mechanism Description | miR-103 and miR-107 reduced homology-directed repair and sensitized cells to various DNA damaging agents, including cisplatin and a PARP inhibitor. Mechanistic analyses revealed that both miR-103 and miR-107 directly target and regulate RAD51 and RAD51D, which is critical for miR-103/107-mediated chemosensitization. | |||
| Key Molecule: Forkhead box K2 (FOXK2) | [51] | |||
| Sensitive Disease | Breast adenocarcinoma [ICD-11: 2C60.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | MDA-MB-231cells | Breast | Homo sapiens (Human) | CVCL_0062 |
| MDA-MB-361 cells | Breast | Homo sapiens (Human) | CVCL_0620 | |
| Experiment for Molecule Alteration |
Western blot assay; qRT-PCR | |||
| Experiment for Drug Resistance |
CCK8 assay; Colony formation assay | |||
| Mechanism Description | We evaluated the effect of?FOXK2?knockdown in combination with conventional chemotherapeutic agents in BC cells.?FOXK2?knockdown significantly enhanced the cytotoxic effect of?doxorubicin, 5-fluorouracil, and?etoposide?in MDA-MB-231?cells and MDA-MB-361?cells. These results show that inhibiting FOXK2 sensitizes FOXK2-overexpressing BC cells to conventional chemotherapeutic agents. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: hsa-mir-103 | [39] | |||
| Sensitive Disease | Ovarian cancer [ICD-11: 2C73.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell proliferation | Inhibition | hsa05200 | ||
| In Vitro Model | PEO1 C4-2 cells | Ovary | Homo sapiens (Human) | N.A. |
| Experiment for Molecule Alteration |
RT-PCR | |||
| Experiment for Drug Resistance |
Survival assay/crystal violet staining assay | |||
| Mechanism Description | miR-103 and miR-107 reduced homology-directed repair and sensitized cells to various DNA damaging agents, including cisplatin and a PARP inhibitor. Mechanistic analyses revealed that both miR-103 and miR-107 directly target and regulate RAD51 and RAD51D, which is critical for miR-103/107-mediated chemosensitization. | |||
| Key Molecule: hsa-miR-107 | [39] | |||
| Sensitive Disease | Ovarian cancer [ICD-11: 2C73.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell proliferation | Inhibition | hsa05200 | ||
| In Vitro Model | PEO1 C4-2 cells | Ovary | Homo sapiens (Human) | N.A. |
| Experiment for Molecule Alteration |
RT-PCR | |||
| Experiment for Drug Resistance |
Survival assay/crystal violet staining assay | |||
| Mechanism Description | miR-103 and miR-107 reduced homology-directed repair and sensitized cells to various DNA damaging agents, including cisplatin and a PARP inhibitor. Mechanistic analyses revealed that both miR-103 and miR-107 directly target and regulate RAD51 and RAD51D, which is critical for miR-103/107-mediated chemosensitization. | |||
|
|
||||
| Key Molecule: DNA repair protein RAD51 homolog 1 (RAD51) | [39] | |||
| Sensitive Disease | Ovarian cancer [ICD-11: 2C73.0] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell proliferation | Inhibition | hsa05200 | ||
| In Vitro Model | PEO1 C4-2 cells | Ovary | Homo sapiens (Human) | N.A. |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Survival assay/crystal violet staining assay | |||
| Mechanism Description | miR-103 and miR-107 reduced homology-directed repair and sensitized cells to various DNA damaging agents, including cisplatin and a PARP inhibitor. Mechanistic analyses revealed that both miR-103 and miR-107 directly target and regulate RAD51 and RAD51D, which is critical for miR-103/107-mediated chemosensitization. | |||
| Key Molecule: DNA repair protein RAD51 homolog 4 (RAD51D) | [39] | |||
| Sensitive Disease | Ovarian cancer [ICD-11: 2C73.0] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell proliferation | Inhibition | hsa05200 | ||
| In Vitro Model | PEO1 C4-2 cells | Ovary | Homo sapiens (Human) | N.A. |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Survival assay/crystal violet staining assay | |||
| Mechanism Description | miR-103 and miR-107 reduced homology-directed repair and sensitized cells to various DNA damaging agents, including cisplatin and a PARP inhibitor. Mechanistic analyses revealed that both miR-103 and miR-107 directly target and regulate RAD51 and RAD51D, which is critical for miR-103/107-mediated chemosensitization. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: hsa-mir-103 | [39] | |||
| Sensitive Disease | Cervical cancer [ICD-11: 2C77.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell proliferation | Inhibition | hsa05200 | ||
| In Vitro Model | Hela cells | Cervix uteri | Homo sapiens (Human) | CVCL_0030 |
| Experiment for Molecule Alteration |
RT-PCR | |||
| Experiment for Drug Resistance |
Survival assay/crystal violet staining assay | |||
| Mechanism Description | miR-103 and miR-107 reduced homology-directed repair and sensitized cells to various DNA damaging agents, including cisplatin and a PARP inhibitor. Mechanistic analyses revealed that both miR-103 and miR-107 directly target and regulate RAD51 and RAD51D, which is critical for miR-103/107-mediated chemosensitization. | |||
| Key Molecule: hsa-miR-107 | [39] | |||
| Sensitive Disease | Cervical cancer [ICD-11: 2C77.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell proliferation | Inhibition | hsa05200 | ||
| In Vitro Model | Hela cells | Cervix uteri | Homo sapiens (Human) | CVCL_0030 |
| Experiment for Molecule Alteration |
RT-PCR | |||
| Experiment for Drug Resistance |
Survival assay/crystal violet staining assay | |||
| Mechanism Description | miR-103 and miR-107 reduced homology-directed repair and sensitized cells to various DNA damaging agents, including cisplatin and a PARP inhibitor. Mechanistic analyses revealed that both miR-103 and miR-107 directly target and regulate RAD51 and RAD51D, which is critical for miR-103/107-mediated chemosensitization. | |||
|
|
||||
| Key Molecule: DNA repair protein RAD51 homolog 1 (RAD51) | [39] | |||
| Sensitive Disease | Cervical cancer [ICD-11: 2C77.0] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell proliferation | Inhibition | hsa05200 | ||
| In Vitro Model | Hela cells | Cervix uteri | Homo sapiens (Human) | CVCL_0030 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Survival assay/crystal violet staining assay | |||
| Mechanism Description | miR-103 and miR-107 reduced homology-directed repair and sensitized cells to various DNA damaging agents, including cisplatin and a PARP inhibitor. Mechanistic analyses revealed that both miR-103 and miR-107 directly target and regulate RAD51 and RAD51D, which is critical for miR-103/107-mediated chemosensitization. | |||
| Key Molecule: DNA repair protein RAD51 homolog 4 (RAD51D) | [39] | |||
| Sensitive Disease | Cervical cancer [ICD-11: 2C77.0] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell proliferation | Inhibition | hsa05200 | ||
| In Vitro Model | Hela cells | Cervix uteri | Homo sapiens (Human) | CVCL_0030 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Survival assay/crystal violet staining assay | |||
| Mechanism Description | miR-103 and miR-107 reduced homology-directed repair and sensitized cells to various DNA damaging agents, including cisplatin and a PARP inhibitor. Mechanistic analyses revealed that both miR-103 and miR-107 directly target and regulate RAD51 and RAD51D, which is critical for miR-103/107-mediated chemosensitization. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Cytochrome P450 family 1 subfamily B member1 (CYP1B1) | [43] | |||
| Sensitive Disease | Kidney cancer [ICD-11: 2C90.1] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | miR27b/CCNG1/p53 signaling pathway | Regulation | N.A. | |
| In Vitro Model | 769-P cells | Kidney | Homo sapiens (Human) | CVCL_1050 |
| 786-O cells | Kidney | Homo sapiens (Human) | CVCL_1051 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CellTiter-Glo luminescent cell viability assay | |||
| Mechanism Description | miR-27b synergizes with anticancer drugs througth enhancing anticancer drug-induced cell death which due to p53 activation and CYP1B1 suppression. | |||
|
|
||||
| Key Molecule: hsa-mir-27b | [43] | |||
| Sensitive Disease | Kidney cancer [ICD-11: 2C90.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | miR27b/CCNG1/p53 signaling pathway | Regulation | N.A. | |
| In Vitro Model | 769-P cells | Kidney | Homo sapiens (Human) | CVCL_1050 |
| 786-O cells | Kidney | Homo sapiens (Human) | CVCL_1051 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
qPCR | |||
| Experiment for Drug Resistance |
CellTiter-Glo luminescent cell viability assay | |||
| Mechanism Description | miR-27b synergizes with anticancer drugs througth enhancing anticancer drug-induced cell death which due to p53 activation and CYP1B1 suppression. | |||
|
|
||||
| Key Molecule: Cyclin-G1 (CCNG1) | [43] | |||
| Sensitive Disease | Kidney cancer [ICD-11: 2C90.1] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| miR27b/CCNG1/p53 signaling pathway | Regulation | N.A. | ||
| In Vitro Model | 769-P cells | Kidney | Homo sapiens (Human) | CVCL_1050 |
| 786-O cells | Kidney | Homo sapiens (Human) | CVCL_1051 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CellTiter-Glo luminescent cell viability assay | |||
| Mechanism Description | miR-27b synergizes with anticancer drugs througth enhancing anticancer drug-induced cell death which due to p53 activation and CYP1B1 suppression. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Interleukin 2 receptor subunit alpha (IL2RA) | [4] | |||
| Resistant Disease | Head and neck squamous cell carcinoma [ICD-11: 2D42.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | PCI-13 cells | Ovary | Homo sapiens (Human) | CVCL_C182 |
| Experiment for Molecule Alteration |
Western blotting assay | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | IL-2Ralpha-expressing cells were significantly more resistant to apoptosis induction by a tripeptidyl proteasome inhibitor (ALLN) and two chemotherapeutic drugs (VP-16 and taxol) than the control or IL-2Rgamma+ cells.IL-2Ralpha overexpression increases cell proliferation rate associated with increasing levels of cell cycle regulatory proteins. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: hsa-mir-29b | [53] | |||
| Sensitive Disease | Negroid cervix epitheloid carcinoma [ICD-11: 2E66.Y] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Hela cells | Cervix uteri | Homo sapiens (Human) | CVCL_0030 |
| Experiment for Molecule Alteration |
RT-PCR | |||
| Experiment for Drug Resistance |
xCELLigence cell viability assay; Flow cytometry assay; Caspase-3 activity assay | |||
| Mechanism Description | microRNA hsa-miR29b potentiates etoposide toxicity in HeLa cells via down-regulation of Mcl-1. | |||
|
|
||||
| Key Molecule: Induced myeloid leukemia cell differentiation protein Mcl-1 (MCL1) | [53] | |||
| Sensitive Disease | Negroid cervix epitheloid carcinoma [ICD-11: 2E66.Y] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Hela cells | Cervix uteri | Homo sapiens (Human) | CVCL_0030 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
xCELLigence cell viability assay; Flow cytometry assay; Caspase-3 activity assay | |||
| Mechanism Description | microRNA hsa-miR29b potentiates etoposide toxicity in HeLa cells via down-regulation of Mcl-1. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Bcl-2-associated agonist of cell death (BAD) | [10] | |||
| Resistant Disease | Pituitary adenoma [ICD-11: 2F37.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Pituitary tumour stem-like cells | Pituitary | Homo sapiens (Human) | N.A. |
| In Vivo Model | NOD/SCID mice xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
WST-1 proliferation assay | |||
| Mechanism Description | Stem cells are generally known to preferentially express antiapoptotic genes, such as BCL-2, cIAP1, NAIP, and XIAP.The expression levels of these antiapoptotic genes in PASC1 were one- to sixfolds higher than those in its daughter cells. | |||
| Key Molecule: Baculoviral IAP repeat containing 2 (BIRC2) | [10] | |||
| Resistant Disease | Pituitary adenoma [ICD-11: 2F37.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Pituitary tumour stem-like cells | Pituitary | Homo sapiens (Human) | N.A. |
| In Vivo Model | NOD/SCID mice xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
WST-1 proliferation assay | |||
| Mechanism Description | Stem cells are generally known to preferentially express antiapoptotic genes, such as BCL-2, cIAP1, NAIP, and XIAP.The expression levels of these antiapoptotic genes in PASC1 were one- to sixfolds higher than those in its daughter cells. | |||
| Key Molecule: Baculoviral IAP repeat-containing protein 1 (BIRC1) | [10] | |||
| Resistant Disease | Pituitary adenoma [ICD-11: 2F37.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Pituitary tumour stem-like cells | Pituitary | Homo sapiens (Human) | N.A. |
| In Vivo Model | NOD/SCID mice xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
WST-1 proliferation assay | |||
| Mechanism Description | Stem cells are generally known to preferentially express antiapoptotic genes, such as BCL-2, cIAP1, NAIP, and XIAP.The expression levels of these antiapoptotic genes in PASC1 were one- to sixfolds higher than those in its daughter cells. | |||
| Key Molecule: E3 ubiquitin-protein ligase XIAP (XIAP) | [10] | |||
| Resistant Disease | Pituitary adenoma [ICD-11: 2F37.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Pituitary tumour stem-like cells | Pituitary | Homo sapiens (Human) | N.A. |
| In Vivo Model | NOD/SCID mice xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
WST-1 proliferation assay | |||
| Mechanism Description | Stem cells are generally known to preferentially express antiapoptotic genes, such as BCL-2, cIAP1, NAIP, and XIAP.The expression levels of these antiapoptotic genes in PASC1 were one- to sixfolds higher than those in its daughter cells. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Neurofibromin (NF1) | [8] | |||
| Resistant Disease | Peripheral nerve sheath tumor [ICD-11: 2F3Y.1] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | RAS signaling pathway | Activation | hsa04014 | |
| In Vitro Model | sNF02.2 cells | Lung | Homo sapiens (Human) | CVCL_K280 |
| Hs 53.T cells | Skin | Homo sapiens (Human) | CVCL_0786 | |
| Experiment for Molecule Alteration |
qRT-PCR; Western blotting assay | |||
| Experiment for Drug Resistance |
Ez-Cytox assay | |||
| Mechanism Description | High expression of Bcl-xL in the MPNST cells was caused by a decreased transcriptional expression of the NF1 gene. Down-regulation of the NF1 gene by RNA interference (RNAi) induced an increase in Bcl-xL expression and a decrease in its sensitivity to apoptosis in the benign neurofibroma cell line and primary normal cells. | |||
ICD-14: Skin diseases
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Multidrug resistance-associated protein 1 (MRP1) | [5] | |||
| Resistant Disease | Hypertrophic scar [ICD-11: EE60.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Hypertrophic scar tissue isolates | N.A. | ||
| Experiment for Molecule Alteration |
Western blotting assay | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Fibroblasts derived from hypertrophic scar and normal skin tissues were first compared for their resistance to verapamil and etoposide phosphate. Scar fibroblasts showed stronger resistance to both verapamil and etoposide than normal fibroblasts, also scar fibroblasts expressed more P-glycoprotein and MRP1 than normal fibroblasts. | |||
| Key Molecule: Multidrug resistance protein 1 (ABCB1) | [5] | |||
| Resistant Disease | Hypertrophic scar [ICD-11: EE60.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Hypertrophic scar tissue isolates | N.A. | ||
| Experiment for Molecule Alteration |
Western blotting assay | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Fibroblasts derived from hypertrophic scar and normal skin tissues were first compared for their resistance to verapamil and etoposide phosphate. Scar fibroblasts showed stronger resistance to both verapamil and etoposide than normal fibroblasts, also scar fibroblasts expressed more P-glycoprotein and MRP1 than normal fibroblasts. | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
