Molecule Information
General Information of the Molecule (ID: Mol00029)
| Name |
Apoptosis regulator Bcl-2 (BCL2)
,Homo sapiens
|
||||
|---|---|---|---|---|---|
| Molecule Type |
Protein
|
||||
| Gene Name |
BCL2
|
||||
| Gene ID | |||||
| Location |
chr18:63123346-63320128[-]
|
||||
| Sequence |
MAHAGRTGYDNREIVMKYIHYKLSQRGYEWDAGDVGAAPPGAAPAPGIFSSQPGHTPHPA
ASRDPVARTSPLQTPAAPGAAAGPALSPVPPVVHLTLRQAGDDFSRRYRRDFAEMSSQLH LTPFTARGRFATVVEELFRDGVNWGRIVAFFEFGGVMCVESVNREMSPLVDNIALWMTEY LNRHLHTWIQDNGGWDAFVELYGPSMRPLFDFSWLSLKTLLSLALVGACITLGAYLGHK Click to Show/Hide
|
||||
| Function |
Suppresses apoptosis in a variety of cell systems including factor-dependent lymphohematopoietic and neural cells. Regulates cell death by controlling the mitochondrial membrane permeability. Appears to function in a feedback loop system with caspases. Inhibits caspase activity either by preventing the release of cytochrome c from the mitochondria and/or by binding to the apoptosis-activating factor (APAF-1). Also acts as an inhibitor of autophagy: interacts with BECN1 and AMBRA1 during non-starvation conditions and inhibits their autophagy function. May attenuate inflammation by impairing NLRP1-inflammasome activation, hence CASP1 activation and IL1B release.
Click to Show/Hide
|
||||
| Uniprot ID | |||||
| Ensembl ID | |||||
| HGNC ID | |||||
| Click to Show/Hide the Complete Species Lineage | |||||
Type(s) of Resistant Mechanism of This Molecule
Drug Resistance Data Categorized by Drug
Approved Drug(s)
26 drug(s) in total
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Prostate cancer [ICD-11: 2C82.0] | [1] | |||
| Resistant Disease | Prostate cancer [ICD-11: 2C82.0] | |||
| Resistant Drug | Docetaxel | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Prostate cancer [ICD-11: 2C82] | |||
| The Specified Disease | Prostate cancer | |||
| The Studied Tissue | Prostate | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.29E-02 Fold-change: 4.09E-02 Z-score: 2.60E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell proliferation | Activation | hsa05200 | ||
| In Vitro Model | DU-145 cells | Prostate | Homo sapiens (Human) | CVCL_0105 |
| PC3 cells | Prostate | Homo sapiens (Human) | CVCL_0035 | |
| 22RV1 cells | Prostate | Homo sapiens (Human) | CVCL_1045 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Acid phosphatase assay | |||
| Mechanism Description | miR-34a regulates BCL-2 and may, in part, regulate response to docetaxel. miR-34a was significantly decreased in prostate cancer versus normal tissues; in biochemical recurrence versus non-recurrence tissue and in metastatic versus primary disease prostate tissue. We confirmed BCL-2 as a target of miR-34a, by manipulating miR-34a expression in our parent and docetaxel resistant cell lines and subsequently assessing BCL-2 levels. Specifically, upon inhibition of miR-34a in sensitive parent cells (PC3 and 22Rv1) we observed an increase in BCL-2 expression, whereas mimicking miR-34a expression in docetaxel-resistant cells (PC3RD and 22Rv1RD) resulted in decreased BCL-2 expression. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Breast cancer [ICD-11: 2C60.3] | [36] | |||
| Sensitive Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Sensitive Drug | Docetaxel | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | MCF-7 cells | Breast | Homo sapiens (Human) | CVCL_0031 |
| MDA-MB-231 cells | Breast | Homo sapiens (Human) | CVCL_0062 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Docetaxel primarily acts in G2-M phase, whereas it has diminished activity in G1 phase. Increased miR-34a expression may therefore be able to inhibit docetaxel activity by arresting cells in G1 phase. | |||
| Disease Class: Gastric cancer [ICD-11: 2B72.1] | [33] | |||
| Sensitive Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Sensitive Drug | Docetaxel | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | p53 signaling pathway | Inhibition | hsa04115 | |
| In Vitro Model | AGS cells | Gastric | Homo sapiens (Human) | CVCL_0139 |
| NCI-N87 cells | Gastric | Homo sapiens (Human) | CVCL_1603 | |
| MkN-45 cells | Gastric | Homo sapiens (Human) | CVCL_0434 | |
| KATO-3 cells | Gastric | Homo sapiens (Human) | CVCL_0371 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | Human gastric cancer kato III cells with miR-34 restoration reduced the expression of target genes Bcl-2, Notch, and HMGA2. MicroRNA miR-34 was recently found to be a direct target of p53, functioning downstream of the p53 pathway as a tumor suppressor, miR-34 impaired cell growth, accumulated the cells in G1 phase, increased caspase-3 activation, and, more significantly, inhibited tumorsphere formation and growth. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Lung cancer [ICD-11: 2C25.5] | [2] | |||
| Sensitive Disease | Lung cancer [ICD-11: 2C25.5] | |||
| Sensitive Drug | Vincristine | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Lung cancer [ICD-11: 2C25] | |||
| The Specified Disease | Lung cancer | |||
| The Studied Tissue | Lung tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 9.45E-01 Fold-change: -4.10E-04 Z-score: -6.86E-02 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| In Vitro Model | A549 cells | Lung | Homo sapiens (Human) | CVCL_0023 |
| A549/CDDP cells | Lung | Homo sapiens (Human) | CVCL_0023 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | The antiapoptotic protein BCL2 is upregulated, whereas miR-181b is downregulated in both SGC7901/VCR and A549/CDDP cells, compared with SGC7901 and A549 cells, respectively. Enforced miR-181b expression reduced BCL2 protein level and sensitized SGC7901/VCR and A549/CDDP cells to VCR-induced and CDDP-induced apoptosis, respectively. And the antiapoptotic protein BCL2 is upregulated, whereas miR-181b is downregulated in both SGC7901/VCR and A549/CDDP cells, compared with SGC7901 and A549 cells, respectively. Enforced miR-181b expression reduced BCL2 protein level and sensitized SGC7901/VCR and A549/CDDP cells to VCR-induced and CDDP-induced apoptosis, respectively. | |||
| Disease Class: Colon cancer [ICD-11: 2B90.1] | [10] | |||
| Sensitive Disease | Colon cancer [ICD-11: 2B90.1] | |||
| Sensitive Drug | Vincristine | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Colon cancer [ICD-11: 2B90] | |||
| The Specified Disease | Colon cancer | |||
| The Studied Tissue | Colon tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 4.01E-51 Fold-change: -1.05E-01 Z-score: -1.95E+01 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| In Vitro Model | HCT8 cells | Colon | Homo sapiens (Human) | CVCL_2478 |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | miR-15a and Mir-16 reverse drug resistance in colon cancer cells, possibly by down-regulating the expression of Bcl-2 protein. | |||
| Disease Class: HPV-related endocervical adenocarcinoma [ICD-11: 2E67.1] | [2], [31] | |||
| Sensitive Disease | HPV-related endocervical adenocarcinoma [ICD-11: 2E67.1] | |||
| Sensitive Drug | Vincristine | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| In Vitro Model | SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 |
| SGC7901/VCR cells | Gastric | Homo sapiens (Human) | CVCL_VU58 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | The antiapoptotic protein BCL2 is upregulated, whereas miR-181b is downregulated in both SGC7901/VCR and A549/CDDP cells, compared with SGC7901 and A549 cells, respectively. Enforced miR-181b expression reduced BCL2 protein level and sensitized SGC7901/VCR and A549/CDDP cells to VCR-induced and CDDP-induced apoptosis, respectively. And the antiapoptotic protein BCL2 is upregulated, whereas miR-181b is downregulated in both SGC7901/VCR and A549/CDDP cells, compared with SGC7901 and A549 cells, respectively. Enforced miR-181b expression reduced BCL2 protein level and sensitized SGC7901/VCR and A549/CDDP cells to VCR-induced and CDDP-induced apoptosis, respectively. | |||
| Disease Class: Gastric adenocarcinoma [ICD-11: 2B72.0] | [2] | |||
| Sensitive Disease | Gastric adenocarcinoma [ICD-11: 2B72.0] | |||
| Sensitive Drug | Vincristine | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| In Vitro Model | SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 |
| SGC7901/VCR cells | Gastric | Homo sapiens (Human) | CVCL_VU58 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | The antiapoptotic protein BCL2 is upregulated, whereas miR-181b is downregulated in both SGC7901/VCR and A549/CDDP cells, compared with SGC7901 and A549 cells, respectively. Enforced miR-181b expression reduced BCL2 protein level and sensitized SGC7901/VCR and A549/CDDP cells to VCR-induced and CDDP-induced apoptosis, respectively. | |||
| Disease Class: Gastric cancer [ICD-11: 2B72.1] | [8] | |||
| Sensitive Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Sensitive Drug | Vincristine | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Mitochondrial signaling pathway | Activation | hsa04217 | ||
| In Vitro Model | SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 |
| SGC7901/VCR cells | Gastric | Homo sapiens (Human) | CVCL_VU58 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miR-15b and miR-16, among the downregulated miRNAs in SGC7901/VCR cells, were demonstrated to play a role in the development of MDR in gastric cancer cells by targeting the antiapoptotic gene BCL2. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Gastric adenocarcinoma [ICD-11: 2B72.0] | [19] | |||
| Resistant Disease | Gastric adenocarcinoma [ICD-11: 2B72.0] | |||
| Resistant Drug | Vincristine | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Fas/FasL signaling pathway | Regulation | N.A. | ||
| In Vitro Model | SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 |
| SGC7901/VCR cells | Gastric | Homo sapiens (Human) | CVCL_VU58 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | The anti-apoptotic protein BCL2 and XIAP were upregulated, while the miR-200bc/429 cluster was downregulated in both SGC7901/VCR and A549/CDDP cells. miR-200bc/429 cluster might play an important role in the development of MDR in human gastric and lung cancer cell lines by targeting the anti-apoptotic genes BCL2 and XIAP. | |||
| Disease Class: Lung cancer [ICD-11: 2C25.5] | [19] | |||
| Resistant Disease | Lung cancer [ICD-11: 2C25.5] | |||
| Resistant Drug | Vincristine | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Fas/FasL signaling pathway | Regulation | N.A. | ||
| In Vitro Model | A549 cells | Lung | Homo sapiens (Human) | CVCL_0023 |
| A549/CDDP cells | Lung | Homo sapiens (Human) | CVCL_0023 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | The anti-apoptotic protein BCL2 and XIAP were upregulated, while the miR-200bc/429 cluster was downregulated in both SGC7901/VCR and A549/CDDP cells. miR-200bc/429 cluster might play an important role in the development of MDR in human gastric and lung cancer cell lines by targeting the anti-apoptotic genes BCL2 and XIAP. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Multiple myeloma [ICD-11: 2A83.0] | [3] | |||
| Sensitive Disease | Multiple myeloma [ICD-11: 2A83.0] | |||
| Sensitive Drug | Bortezomib | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Multiple myeloma [ICD-11: 2A83] | |||
| The Specified Disease | Myeloma | |||
| The Studied Tissue | Peripheral blood | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 6.24E-01 Fold-change: -1.27E-02 Z-score: -4.96E-01 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell colony | Inhibition | hsa05200 | ||
| Cell proliferation | Inhibition | hsa05200 | ||
| Cell viability | Inhibition | hsa05200 | ||
| In Vitro Model | U266 cells | Bone marrow | Homo sapiens (Human) | CVCL_0566 |
| RPMI-8226 cells | Peripheral blood | Homo sapiens (Human) | CVCL_0014 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay; Flow cytometry assay | |||
| Mechanism Description | microRNA-497 inhibits multiple myeloma growth and increases susceptibility to bortezomib by targeting Bcl-2. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Multiple myeloma [ICD-11: 2A83.0] | [12] | |||
| Resistant Disease | Multiple myeloma [ICD-11: 2A83.0] | |||
| Resistant Drug | Bortezomib | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Mechanism Description | Our findings demonstrate miR-34c-5p is differentially expressed between bortezomib-sensitive and -resistant MM cells. Inhibiting miR-34c-5p re-sensitized resistant cells to bortezomib by modulating Bax/Bcl-2 expression, suggesting this miRNA regulates apoptosis and drug resistance and may be a promising therapeutic target for overcoming proteasome inhibitor resistance in MM. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Hepatocellular carcinoma [ICD-11: 2C12.2] | [4] | |||
| Sensitive Disease | Hepatocellular carcinoma [ICD-11: 2C12.2] | |||
| Sensitive Drug | Sorafenib | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Liver cancer [ICD-11: 2C12] | |||
| The Specified Disease | Liver cancer | |||
| The Studied Tissue | Liver tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.81E-06 Fold-change: -5.23E-02 Z-score: -5.27E+00 |
|||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell viability | Inhibition | hsa05200 | ||
| In Vitro Model | Huh-7 cells | Liver | Homo sapiens (Human) | CVCL_0336 |
| HL-7702 cells | Liver | Homo sapiens (Human) | CVCL_6926 | |
| MHCC97-H cells | Liver | Homo sapiens (Human) | CVCL_4972 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | The restoration of miR-34a reduced cell viability, promoted cell apoptosis and potentiated sorafenib-induced apoptosis and toxicity in HCC cell lines by inhibiting Bcl-2 expression. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Pancreatic ductal adenocarcinoma [ICD-11: 2C10.0] | [5] | |||
| Sensitive Disease | Pancreatic ductal adenocarcinoma [ICD-11: 2C10.0] | |||
| Sensitive Drug | Gemcitabine | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Pancreatic cancer [ICD-11: 2C10] | |||
| The Specified Disease | Pancreatic cancer | |||
| The Studied Tissue | Pancreas | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.85E-02 Fold-change: -5.33E-02 Z-score: -2.60E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| In Vitro Model | SW1990 cells | Pancreas | Homo sapiens (Human) | CVCL_1723 |
| CFPAC1 cells | Pancreas | Homo sapiens (Human) | CVCL_1119 | |
| In Vivo Model | BALB/c nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | BCL-2 facilitates cell survival against chemotherapy via the blockage of Bax/Bak-induced apoptosis, miRNA-181b sensitizes PDAC cells to gemcitabine by targeting BCL-2. | |||
| Disease Class: Malignant pleural mesothelioma [ICD-11: 2C26.0] | [53] | |||
| Sensitive Disease | Malignant pleural mesothelioma [ICD-11: 2C26.0] | |||
| Sensitive Drug | Gemcitabine | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | MET-5A cells | Lung | Homo sapiens (Human) | CVCL_3749 |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay; Colony formation assay | |||
| Mechanism Description | Growth inhibition caused by miR-16 correlated with downregulation of target genes including Bcl-2 and CCND1, and miR-16 re-expression sensitised MPM cells to pemetrexed and gemcitabine. | |||
| Disease Class: Gastric cancer [ICD-11: 2B72.1] | [33] | |||
| Sensitive Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Sensitive Drug | Gemcitabine | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | p53 signaling pathway | Inhibition | hsa04115 | |
| In Vitro Model | AGS cells | Gastric | Homo sapiens (Human) | CVCL_0139 |
| NCI-N87 cells | Gastric | Homo sapiens (Human) | CVCL_1603 | |
| MkN-45 cells | Gastric | Homo sapiens (Human) | CVCL_0434 | |
| KATO-3 cells | Gastric | Homo sapiens (Human) | CVCL_0371 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | Human gastric cancer kato III cells with miR-34 restoration reduced the expression of target genes Bcl-2, Notch, and HMGA2. MicroRNA miR-34 was recently found to be a direct target of p53, functioning downstream of the p53 pathway as a tumor suppressor, miR-34 impaired cell growth, accumulated the cells in G1 phase, increased caspase-3 activation, and, more significantly, inhibited tumorsphere formation and growth. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Pancreatic cancer [ICD-11: 2C10.3] | [52] | |||
| Resistant Disease | Pancreatic cancer [ICD-11: 2C10.3] | |||
| Resistant Drug | Gemcitabine | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell proliferation | Activation | hsa05200 | ||
| In Vitro Model | MIA PaCa-2 cells | Pancreas | Homo sapiens (Human) | CVCL_0428 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | Upregulation of Bcl-2 expression was detected in cells transfected with miR-21 mimics, accompanied by downregulated Bax expression, less apoptosis, lower caspase-3 activity, decreased chemosensitivity to gemcitabine and increased proliferation. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Breast cancer [ICD-11: 2C60.3] | [6] | |||
| Resistant Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Resistant Drug | Paclitaxel | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Breast cancer [ICD-11: 2C60] | |||
| The Specified Disease | Breast cancer | |||
| The Studied Tissue | Breast tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.08E-24 Fold-change: -5.64E-02 Z-score: -1.09E+01 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| In Vitro Model | MCF-7 cells | Breast | Homo sapiens (Human) | CVCL_0031 |
| SkBR3 cells | Breast | Homo sapiens (Human) | CVCL_0033 | |
| MDA-MB-231 cells | Breast | Homo sapiens (Human) | CVCL_0062 | |
| BT474 cells | Breast | Homo sapiens (Human) | CVCL_0179 | |
| MDA-MB-436 cells | Breast | Homo sapiens (Human) | CVCL_0623 | |
| MDA-MB-435 cells | Breast | Homo sapiens (Human) | CVCL_0417 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Celltiter 96 aqueous one solution cell proliferation assay | |||
| Mechanism Description | miR-125b was up-regulated in Taxol-resistant cells, causing a marked inhibition of Taxol-induced cytotoxicity and apoptosis and a subsequent increase in the resistance to Taxol in cancer cells. The pro-apoptotic Bcl-2 antagonist killer 1 (Bak1) is a direct target of miR-125b. Down-regulation of Bak1 suppressed Taxol-induced apoptosis and led to an increased resistance to Taxol. | |||
| Disease Class: Lung adenocarcinoma [ICD-11: 2C25.0] | [58] | |||
| Resistant Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | |||
| Resistant Drug | Paclitaxel | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Mitochondrial signaling pathway | Activation | hsa04217 | |
| In Vitro Model | A549 cells | Lung | Homo sapiens (Human) | CVCL_0023 |
| A549/Taxol cells | Lung | Homo sapiens (Human) | CVCL_W218 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay; Flow cytometry assay; Transwell Invasion assay | |||
| Mechanism Description | ANRIL, also known as CDkN2B antisense RNA1, was origi.lly identified in the familial melanoma patients, it is located within the CDkN2B-CDkN2A gene cluster at chromosome 9p21. ANRIL decreases Bcl-2 expression and increases PARP expression. | |||
| Disease Class: Lung adenocarcinoma [ICD-11: 2C25.0] | [58] | |||
| Resistant Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | |||
| Resistant Drug | Paclitaxel | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | A549 cells | Lung | Homo sapiens (Human) | CVCL_0023 |
| A549/Taxol cells | Lung | Homo sapiens (Human) | CVCL_W218 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay; Flow cytometry assay; Transwell Invasion assay | |||
| Mechanism Description | ANRIL functioning as a potential oncogene was up-regulated in LAD, and promoted the acquisition of chemo-resistance in paclitaxel partly through the mitochondrial pathway by modulating the expression of apoptosis-related protein cleaved-PARP and Bcl-2. ANRIL decreases Bcl-2 expression and increases PARP expression. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Non-small cell lung cancer [ICD-11: 2C25.Y] | [59] | |||
| Sensitive Disease | Non-small cell lung cancer [ICD-11: 2C25.Y] | |||
| Sensitive Drug | Paclitaxel | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| In Vitro Model | A549 cells | Lung | Homo sapiens (Human) | CVCL_0023 |
| H460 cells | Lung | Homo sapiens (Human) | CVCL_0459 | |
| A549/PR cells | Lung | Homo sapiens (Human) | CVCL_0023 | |
| H460/PR cells | Lung | Homo sapiens (Human) | CVCL_0459 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Luciferase reporter assay; Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay; Flow cytometric analysis | |||
| Mechanism Description | miR30a-5p increases paclitaxel sensitivity by promoting chemotherapy-induced apoptosis via downregulating BCL-2. | |||
| Disease Class: Breast cancer [ICD-11: 2C60.3] | [60] | |||
| Sensitive Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Sensitive Drug | Paclitaxel | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| In Vitro Model | MCF-7 cells | Breast | Homo sapiens (Human) | CVCL_0031 |
| SkBR3 cells | Breast | Homo sapiens (Human) | CVCL_0033 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay; Flow cytometry assay | |||
| Mechanism Description | miR-21 inhibitors induced sensitivity of MCF-7/PR and SkBR-3/PR cells to paclitaxel. And miR-21 mimic can increase the expression of MDR1, Bcl-2/Bax and change cell morphology from parental cells to resistant cells. | |||
| Disease Class: Lung cancer [ICD-11: 2C25.5] | [61] | |||
| Sensitive Disease | Lung cancer [ICD-11: 2C25.5] | |||
| Sensitive Drug | Paclitaxel | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| PI3K/AKT/mTOR signaling pathway | Regulation | N.A. | ||
| In Vitro Model | A549 cells | Lung | Homo sapiens (Human) | CVCL_0023 |
| H1299 cells | Lung | Homo sapiens (Human) | CVCL_0060 | |
| NCl-H596 cells | Lung | Homo sapiens (Human) | CVCL_1571 | |
| NCI-H1734 cells | Lung | Homo sapiens (Human) | CVCL_1491 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miR-16 was also significantly downregulated in paclitaxel resistant lung cancer cells. anti-apoptotic protein Bcl-2 was directly targeted miR-16 in paclitaxel resistant lung cancer cells. the combined overexpression of miR-16 and miR-17 and subsequent paclitaxel treatment greatly sensitized paclitaxel resistant lung cancer cells to paclitaxel by inducing apoptosis via caspase-3 mediated pathway. Combined overexpression of miR-16 and miR-17 greatly reduced Beclin-1 and Bcl-2 expressions respectively. though miR-17 and miR-16 had no common target, both miR-16 and miR-17 jointly played roles in the development of paclitaxel resistance in lung cancer. miR-17 overexpression reduced cytoprotective autophagy by targeting Beclin-1, whereas overexpression of miR-16 potentiated paclitaxel induced apoptotic cell death by inhibiting anti-apoptotic protein Bcl-2. | |||
| Disease Class: Prostate cancer [ICD-11: 2C82.0] | [62] | |||
| Sensitive Disease | Prostate cancer [ICD-11: 2C82.0] | |||
| Sensitive Drug | Paclitaxel | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell viability | Inhibition | hsa05200 | |
| In Vitro Model | PC3 cells | Prostate | Homo sapiens (Human) | CVCL_0035 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Trypan blue dye exclusion assay | |||
| Mechanism Description | SIRT1 plays crucial roles in various cellular processes including cell survival under genotoxic and oxidative stresses. Bcl2I is an anti-apoptotic factor. In PC3PR cells, reduced expression of miR-34a confers paclitaxel resistance via up-regulating SIRT1 and Bcl2 expression. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Neuroblastoma [ICD-11: 2A00.11] | [7] | |||
| Sensitive Disease | Neuroblastoma [ICD-11: 2A00.11] | |||
| Sensitive Drug | Cisplatin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Brain cancer [ICD-11: 2A00] | |||
| The Specified Disease | Brain cancer | |||
| The Studied Tissue | Nervous tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.33E-53 Fold-change: -6.17E-02 Z-score: -1.67E+01 |
|||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| PI3K/AKT signaling pathway | Inhibition | hsa04151 | ||
| In Vitro Model | Kelly cells | Adrenal | Homo sapiens (Human) | CVCL_2092 |
| Sk-N-AS cells | Adrenal | Homo sapiens (Human) | CVCL_1700 | |
| SH-SY5Y cells | Abdomen | Homo sapiens (Human) | CVCL_0019 | |
| In Vivo Model | Orthotopic xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTS assay | |||
| Mechanism Description | miR-204 direct targeting of the 3' UTR of BCL2 and NTRk2 (TrkB). BCL2 has a critical role in ensuring the survival of early developing cell types, NTRk2 is also a well-established pro-survival oncogene in neuroblastoma, signalling the activation of the PI3k/AkT pathway, a significant mechanism of drug resistance in neuroblastoma. Ectopic miR-204 expression significantly increased sensitivity to cisplatin and etoposide in vitro. | |||
| Disease Class: Ovarian cancer [ICD-11: 2C73.0] | [9] | |||
| Sensitive Disease | Ovarian cancer [ICD-11: 2C73.0] | |||
| Sensitive Drug | Cisplatin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Ovarian cancer [ICD-11: 2C73] | |||
| The Specified Disease | Ovarian cancer | |||
| The Studied Tissue | Ovarian tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 2.42E-03 Fold-change: -9.65E-02 Z-score: -4.31E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| In Vitro Model | SkOV3 cells | Ovary | Homo sapiens (Human) | CVCL_0532 |
| HEK293T cells | Kidney | Homo sapiens (Human) | CVCL_0063 | |
| OVCAR3 cells | Ovary | Homo sapiens (Human) | CVCL_0465 | |
| Experiment for Molecule Alteration |
Western blot analysis; Dual luciferase reporter assay | |||
| Experiment for Drug Resistance |
MTT and DAPI assays | |||
| Mechanism Description | miR509-3p could sensitize ovarian cancer cells to cisplatin treatment by targeting multiple anti-apoptosis genes including BCL2 and promoteing apoptosis in cancer cells. | |||
| Disease Class: Hepatocellular carcinoma [ICD-11: 2C12.2] | [21] | |||
| Sensitive Disease | Hepatocellular carcinoma [ICD-11: 2C12.2] | |||
| Sensitive Drug | Cisplatin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | BEL-7402 cells | Liver | Homo sapiens (Human) | CVCL_5492 |
| HL-7702 cells | Liver | Homo sapiens (Human) | CVCL_6926 | |
| Experiment for Molecule Alteration |
Western blot analysis; Dual luciferase activity assay; qRT-PCR | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miR503 may enhance the sensitivity of BEL-7402 cells to cisplatin and inhibit the cell proliferation by targeting bcl-2. miR503 could interact with bcl-2 and inhibit its expression. | |||
| Disease Class: Lung adenocarcinoma [ICD-11: 2C25.0] | [22] | |||
| Sensitive Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | |||
| Sensitive Drug | Cisplatin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| In Vitro Model | SPC-A1 cells | Lung | Homo sapiens (Human) | CVCL_6955 |
| Experiment for Molecule Alteration |
qRT-PCR; Western blot analysis; Luciferase reporter assay | |||
| Experiment for Drug Resistance |
MTT assay; Flow cytometric analysis | |||
| Mechanism Description | Upregulation of miR7 increases the sensitivity of LA cells to CDDP via induction of apoptosis by targeting Bcl-2. | |||
| Disease Class: Nasopharyngeal carcinoma [ICD-11: 2B6B.0] | [23] | |||
| Sensitive Disease | Nasopharyngeal carcinoma [ICD-11: 2B6B.0] | |||
| Sensitive Drug | Cisplatin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | CNE2 cells | Nasopharynx | Homo sapiens (Human) | CVCL_6889 |
| CNE2/DDP cells | Nasopharynx | Homo sapiens (Human) | CVCL_6889 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTS assay; Flow cytometry assay-directed annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) assay | |||
| Mechanism Description | microRNA-125b reverses the multidrug resistance of nasopharyngeal carcinoma cells via targeting of Bcl-2. | |||
| Disease Class: Gallbladder cancer [ICD-11: 2C13.0] | [24] | |||
| Sensitive Disease | Gallbladder cancer [ICD-11: 2C13.0] | |||
| Sensitive Drug | Cisplatin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | miR125b-5p/BCL2 signaling pathway | Regulation | N.A. | |
| In Vitro Model | GBC-SD cells | Gallbladder | Homo sapiens (Human) | CVCL_6903 |
| NOZ cells | Gallbladder | Homo sapiens (Human) | CVCL_3079 | |
| HEK293 FT cells | Kidney | Homo sapiens (Human) | CVCL_6911 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTS assay; Annexin V/PI Apoptosis Detection assay | |||
| Mechanism Description | miR125b-5p enhances chemotherapy sensitivity to cisplatin by down-regulating Bcl2 in gallbladder cancer. | |||
| Disease Class: Osteosarcoma [ICD-11: 2B51.0] | [25], [26] | |||
| Sensitive Disease | Osteosarcoma [ICD-11: 2B51.0] | |||
| Sensitive Drug | Cisplatin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell proliferation | Inhibition | hsa05200 | ||
| p53/p38/MAPK signaling pathway | Regulation | N.A. | ||
| In Vitro Model | MG63 cells | Bone marrow | Homo sapiens (Human) | CVCL_0426 |
| SAOS-2 cells | Bone marrow | Homo sapiens (Human) | CVCL_0548 | |
| U2OS cells | Bone | Homo sapiens (Human) | CVCL_0042 | |
| HOS cells | Bone | Homo sapiens (Human) | CVCL_0312 | |
| 3AB-OS CSC cells | Bone marrow | Homo sapiens (Human) | CVCL_LM95 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
RT-PCR; Western blot analysis | |||
| Experiment for Drug Resistance |
Trypan blue assay; Flow cytometry assay; CCK8 assay | |||
| Mechanism Description | miR-29b-1 overexpression sensitized 3AB-OS cells to chemotherapeutic drug-induced apoptosis miR-29b-1 negatively regulated the expression of Bcl-2. Overexpression of miR-125b inhibited proliferation, migration, and invasion of OS cells and reduced the chemotherapy resistance of OS cells to cisplatin by targeting Bcl-2. | |||
| Disease Class: Breast cancer [ICD-11: 2C60.3] | [27] | |||
| Sensitive Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Sensitive Drug | Cisplatin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell invasion | Inhibition | hsa05200 | ||
| Cell migration | Inhibition | hsa04670 | ||
| In Vitro Model | Hs-578T cells | Breast | Homo sapiens (Human) | CVCL_0332 |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Acid phosphatase assay | |||
| Mechanism Description | When delivered directly by transfection the STAT5B and Hsp90 expression levels were reduced, but response to anti-Hsp90 drugs was not augmented. However, cellular growth was reduced and cisplatin-induced apoptosis was (+). Delivery via miR-134-enriched EVs also reduced STAT5B and Hsp90 expression, had no apparent effects on proliferation, but cellular migration and invasion were reduced and sensitivity to anti-Hsp90 drugs was (+). | |||
| Disease Class: Glioma [ICD-11: 2A00.1] | [28] | |||
| Sensitive Disease | Glioma [ICD-11: 2A00.1] | |||
| Sensitive Drug | Cisplatin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell invasion | Inhibition | hsa05200 | ||
| Cell migration | Inhibition | hsa04670 | ||
| Cell proliferation | Inhibition | hsa05200 | ||
| In Vitro Model | U251 cells | Brain | Homo sapiens (Human) | CVCL_0021 |
| U87 cells | Brain | Homo sapiens (Human) | CVCL_0022 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Bcl-2 was a direct target of miR 873, and miR 873 decreased the level of the Bcl-2 protein in cisplatin-resistant glioma cells. Notably, re-expression of Bcl-2 attenuated the function of miR 873 in cisplatin-resistant glioma cells and the sensitivity of the cells to cisplatin. | |||
| Disease Class: Non-small cell lung cancer [ICD-11: 2C25.Y] | [29] | |||
| Sensitive Disease | Non-small cell lung cancer [ICD-11: 2C25.Y] | |||
| Sensitive Drug | Cisplatin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| In Vitro Model | A549 cells | Lung | Homo sapiens (Human) | CVCL_0023 |
| Sk-MES-1 cells | Lung | Homo sapiens (Human) | CVCL_0630 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | Down-regulation of miR-21 inhibited growth, colony formation, antiapoptotic Bcl-2 expression and promoted proapoptotic Bax and caspase-9 expression in A549 cells treated with DDP. Upregulation of miR-21 promoted growth and colony formation in Sk-MES-1 cells treated with DDP. Furthermore, downregulation of miR-21 reduced growth of implanted tumors, suggesting that miR-21 inhibition could enhance the sensitivity of A549 cells to DDP in vivo. These data suggest an appropriate combination of DDP and miR-21 regulation might be a potential approach to lung cancer therapy. Combined DDP application with miR-21 downregulation for the treatment of lung cancer would help achieve effective treatment and reduce DDP side effects. | |||
| Disease Class: Gastric cancer [ICD-11: 2B72.1] | [30] | |||
| Sensitive Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Sensitive Drug | Cisplatin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell proliferation | Inhibition | hsa05200 | ||
| IGF1R/IRS1 signaling pathway | Regulation | N.A. | ||
| In Vitro Model | SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay; Transwell assay | |||
| Mechanism Description | Enforced miR-1271 expression repressed the protein levels of its targets, inhibited proliferation of SGC7901/DDP cells, and sensitized SGC7901/DDP cells to DDP-induced apoptosis. Overall, on the basis of the results of our study, we proposed that miR-1271 could regulate cisplatin resistance in human gastric cancer cells, at least partially, via targeting the IGF1R/IRS1 pathway. | |||
| Disease Class: HPV-related endocervical adenocarcinoma [ICD-11: 2E67.1] | [2], [31] | |||
| Sensitive Disease | HPV-related endocervical adenocarcinoma [ICD-11: 2E67.1] | |||
| Sensitive Drug | Cisplatin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| In Vitro Model | SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 |
| SGC7901/VCR cells | Gastric | Homo sapiens (Human) | CVCL_VU58 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | The antiapoptotic protein BCL2 is upregulated, whereas miR-181b is downregulated in both SGC7901/VCR and A549/CDDP cells, compared with SGC7901 and A549 cells, respectively. Enforced miR-181b expression reduced BCL2 protein level and sensitized SGC7901/VCR and A549/CDDP cells to VCR-induced and CDDP-induced apoptosis, respectively. And the antiapoptotic protein BCL2 is upregulated, whereas miR-181b is downregulated in both SGC7901/VCR and A549/CDDP cells, compared with SGC7901 and A549 cells, respectively. Enforced miR-181b expression reduced BCL2 protein level and sensitized SGC7901/VCR and A549/CDDP cells to VCR-induced and CDDP-induced apoptosis, respectively. | |||
| Disease Class: Lung cancer [ICD-11: 2C25.5] | [2], [31] | |||
| Sensitive Disease | Lung cancer [ICD-11: 2C25.5] | |||
| Sensitive Drug | Cisplatin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| In Vitro Model | A549 cells | Lung | Homo sapiens (Human) | CVCL_0023 |
| A549/CDDP cells | Lung | Homo sapiens (Human) | CVCL_0023 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | The antiapoptotic protein BCL2 is upregulated, whereas miR-181b is downregulated in both SGC7901/VCR and A549/CDDP cells, compared with SGC7901 and A549 cells, respectively. Enforced miR-181b expression reduced BCL2 protein level and sensitized SGC7901/VCR and A549/CDDP cells to VCR-induced and CDDP-induced apoptosis, respectively. And the antiapoptotic protein BCL2 is upregulated, whereas miR-181b is downregulated in both SGC7901/VCR and A549/CDDP cells, compared with SGC7901 and A549 cells, respectively. Enforced miR-181b expression reduced BCL2 protein level and sensitized SGC7901/VCR and A549/CDDP cells to VCR-induced and CDDP-induced apoptosis, respectively. | |||
| Disease Class: Nasopharyngeal carcinoma [ICD-11: 2B6B.0] | [32] | |||
| Sensitive Disease | Nasopharyngeal carcinoma [ICD-11: 2B6B.0] | |||
| Sensitive Drug | Cisplatin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| In Vitro Model | 5-8F cells | Nasopharynx | Homo sapiens (Human) | CVCL_C528 |
| CNE2 cells | Nasopharynx | Homo sapiens (Human) | CVCL_6889 | |
| C666-1 cells | Throat | Homo sapiens (Human) | CVCL_7949 | |
| CNE1 cells | Throat | Homo sapiens (Human) | CVCL_6888 | |
| HONE1 cells | Throat | Homo sapiens (Human) | CVCL_8706 | |
| 6-10B cells | Nasopharynx | Homo sapiens (Human) | CVCL_C529 | |
| SUNE-1 cells | Nasopharynx | Homo sapiens (Human) | CVCL_6946 | |
| HNE-2 cells | Nasopharynx | Homo sapiens (Human) | CVCL_FA07 | |
| In Vivo Model | SCID-Beige nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miR-29c repressed expression of anti-apoptotic factors, Mcl-1 and Bcl-2 in NPC tissues and cell lines, cause the resstance to Cisplatin. | |||
| Disease Class: Gastric adenocarcinoma [ICD-11: 2B72.0] | [2] | |||
| Sensitive Disease | Gastric adenocarcinoma [ICD-11: 2B72.0] | |||
| Sensitive Drug | Cisplatin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| In Vitro Model | SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 |
| SGC7901/VCR cells | Gastric | Homo sapiens (Human) | CVCL_VU58 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | The antiapoptotic protein BCL2 is upregulated, whereas miR-181b is downregulated in both SGC7901/VCR and A549/CDDP cells, compared with SGC7901 and A549 cells, respectively. Enforced miR-181b expression reduced BCL2 protein level and sensitized SGC7901/VCR and A549/CDDP cells to VCR-induced and CDDP-induced apoptosis, respectively. | |||
| Disease Class: Gastric cancer [ICD-11: 2B72.1] | [33] | |||
| Sensitive Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Sensitive Drug | Cisplatin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | p53 signaling pathway | Inhibition | hsa04115 | |
| In Vitro Model | AGS cells | Gastric | Homo sapiens (Human) | CVCL_0139 |
| NCI-N87 cells | Gastric | Homo sapiens (Human) | CVCL_1603 | |
| MkN-45 cells | Gastric | Homo sapiens (Human) | CVCL_0434 | |
| KATO-3 cells | Gastric | Homo sapiens (Human) | CVCL_0371 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | Human gastric cancer kato III cells with miR-34 restoration reduced the expression of target genes Bcl-2, Notch, and HMGA2. MicroRNA miR-34 was recently found to be a direct target of p53, functioning downstream of the p53 pathway as a tumor suppressor, miR-34 impaired cell growth, accumulated the cells in G1 phase, increased caspase-3 activation, and, more significantly, inhibited tumorsphere formation and growth. | |||
| Disease Class: Gastric cancer [ICD-11: 2B72.1] | [8] | |||
| Sensitive Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Sensitive Drug | Cisplatin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Mitochondrial signaling pathway | Activation | hsa04217 | ||
| In Vitro Model | SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 |
| SGC7901/VCR cells | Gastric | Homo sapiens (Human) | CVCL_VU58 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miR-15b and miR-16, among the downregulated miRNAs in SGC7901/VCR cells, were demonstrated to play a role in the development of MDR in gastric cancer cells by targeting the antiapoptotic gene BCL2. | |||
|
|
||||
| Disease Class: Lung small cell carcinoma [ICD-11: 2C25.2] | [20] | |||
| Sensitive Disease | Lung small cell carcinoma [ICD-11: 2C25.2] | |||
| Sensitive Drug | Cisplatin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell proliferation | Inhibition | hsa05200 | ||
| In Vitro Model | 16HBE cells | Lung | Homo sapiens (Human) | CVCL_0112 |
| H446 cells | Lung | Homo sapiens (Human) | CVCL_1562 | |
| H69 cells | Lung | Homo sapiens (Human) | CVCL_8121 | |
| H69AR cells | Lung | Homo sapiens (Human) | CVCL_3513 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Luciferase reporter assay; RT-qPCR | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | Long non-coding RNA HOTTIP promotes BCL-2 expression and induces chemoresistance in small cell lung cancer by sponging miR216a. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Gastric cancer [ICD-11: 2B72.1] | [14] | |||
| Resistant Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Resistant Drug | Cisplatin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| In Vitro Model | SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 |
| BGC823 cells | Gastric | Homo sapiens (Human) | CVCL_3360 | |
| Experiment for Molecule Alteration |
Western blot analysis; RT-qPCR | |||
| Experiment for Drug Resistance |
MTT assay; Flow cytometry assay | |||
| Mechanism Description | LncRNA SNHG5 promotes cisplatin resistance in gastric cancer via inhibiting cell apoptosis and upregulating drug resistance-related genes. | |||
| Disease Class: Cervical cancer [ICD-11: 2C77.0] | [15] | |||
| Resistant Disease | Cervical cancer [ICD-11: 2C77.0] | |||
| Resistant Drug | Cisplatin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| In Vitro Model | Hela cells | Cervix uteri | Homo sapiens (Human) | CVCL_0030 |
| Siha cells | Cervix uteri | Homo sapiens (Human) | CVCL_0032 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Flow cytometry assay | |||
| Mechanism Description | There was a protective role of miR-7-5p in cervical cancer cells treated with cisplatin and that miR-7-5p expression.miR-7-5p reduced energy consumption via inhibiting PARP-1 expression, and miR-7-5p increased energy generation by suppressing the expression of Bcl-2. | |||
| Disease Class: Osteosarcoma [ICD-11: 2B51.0] | [16] | |||
| Resistant Disease | Osteosarcoma [ICD-11: 2B51.0] | |||
| Resistant Drug | Cisplatin | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | BCL2/cyclin D1 signaling pathway | Inhibition | hsa04210 | |
| Cell apoptosis | Inhibition | hsa04210 | ||
| Cell viability | Activation | hsa05200 | ||
| In Vitro Model | MG63 cells | Bone marrow | Homo sapiens (Human) | CVCL_0426 |
| SAOS-2 cells | Bone marrow | Homo sapiens (Human) | CVCL_0548 | |
| HOS cells | Bone | Homo sapiens (Human) | CVCL_0312 | |
| 143B cells | Bone | Homo sapiens (Human) | CVCL_2270 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay; Flow cytometry assay | |||
| Mechanism Description | The miR-34c inhibitor restored the BCL-2 and cyclin D1 levels in MG63 and HOS cell line, which implicated that NEAT1 inhibited the tumor suppressor miR-34c and up-regulated cell survival signals for the development of OS. | |||
| Disease Class: Non-small cell lung cancer [ICD-11: 2C25.Y] | [17] | |||
| Resistant Disease | Non-small cell lung cancer [ICD-11: 2C25.Y] | |||
| Resistant Drug | Cisplatin | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell viability | Activation | hsa05200 | ||
| In Vitro Model | A549 cells | Lung | Homo sapiens (Human) | CVCL_0023 |
| H1299 cells | Lung | Homo sapiens (Human) | CVCL_0060 | |
| NCI-H358 cells | Lung | Homo sapiens (Human) | CVCL_1559 | |
| CL1-0 cells | Lung | Homo sapiens (Human) | CVCL_3871 | |
| H23 cells | Lung | Homo sapiens (Human) | CVCL_1547 | |
| TL4 cells | Lung | Homo sapiens (Human) | N.A. | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay; Flow cytometry assay | |||
| Mechanism Description | Patients with tumors expressing low miR-630, high Bcl-2, and a combination of both were more likely than their counterparts to show unfavorable responses to cisplatin-based chemotherapy. | |||
| Disease Class: Non-small cell lung cancer [ICD-11: 2C25.Y] | [18] | |||
| Resistant Disease | Non-small cell lung cancer [ICD-11: 2C25.Y] | |||
| Resistant Drug | Cisplatin | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | A549 cells | Lung | Homo sapiens (Human) | CVCL_0023 |
| A549/CDDP cells | Lung | Homo sapiens (Human) | CVCL_0023 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miR-21 decreased the expression of PTEN and increased Bcl-2 in A549. Upregulation of miR-21 induces cholangiocarcinoma cell survival and gemcitabine resistance primarily through targeting the PTEN dependent PI3k/Akt pathway. Inhibition of miR-21 was shown to increase the sensitivity to topotecan in breast cancer cells partly by regulating BCL2 induced anti-apoptosis indirectly in MCF-7 cells. | |||
| Disease Class: Gastric adenocarcinoma [ICD-11: 2B72.0] | [19] | |||
| Resistant Disease | Gastric adenocarcinoma [ICD-11: 2B72.0] | |||
| Resistant Drug | Cisplatin | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Fas/FasL signaling pathway | Regulation | N.A. | ||
| In Vitro Model | SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 |
| SGC7901/VCR cells | Gastric | Homo sapiens (Human) | CVCL_VU58 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | The anti-apoptotic protein BCL2 and XIAP were upregulated, while the miR-200bc/429 cluster was downregulated in both SGC7901/VCR and A549/CDDP cells. miR-200bc/429 cluster might play an important role in the development of MDR in human gastric and lung cancer cell lines by targeting the anti-apoptotic genes BCL2 and XIAP. | |||
| Disease Class: Lung cancer [ICD-11: 2C25.5] | [19] | |||
| Resistant Disease | Lung cancer [ICD-11: 2C25.5] | |||
| Resistant Drug | Cisplatin | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Fas/FasL signaling pathway | Regulation | N.A. | ||
| In Vitro Model | A549 cells | Lung | Homo sapiens (Human) | CVCL_0023 |
| A549/CDDP cells | Lung | Homo sapiens (Human) | CVCL_0023 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | The anti-apoptotic protein BCL2 and XIAP were upregulated, while the miR-200bc/429 cluster was downregulated in both SGC7901/VCR and A549/CDDP cells. miR-200bc/429 cluster might play an important role in the development of MDR in human gastric and lung cancer cell lines by targeting the anti-apoptotic genes BCL2 and XIAP. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Gastric cancer [ICD-11: 2B72.1] | [8] | |||
| Sensitive Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Sensitive Drug | Doxorubicin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Gastric cancer [ICD-11: 2B72] | |||
| The Specified Disease | Gastric cancer | |||
| The Studied Tissue | Gastric tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 5.02E-02 Fold-change: -6.34E-02 Z-score: -4.00E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Mitochondrial signaling pathway | Activation | hsa04217 | ||
| In Vitro Model | SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 |
| SGC7901/VCR cells | Gastric | Homo sapiens (Human) | CVCL_VU58 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miR-15b and miR-16, among the downregulated miRNAs in SGC7901/VCR cells, were demonstrated to play a role in the development of MDR in gastric cancer cells by targeting the antiapoptotic gene BCL2. | |||
| Disease Class: Gastric cancer [ICD-11: 2B72.1] | [39] | |||
| Sensitive Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Sensitive Drug | Doxorubicin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| In Vitro Model | SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Higher miR-218 levels increased the level of Bax and reduced the level of Bcl-2 and miR-218 inhibits multidrug resistance (MDR) of gastric cancer cells by targeting Hedgehog/smoothened. | |||
| Disease Class: Glioma [ICD-11: 2A00.1] | [40] | |||
| Sensitive Disease | Glioma [ICD-11: 2A00.1] | |||
| Sensitive Drug | Doxorubicin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | AKT signaling pathway | Inhibition | hsa04151 | |
| Cell apoptosis | Activation | hsa04210 | ||
| Cell proliferation | Inhibition | hsa05200 | ||
| In Vitro Model | U251 cells | Brain | Homo sapiens (Human) | CVCL_0021 |
| U87-MG cells | Brain | Homo sapiens (Human) | CVCL_0022 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTS assay; Flow cytometry assay | |||
| Mechanism Description | microRNA-127 silencing significantly affects cell growth and increases the sensitivity to adriamycin. microRNA-127 silencing arrests the cell cycle, potentiates adriamycin-induced apoptosis, and increases cellular Rh-123 uptake. microRNA-127 silencing down-regulates MDR1, MRP1, Runx2, Bcl-2, Survivin and ErbB4 expression while up-regulates p53 expression. microRNA-127 silencing inhibits AkT phosphorylation. | |||
| Disease Class: Breast cancer [ICD-11: 2C60.3] | [41] | |||
| Sensitive Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Sensitive Drug | Doxorubicin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| c-Jun signaling pathway | Inhibition | hsa04210 | ||
| In Vitro Model | MDA-MB-468 cells | Breast | Homo sapiens (Human) | CVCL_0419 |
| Experiment for Molecule Alteration |
Immunoblotting techniques assay | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | HA/CD44 activates c-Jun signaling which, in turn, stimulates miR-21 expression and function. These events lead to the production of an anti-apoptosis protein, Bcl-2 and upregulation of survival proteins (IAPs) and Doxorubicin chemoresistance in MDA-MB-468 cells. cells. Inhibition of c-Jun signaling or silencing miR-21 expression/function not only results in Bcl-2 downregulation, but also causes a reduction of survival protein expression and enhances chemosensitivity to Doxorubicin. | |||
| Disease Class: Gastric adenocarcinoma [ICD-11: 2B72.0] | [2] | |||
| Sensitive Disease | Gastric adenocarcinoma [ICD-11: 2B72.0] | |||
| Sensitive Drug | Doxorubicin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| In Vitro Model | SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 |
| SGC7901/VCR cells | Gastric | Homo sapiens (Human) | CVCL_VU58 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | The antiapoptotic protein BCL2 is upregulated, whereas miR-181b is downregulated in both SGC7901/VCR and A549/CDDP cells, compared with SGC7901 and A549 cells, respectively. Enforced miR-181b expression reduced BCL2 protein level and sensitized SGC7901/VCR and A549/CDDP cells to VCR-induced and CDDP-induced apoptosis, respectively. | |||
| Disease Class: Lung cancer [ICD-11: 2C25.5] | [2] | |||
| Sensitive Disease | Lung cancer [ICD-11: 2C25.5] | |||
| Sensitive Drug | Doxorubicin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| In Vitro Model | A549 cells | Lung | Homo sapiens (Human) | CVCL_0023 |
| A549/CDDP cells | Lung | Homo sapiens (Human) | CVCL_0023 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | The antiapoptotic protein BCL2 is upregulated, whereas miR-181b is downregulated in both SGC7901/VCR and A549/CDDP cells, compared with SGC7901 and A549 cells, respectively. Enforced miR-181b expression reduced BCL2 protein level and sensitized SGC7901/VCR and A549/CDDP cells to VCR-induced and CDDP-induced apoptosis, respectively. | |||
| Disease Class: Gastric cancer [ICD-11: 2B72.1] | [33] | |||
| Sensitive Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Sensitive Drug | Doxorubicin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | p53 signaling pathway | Inhibition | hsa04115 | |
| In Vitro Model | AGS cells | Gastric | Homo sapiens (Human) | CVCL_0139 |
| NCI-N87 cells | Gastric | Homo sapiens (Human) | CVCL_1603 | |
| MkN-45 cells | Gastric | Homo sapiens (Human) | CVCL_0434 | |
| KATO-3 cells | Gastric | Homo sapiens (Human) | CVCL_0371 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | Human gastric cancer kato III cells with miR-34 restoration reduced the expression of target genes Bcl-2, Notch, and HMGA2. MicroRNA miR-34 was recently found to be a direct target of p53, functioning downstream of the p53 pathway as a tumor suppressor, miR-34 impaired cell growth, accumulated the cells in G1 phase, increased caspase-3 activation, and, more significantly, inhibited tumorsphere formation and growth. | |||
|
|
||||
| Disease Class: Lung small cell carcinoma [ICD-11: 2C25.2] | [20] | |||
| Sensitive Disease | Lung small cell carcinoma [ICD-11: 2C25.2] | |||
| Sensitive Drug | Doxorubicin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell proliferation | Inhibition | hsa05200 | ||
| In Vitro Model | 16HBE cells | Lung | Homo sapiens (Human) | CVCL_0112 |
| H446 cells | Lung | Homo sapiens (Human) | CVCL_1562 | |
| H69 cells | Lung | Homo sapiens (Human) | CVCL_8121 | |
| H69AR cells | Lung | Homo sapiens (Human) | CVCL_3513 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Luciferase reporter assay; RT-qPCR | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | Long non-coding RNA HOTTIP promotes BCL-2 expression and induces chemoresistance in small cell lung cancer by sponging miR216a. HOTTIP functioned as an oncogene in SCLC progression by binding miR216a and abrogating its tumor-suppressive function in this setting. HOTTIP also increased the expression of anti-apoptotic factor BCL-2, a target gene of miR216a, and jointly enhanced chemoresistance of SCLC by regulating BCL-2. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Osteosarcoma [ICD-11: 2B51.0] | [37] | |||
| Resistant Disease | Osteosarcoma [ICD-11: 2B51.0] | |||
| Resistant Drug | Doxorubicin | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell proliferation | Inhibition | hsa05200 | ||
| In Vitro Model | SAOS-2 cells | Bone marrow | Homo sapiens (Human) | CVCL_0548 |
| U2OS cells | Bone | Homo sapiens (Human) | CVCL_0042 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay; Matrigel colony formation assay; Hoechst33342 staining assay | |||
| Mechanism Description | In chemoresistant SAOS-2 and U2OS osteosarcomas cells, miR-143 levels were significantly downregulated and accompanied by increases in ATG2B, Bcl-2, and/or LC3-II protein levels, high rate of ALDH1+CD133+ cells, and an increase in Matrigel colony formation ability. H2O2 upregulated p53 and miR-143, but downregulated ATG2B, Bcl-2, and LC3-I expression in U2OS cells (wild-type p53) but not in SAOS-2 (p53-null) cells. Forced miR-143 expression significantly reversed chemoresistance as well as downregulation of ATG2B, LC3-I, and Bcl-2 expression in SAOS-2- and U2OS-resistant cells. Forced miR-143 expression significantly inhibited tumor growth in xenograft SAOS-2-Dox and U2OS-Dox animal models. Loss of miR-143 expression is associated with poor prognosis of patients with osteosarcoma underlying chemotherapy. The chemoresistance of osteosarcoma tumor cells to doxorubicin is associated with the downregulation of miR-143 expression, activation of ALDH1+CD133+ cells, activation of autophagy, and inhibition of cell death. | |||
| Disease Class: Breast cancer [ICD-11: 2C60.3] | [38] | |||
| Resistant Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Resistant Drug | Doxorubicin | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| In Vitro Model | MCF-7 cells | Breast | Homo sapiens (Human) | CVCL_0031 |
| MCF-7/ADR cells | Breast | Homo sapiens (Human) | CVCL_1452 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | miR-181a played an important role in chemosensitivity to adriamycin in MCF-7 and MCF-7/ADR cells via targeting Bcl-2. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Colorectal cancer [ICD-11: 2B91.1] | [11] | |||
| Sensitive Disease | Colorectal cancer [ICD-11: 2B91.1] | |||
| Sensitive Drug | Berberine | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell viability | Inhibition | hsa05200 | ||
| In Vitro Model | HT29 Cells | Colon | Homo sapiens (Human) | CVCL_A8EZ |
| HCT116 cells | Colon | Homo sapiens (Human) | CVCL_0291 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay; Flow cytometry assay | |||
| Mechanism Description | LncRNA CASC2 mediates the berberine-induced pro-apoptotic effect via inhibition of Bcl-2 expression at the post-transcriptional level. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Colon cancer [ICD-11: 2B90.1] | [13] | |||
| Sensitive Disease | Colon cancer [ICD-11: 2B90.1] | |||
| Sensitive Drug | Cetuximab | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell invasion | Inhibition | hsa05200 | ||
| Cell migration | Inhibition | hsa04670 | ||
| Cell proliferation | Inhibition | hsa05200 | ||
| EGFR/RAS/MAPK signaling pathway | Regulation | N.A. | ||
| In Vitro Model | SW480 cells | Colon | Homo sapiens (Human) | CVCL_0546 |
| HCT116 cells | Colon | Homo sapiens (Human) | CVCL_0291 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTS assay | |||
| Mechanism Description | The extent of caspase and nuclear fragmentation inhibition was higher in cells overexpressing miR-143 or miR-145, which also display reduced Bcl-2 protein steady-state levels. restoration of miR-143 or miR-145 reduces the aggressiveness of mutant kRAS HCT116 cells. In addition, forced expression of these miRNAs in both mutant and wild-type kRAS colon cancer cells increased their sensitivity to cetuximab by increasing cetuximab-mediated ADCC. Moreover, increased levels of effector cell-mediated caspase-dependent apoptosis were observed for mutant kRAS HCT116 miRNAs-overexpressing cells. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Leukemia [ICD-11: 2B33.6] | [34] | |||
| Sensitive Disease | Leukemia [ICD-11: 2B33.6] | |||
| Sensitive Drug | Cytarabine | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | HL60 cells | Peripheral blood | Homo sapiens (Human) | CVCL_0002 |
| HL-60/Ara-C-resistant cells | Blood | Homo sapiens (Human) | CVCL_1736 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Bcl-2 was conWrmed as adirect miR-181a target by immunoblot analysis andreporter gene assays. knockdown of Bcl-2 mimicked theeVect of enforced miR-181a expression by reducing cellviability. In addition, the apoptosis pathway was activated by cytochrome C release and caspase 9/caspase 3 activationafter miR-181a overexpression. Down-regulation of miR-181a and upregulation of Bcl-2in leukaemia cells confer resistance to Ara-C-based ther-apy. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Leukemia [ICD-11: 2B33.6] | [35] | |||
| Sensitive Disease | Leukemia [ICD-11: 2B33.6] | |||
| Sensitive Drug | Daunorubicin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| In Vitro Model | K562 cells | Blood | Homo sapiens (Human) | CVCL_0004 |
| K562/A02 cells | Blood | Homo sapiens (Human) | CVCL_0368 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Anti-apoptotic BCL-2 contributes to the survival and chemoresistance of quiescent leukemia CD34+ cells, leukemia cells with decreased miR-181a expression and elevated BCL-2 protein expression were more resistant to DNR than the control cells. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Lung small cell carcinoma [ICD-11: 2C25.2] | [20] | |||
| Sensitive Disease | Lung small cell carcinoma [ICD-11: 2C25.2] | |||
| Sensitive Drug | Etoposide | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell proliferation | Inhibition | hsa05200 | ||
| In Vitro Model | 16HBE cells | Lung | Homo sapiens (Human) | CVCL_0112 |
| H446 cells | Lung | Homo sapiens (Human) | CVCL_1562 | |
| H69 cells | Lung | Homo sapiens (Human) | CVCL_8121 | |
| H69AR cells | Lung | Homo sapiens (Human) | CVCL_3513 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Luciferase reporter assay; RT-qPCR | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | Long non-coding RNA HOTTIP promotes BCL-2 expression and induces chemoresistance in small cell lung cancer by sponging miR216a. | |||
|
|
||||
| Disease Class: Neuroblastoma [ICD-11: 2A00.11] | [7] | |||
| Sensitive Disease | Neuroblastoma [ICD-11: 2A00.11] | |||
| Sensitive Drug | Etoposide | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| PI3K/AKT signaling pathway | Inhibition | hsa04151 | ||
| In Vitro Model | Kelly cells | Adrenal | Homo sapiens (Human) | CVCL_2092 |
| Sk-N-AS cells | Adrenal | Homo sapiens (Human) | CVCL_1700 | |
| SH-SY5Y cells | Abdomen | Homo sapiens (Human) | CVCL_0019 | |
| In Vivo Model | Orthotopic xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTS assay | |||
| Mechanism Description | miR-204 direct targeting of the 3' UTR of BCL2 and NTRk2 (TrkB). BCL2 has a critical role in ensuring the survival of early developing cell types, NTRk2 is also a well-established pro-survival oncogene in neuroblastoma, signalling the activation of the PI3k/AkT pathway, a significant mechanism of drug resistance in neuroblastoma. Ectopic miR-204 expression significantly increased sensitivity to cisplatin and etoposide in vitro. | |||
| Disease Class: Gastric adenocarcinoma [ICD-11: 2B72.0] | [2] | |||
| Sensitive Disease | Gastric adenocarcinoma [ICD-11: 2B72.0] | |||
| Sensitive Drug | Etoposide | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| In Vitro Model | SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 |
| SGC7901/VCR cells | Gastric | Homo sapiens (Human) | CVCL_VU58 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | The antiapoptotic protein BCL2 is upregulated, whereas miR-181b is downregulated in both SGC7901/VCR and A549/CDDP cells, compared with SGC7901 and A549 cells, respectively. Enforced miR-181b expression reduced BCL2 protein level and sensitized SGC7901/VCR and A549/CDDP cells to VCR-induced and CDDP-induced apoptosis, respectively. | |||
| Disease Class: Lung cancer [ICD-11: 2C25.5] | [2] | |||
| Sensitive Disease | Lung cancer [ICD-11: 2C25.5] | |||
| Sensitive Drug | Etoposide | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| In Vitro Model | A549 cells | Lung | Homo sapiens (Human) | CVCL_0023 |
| A549/CDDP cells | Lung | Homo sapiens (Human) | CVCL_0023 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | The antiapoptotic protein BCL2 is upregulated, whereas miR-181b is downregulated in both SGC7901/VCR and A549/CDDP cells, compared with SGC7901 and A549 cells, respectively. Enforced miR-181b expression reduced BCL2 protein level and sensitized SGC7901/VCR and A549/CDDP cells to VCR-induced and CDDP-induced apoptosis, respectively. | |||
| Disease Class: Gastric cancer [ICD-11: 2B72.1] | [8] | |||
| Sensitive Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Sensitive Drug | Etoposide | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Mitochondrial signaling pathway | Activation | hsa04217 | ||
| In Vitro Model | SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 |
| SGC7901/VCR cells | Gastric | Homo sapiens (Human) | CVCL_VU58 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miR-15b and miR-16, among the downregulated miRNAs in SGC7901/VCR cells, were demonstrated to play a role in the development of MDR in gastric cancer cells by targeting the antiapoptotic gene BCL2. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Chronic lymphocytic leukemia [ICD-11: 2A82.0] | [42] | |||
| Sensitive Disease | Chronic lymphocytic leukemia [ICD-11: 2A82.0] | |||
| Sensitive Drug | Fludarabine | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| In Vitro Model | CLL B cells | Lymph | Homo sapiens (Human) | N.A. |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Flow cytometry assay | |||
| Mechanism Description | miR-181a and miR-181b directly inhibit the expression of BCL-2, MCL-1 and XIAP by binding to the target sequence, sensitizes CLL cells to fludarabine-induced apoptosis. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Gastric cancer [ICD-11: 2B72.1] | [43] | |||
| Resistant Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Resistant Drug | Fluorouracil | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| In Vitro Model | SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 |
| In Vivo Model | Nod/SCID mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis; RT-qPCR | |||
| Experiment for Drug Resistance |
CCK8 assay; Flow cytometry assay | |||
| Mechanism Description | LncRNA PVT1 can inhibit apoptosis and enhance the 5-Fu resistance via Increasing Bcl2 expression in Gastric Cancer. | |||
| Disease Class: Colorectal cancer [ICD-11: 2B91.1] | [44] | |||
| Resistant Disease | Colorectal cancer [ICD-11: 2B91.1] | |||
| Resistant Drug | Fluorouracil | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell viability | Activation | hsa05200 | ||
| In Vitro Model | HCT116 cells | Colon | Homo sapiens (Human) | CVCL_0291 |
| HCT-8 cells | Colon | Homo sapiens (Human) | CVCL_2478 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay; Flow cytometry assay | |||
| Mechanism Description | The overexpression of PVT1 increased the mRNA and protein expression levels of multidrug resistance associated protein 1, P glycoprotein, serine/threonine protein kinase mTOR and apoptosis regulator Bcl2. | |||
| Disease Class: Colon cancer [ICD-11: 2B90.1] | [45] | |||
| Resistant Disease | Colon cancer [ICD-11: 2B90.1] | |||
| Resistant Drug | Fluorouracil | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell proliferation | Activation | hsa05200 | ||
| In Vitro Model | HCT116 cells | Colon | Homo sapiens (Human) | CVCL_0291 |
| RkO cells | Colon | Homo sapiens (Human) | CVCL_0504 | |
| In Vivo Model | SCID mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTS assay; Flow cytometry assay | |||
| Mechanism Description | miR-206 downregulation modulates 5-FU resistance in HCT116 cells by upregulating Bcl-2. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Colorectal cancer [ICD-11: 2B91.1] | [46] | |||
| Sensitive Disease | Colorectal cancer [ICD-11: 2B91.1] | |||
| Sensitive Drug | Fluorouracil | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | BCL2 signaling pathway | Regulation | N.A. | |
| Cell apoptosis | Activation | hsa04210 | ||
| Cell invasion | Inhibition | hsa05200 | ||
| Cell migration | Inhibition | hsa04670 | ||
| In Vitro Model | SW480 cells | Colon | Homo sapiens (Human) | CVCL_0546 |
| SW620 cells | Colon | Homo sapiens (Human) | CVCL_0547 | |
| HCT116 cells | Colon | Homo sapiens (Human) | CVCL_0291 | |
| LOVO cells | Colon | Homo sapiens (Human) | CVCL_0399 | |
| RkO cells | Colon | Homo sapiens (Human) | CVCL_0504 | |
| HT-29 cells | Colon | Homo sapiens (Human) | CVCL_0320 | |
| LS174T cells | Colon | Homo sapiens (Human) | CVCL_1384 | |
| COLO205 cells | Colon | Homo sapiens (Human) | CVCL_F402 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay; Transwell assay | |||
| Mechanism Description | BCL2 is a direct target of miR-139-5p in colorectal cancer cells and showed that the tumour suppressor activity of miR-139-5p is mediated by the modulation of BCL2 expression. BCL2 family proteins regulate and contribute to programmed cell death or apoptosis. The cell apoptosis results showed the induction of apoptotic cells contributed greatly to 5-Fu and OXA drug sensitivity, which was consistent with the multidrug resistance mechanisms. miR-139-5p is downregulated in colorectal cancer cells and tissues, and its inhibitory effects on cell migration, invasion, and drug sensitivity are mediated by the downregulation of its target BCL2. | |||
| Disease Class: Gastric cancer [ICD-11: 2B72.1] | [39] | |||
| Sensitive Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Sensitive Drug | Fluorouracil | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| In Vitro Model | SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Higher miR-218 levels increased the level of Bax and reduced the level of Bcl-2 and miR-218 inhibits multidrug resistance (MDR) of gastric cancer cells by targeting Hedgehog/smoothened. | |||
| Disease Class: Colorectal cancer [ICD-11: 2B91.1] | [47] | |||
| Sensitive Disease | Colorectal cancer [ICD-11: 2B91.1] | |||
| Sensitive Drug | Fluorouracil | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell proliferation | Inhibition | hsa05200 | ||
| In Vitro Model | HCT116 cells | Colon | Homo sapiens (Human) | CVCL_0291 |
| In Vivo Model | CRC nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis; Immunofluorescence analysis | |||
| Experiment for Drug Resistance |
WST assay | |||
| Mechanism Description | microRNA-129 (miR-129) trigger apoptosis by suppressing key anti-apoptotic protein, B-cell lymphoma 2 (BCL2), enhanced the cytotoxic effect of 5-fluorouracil both in vitro and in vivo. | |||
| Disease Class: Gastric cancer [ICD-11: 2B72.1] | [48] | |||
| Sensitive Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Sensitive Drug | Fluorouracil | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| In Vitro Model | NCI-N87 cells | Gastric | Homo sapiens (Human) | CVCL_1603 |
| GTL-16 cells | Gastric | Homo sapiens (Human) | CVCL_7668 | |
| In Vivo Model | CD1 nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Immunohistochemistry assay | |||
| Experiment for Drug Resistance |
Flow cytometry assay | |||
| Mechanism Description | miR-204 targeted Bcl-2 messenger RNA and increased responsiveness of GC cells to 5-fluorouracil and oxaliplatin treatment. Ectopic expression of Bcl-2 protein counteracted miR-204 pro-apoptotic activity in response to 5-fluorouracil. | |||
| Disease Class: Gastric adenocarcinoma [ICD-11: 2B72.0] | [2] | |||
| Sensitive Disease | Gastric adenocarcinoma [ICD-11: 2B72.0] | |||
| Sensitive Drug | Fluorouracil | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| In Vitro Model | SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 |
| SGC7901/VCR cells | Gastric | Homo sapiens (Human) | CVCL_VU58 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | The antiapoptotic protein BCL2 is upregulated, whereas miR-181b is downregulated in both SGC7901/VCR and A549/CDDP cells, compared with SGC7901 and A549 cells, respectively. Enforced miR-181b expression reduced BCL2 protein level and sensitized SGC7901/VCR and A549/CDDP cells to VCR-induced and CDDP-induced apoptosis, respectively. | |||
| Disease Class: Lung cancer [ICD-11: 2C25.5] | [2] | |||
| Sensitive Disease | Lung cancer [ICD-11: 2C25.5] | |||
| Sensitive Drug | Fluorouracil | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| In Vitro Model | A549 cells | Lung | Homo sapiens (Human) | CVCL_0023 |
| A549/CDDP cells | Lung | Homo sapiens (Human) | CVCL_0023 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | The antiapoptotic protein BCL2 is upregulated, whereas miR-181b is downregulated in both SGC7901/VCR and A549/CDDP cells, compared with SGC7901 and A549 cells, respectively. Enforced miR-181b expression reduced BCL2 protein level and sensitized SGC7901/VCR and A549/CDDP cells to VCR-induced and CDDP-induced apoptosis, respectively. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Breast cancer [ICD-11: 2C60.3] | [49] | |||
| Sensitive Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Sensitive Drug | Fulvestrant | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell invasion | Inhibition | hsa05200 | ||
| Cell proliferation | Inhibition | hsa05200 | ||
| PI3K/AKT/mTOR signaling pathway | Regulation | N.A. | ||
| In Vitro Model | MCF-7 cells | Breast | Homo sapiens (Human) | CVCL_0031 |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miR-21 is a miRNA that is overexpressed in most tumor types, and acts as an oncogene by targeting many suppressor genes related to proliferation, apoptosis, and invasion. miR-21 facilitates tumor growth and invasion by targeting programmed cell death 4 (PDCD4), PTEN, and Bcl-2. silencing of miR-21 sensitized ER+ breast cancer cells to TAM and FUL induced cell apoptosis. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Esophageal squamous cell carcinoma [ICD-11: 2B70.3] | [50] | |||
| Resistant Disease | Esophageal squamous cell carcinoma [ICD-11: 2B70.3] | |||
| Resistant Drug | Gefitinib | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell colony | Activation | hsa05200 | ||
| Cell viability | Activation | hsa05200 | ||
| miR129/BCL2 signaling pathway | Regulation | N.A. | ||
| In Vitro Model | TE-1 cells | Esophagus | Homo sapiens (Human) | CVCL_1759 |
| KYSE-450 cells | Esophagus | Homo sapiens (Human) | CVCL_1353 | |
| TE6 cells | Esophageal | Homo sapiens (Human) | CVCL_1765 | |
| TE8 cells | Esophageal | Homo sapiens (Human) | CVCL_1766 | |
| TTn cells | Esophageal | Homo sapiens (Human) | CVCL_3175 | |
| In Vivo Model | BALB/c nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay; TUNEL assay | |||
| Mechanism Description | Exosome-mediated transfer of PART1 promoted gefitinib resistance by competitively binding to miR-129 to facilitate Bcl-2 expression in ESCC cells. | |||
|
|
||||
| Disease Class: Non-small cell lung cancer [ICD-11: 2C25.Y] | [51] | |||
| Resistant Disease | Non-small cell lung cancer [ICD-11: 2C25.Y] | |||
| Resistant Drug | Gefitinib | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell viability | Activation | hsa05200 | ||
| In Vitro Model | PC9 cells | Lung | Homo sapiens (Human) | CVCL_B260 |
| In Vivo Model | BALB/c nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Flow cytometry assay | |||
| Experiment for Drug Resistance |
CCK8 assay; Flow cytometry assay | |||
| Mechanism Description | miR-214 level was upregulated in gefitinib-resistant PC-9GR cells and their derived exosomes while anti-apoptotic protein of bcl-2 is uoregulated. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Diabetic retinopathy [ICD-11: 9B71.0] | [54] | |||
| Sensitive Disease | Diabetic retinopathy [ICD-11: 9B71.0] | |||
| Sensitive Drug | Idebenone | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | PI3K signaling pathway | Regulation | N.A. | |
| Akt signaling pathway | Regulation | N.A. | ||
| In Vitro Model | RF/6A cells | Chorioretinal | Homo sapiens (Human) | CVCL_4552 |
| In Vivo Model | Diabetic rats model | Rattus norvegicus | ||
| Experiment for Molecule Alteration |
Western blot assay | |||
| Experiment for Drug Resistance |
Ocular fundus ultrasound testing; Histological assay; Cell proliferation assay | |||
| Mechanism Description | IDE regulated the autophagy of retina cells to alleviate diabetic retinopathy via regulating the PI3K signaling pathway.The PI3K/Akt/mTOR signaling pathway acts as the mediator of IDE in alleviating DR.IDE treatment suppressed the activation of the PI3K/Akt/mTOR signaling pathway, and PI3K signaling repressed the protective role of IDE in DR, explaining the IDE-suppressed autophagy in DR. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Gastrointestinal stromal tumor [ICD-11: 2B5B.0] | [55] | |||
| Sensitive Disease | Gastrointestinal stromal tumor [ICD-11: 2B5B.0] | |||
| Sensitive Drug | Imatinib | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | GIST-T1 cells | Gastric | Homo sapiens (Human) | CVCL_4976 |
| Experiment for Molecule Alteration |
RT-qPCR; Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay; Annexin V-FITC Apoptosis Detection assay | |||
| Mechanism Description | miRNA-21 sensitizes gastrointesti.l stromal tumors (GISTs) cells to Imatinib via targeting B-cell lymphoma 2 (Bcl-2), miRNA-21 suppressed Bcl-2 expression in GIST cells and could function as a potent tumor suppressor in GIST. | |||
| Disease Class: Chronic myeloid leukemia [ICD-11: 2A20.0] | [56] | |||
| Sensitive Disease | Chronic myeloid leukemia [ICD-11: 2A20.0] | |||
| Sensitive Drug | Imatinib | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| p53 signaling pathway | Regulation | N.A. | ||
| In Vitro Model | K562 cells | Blood | Homo sapiens (Human) | CVCL_0004 |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Flow cytometry assay | |||
| Mechanism Description | BCL-2, MCL-1 and XIAP were the target genes of miR-130a. BCL-2, MCL-1, TCL-1 and XIAP protein levels were significantly higher in patients with drug-sensitive CML cells. Transfected miR-130a mimics significantly decreased the protein expression of BCL-1, MCL-1 and XIAP. Transfected miR-130a significantly increased the CML sensitivity to Gleevec. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Colorectal cancer [ICD-11: 2B91.1] | [46] | |||
| Sensitive Disease | Colorectal cancer [ICD-11: 2B91.1] | |||
| Sensitive Drug | Oxaliplatin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | BCL2 signaling pathway | Regulation | N.A. | |
| Cell apoptosis | Activation | hsa04210 | ||
| Cell invasion | Inhibition | hsa05200 | ||
| Cell migration | Inhibition | hsa04670 | ||
| In Vitro Model | SW480 cells | Colon | Homo sapiens (Human) | CVCL_0546 |
| SW620 cells | Colon | Homo sapiens (Human) | CVCL_0547 | |
| HCT116 cells | Colon | Homo sapiens (Human) | CVCL_0291 | |
| LOVO cells | Colon | Homo sapiens (Human) | CVCL_0399 | |
| RkO cells | Colon | Homo sapiens (Human) | CVCL_0504 | |
| HT-29 cells | Colon | Homo sapiens (Human) | CVCL_0320 | |
| LS174T cells | Colon | Homo sapiens (Human) | CVCL_1384 | |
| COLO205 cells | Colon | Homo sapiens (Human) | CVCL_F402 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay; Transwell assay | |||
| Mechanism Description | BCL2 is a direct target of miR-139-5p in colorectal cancer cells and showed that the tumour suppressor activity of miR-139-5p is mediated by the modulation of BCL2 expression. BCL2 family proteins regulate and contribute to programmed cell death or apoptosis. The cell apoptosis results showed the induction of apoptotic cells contributed greatly to 5-Fu and OXA drug sensitivity, which was consistent with the multidrug resistance mechanisms. miR-139-5p is downregulated in colorectal cancer cells and tissues, and its inhibitory effects on cell migration, invasion, and drug sensitivity are mediated by the downregulation of its target BCL2. | |||
| Disease Class: Gastric cancer [ICD-11: 2B72.1] | [39] | |||
| Sensitive Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Sensitive Drug | Oxaliplatin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| In Vitro Model | SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Higher miR-218 levels increased the level of Bax and reduced the level of Bcl-2 and miR-218 inhibits multidrug resistance (MDR) of gastric cancer cells by targeting Hedgehog/smoothened. | |||
| Disease Class: Gastric cancer [ICD-11: 2B72.1] | [48] | |||
| Sensitive Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Sensitive Drug | Oxaliplatin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| In Vitro Model | NCI-N87 cells | Gastric | Homo sapiens (Human) | CVCL_1603 |
| GTL-16 cells | Gastric | Homo sapiens (Human) | CVCL_7668 | |
| In Vivo Model | CD1 nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Immunohistochemistry assay | |||
| Experiment for Drug Resistance |
Flow cytometry assay | |||
| Mechanism Description | miR-204 targeted Bcl-2 messenger RNA and increased responsiveness of GC cells to 5-fluorouracil and oxaliplatin treatment. Ectopic expression of Bcl-2 protein counteracted miR-204 pro-apoptotic activity in response to 5-fluorouracil. | |||
| Disease Class: Colorectal carcinoma [ICD-11: 2B91.3] | [57] | |||
| Sensitive Disease | Colorectal carcinoma [ICD-11: 2B91.3] | |||
| Sensitive Drug | Oxaliplatin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| In Vitro Model | HCT116 cells | Colon | Homo sapiens (Human) | CVCL_0291 |
| HCT-116/L-OHP cells | Kidney | Homo sapiens (Human) | CVCL_0291 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Elevated levels of miR-1915 in the mimics-transfected HCT116/L-OHP cells reduced Bcl-2 protein level and the luciferase activity of a Bcl-2 3'-untranslated region-based reporter, and also sensitized these cells to some anticancer drugs. miR-1915 could play a role in the development of MDR in colorectal carcinoma cells at least in part by modulation of apoptosis via targeting Bcl-2. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Malignant pleural mesothelioma [ICD-11: 2C26.0] | [53] | |||
| Sensitive Disease | Malignant pleural mesothelioma [ICD-11: 2C26.0] | |||
| Sensitive Drug | Pemetrexed | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | MET-5A cells | Lung | Homo sapiens (Human) | CVCL_3749 |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay; Colony formation assay | |||
| Mechanism Description | Growth inhibition caused by miR-16 correlated with downregulation of target genes including Bcl-2 and CCND1, and miR-16 re-expression sensitised MPM cells to pemetrexed and gemcitabine. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Hereditary angioedema [ICD-11: 4B05.0] | [63] | |||
| Resistant Disease | Hereditary angioedema [ICD-11: 4B05.0] | |||
| Resistant Drug | Stanozolol | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Discovered Using In-vivo Testing Model | |||
| In Vivo Model | Sprague Dawley male rats model | Rattus norvegicus | ||
| Experiment for Molecule Alteration |
Gene expression analysis | |||
| Mechanism Description | Stanozolol can increases levels of Bax, Bcl-2, P53, caspase 3 and Bax/Bcl-2 ratio. Resistance training, 50 and 100 mg/kg Tribulus terrestris and resistance training along with Tribulus terrestris can decrease the Bax, Bcl-2, P53, caspase 3 and Bax/Bcl-2 ratio in rats exposed to Stanozolol. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Breast cancer [ICD-11: 2C60.3] | [49] | |||
| Sensitive Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Sensitive Drug | Tamoxifen | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell migration | Inhibition | hsa04670 | ||
| Cell proliferation | Inhibition | hsa05200 | ||
| PI3K/AKT/mTOR signaling pathway | Regulation | N.A. | ||
| In Vitro Model | MCF-7 cells | Breast | Homo sapiens (Human) | CVCL_0031 |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miR-21 is a miRNA that is overexpressed in most tumor types, and acts as an oncogene by targeting many suppressor genes related to proliferation, apoptosis, and invasion. miR-21 facilitates tumor growth and invasion by targeting programmed cell death 4 (PDCD4), PTEN, and Bcl-2. silencing of miR-21 sensitized ER+ breast cancer cells to TAM and FUL induced cell apoptosis. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Glioma [ICD-11: 2A00.1] | [64] | |||
| Resistant Disease | Glioma [ICD-11: 2A00.1] | |||
| Resistant Drug | Temozolomide | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | IGF1R/IRS1 signaling pathway | Activation | hsa04212 | |
| In Vitro Model | U251 cells | Brain | Homo sapiens (Human) | CVCL_0021 |
| U87 cells | Brain | Homo sapiens (Human) | CVCL_0022 | |
| U138 cells | Brain | Homo sapiens (Human) | CVCL_0020 | |
| HEK293 cells | Kidney | Homo sapiens (Human) | CVCL_0045 | |
| NHA cells | Brain | Homo sapiens (Human) | N.A. | |
| LN382 cells | Brain | Homo sapiens (Human) | CVCL_3956 | |
| SF295 cells | Brain | Homo sapiens (Human) | CVCL_1690 | |
| SHG-44 cells | Brain | Homo sapiens (Human) | CVCL_6728 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | Up-regulation of miR-497 confers resistance to temozolomide in human glioma cells by targeting mTOR/Bcl-2. The silencing of miR-497 decreased the protein levels of IGF1R/IRS1 pathway-related proteins, that is, IGF1R, IRS1, mTOR, and Bcl-2. | |||
| Disease Class: Glioma [ICD-11: 2A00.1] | [65] | |||
| Resistant Disease | Glioma [ICD-11: 2A00.1] | |||
| Resistant Drug | Temozolomide | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell proliferation | Activation | hsa05200 | ||
| In Vitro Model | A172 cells | Brain | Homo sapiens (Human) | CVCL_0131 |
| U138-MG cells | Brain | Homo sapiens (Human) | CVCL_0020 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | The mechanism responsible for resistance of glioma cells to temozolomide was associated with miR-16-mediated downregulation of Bcl-2. miR-16 may function as an important modifier of the response of glioma cells to temozolomide. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Glioma [ICD-11: 2A00.1] | [66] | |||
| Sensitive Disease | Glioma [ICD-11: 2A00.1] | |||
| Sensitive Drug | Temozolomide | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell invasion | Inhibition | hsa05200 | ||
| Cell proliferation | Inhibition | hsa05200 | ||
| Cell viability | Inhibition | hsa05200 | ||
| In Vitro Model | U251 cells | Brain | Homo sapiens (Human) | CVCL_0021 |
| U87MG cells | Brain | Homo sapiens (Human) | CVCL_GP63 | |
| In Vivo Model | BALB/c nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay; Flow cytometry assay; Transwell assay | |||
| Mechanism Description | Upregulation of miR-181b-5p targets Bcl-2 directly and may function as an important modifier to sensitize glioma cells to TMZ. | |||
| Disease Class: Glioblastoma [ICD-11: 2A00.02] | [67] | |||
| Sensitive Disease | Glioblastoma [ICD-11: 2A00.02] | |||
| Sensitive Drug | Temozolomide | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell viability | Inhibition | hsa05200 | ||
| In Vitro Model | A172 cells | Brain | Homo sapiens (Human) | CVCL_0131 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay; Flow cytometry assay | |||
| Mechanism Description | The chemoresistant cell survival mediated with Bcl-2 was inhibited by overexpression of miR-1271 and was enhanced by depletion of miR-1271. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Breast cancer [ICD-11: 2C60.3] | [68] | |||
| Resistant Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Resistant Drug | Trastuzumab | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | BCL2/Bax apoptosis signaling pathway | Activation | hsa04210 | |
| Cell apoptosis | Inhibition | hsa04210 | ||
| Cell invasion | Activation | hsa05200 | ||
| Cell viability | Activation | hsa05200 | ||
| In Vitro Model | SkBR3 cells | Breast | Homo sapiens (Human) | CVCL_0033 |
| BT474 cells | Breast | Homo sapiens (Human) | CVCL_0179 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay; Flow cytometry assay | |||
| Mechanism Description | Exosomal LncRNA-SNHG14 may induce trastuzumab resistance through inhibiting apoptotic proteins and cell apoptosis via Bcl-2/Bax pathway. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Lymphoma [ICD-11: 2A90- 2A85] | [69] | |||
| Resistant Disease | Lymphoma [ICD-11: 2A90- 2A85] | |||
| Resistant Drug | Venetoclax | |||
| Molecule Alteration | Missense mutation | p.F104I (c.310T>A) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Disease Class: Chronic lymphocytic leukemia [ICD-11: 2A82.0] | [70] | |||
| Resistant Disease | Chronic lymphocytic leukemia [ICD-11: 2A82.0] | |||
| Resistant Drug | Venetoclax | |||
| Molecule Alteration | Missense mutation | p.G101V (c.302G>T) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | KMS-12-PE cells | Pleural effusion | Homo sapiens (Human) | CVCL_1333 |
| Experiment for Drug Resistance |
CellTiter-Glo assay; IC50 assay | |||
Disease- and Tissue-specific Abundances of This Molecule
ICD Disease Classification 02

| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Nervous tissue | |
| The Specified Disease | Brain cancer | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.33E-53; Fold-change: -2.89E-01; Z-score: -1.09E+00 | |
|
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |

|
Click to View the Clearer Original Diagram |
| The Studied Tissue | Brainstem tissue | |
| The Specified Disease | Glioma | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 6.38E-01; Fold-change: -2.32E-01; Z-score: -4.42E-01 | |
|
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |

|
Click to View the Clearer Original Diagram |
| The Studied Tissue | White matter | |
| The Specified Disease | Glioma | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.61E-03; Fold-change: -1.06E+00; Z-score: -1.89E+00 | |
|
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |
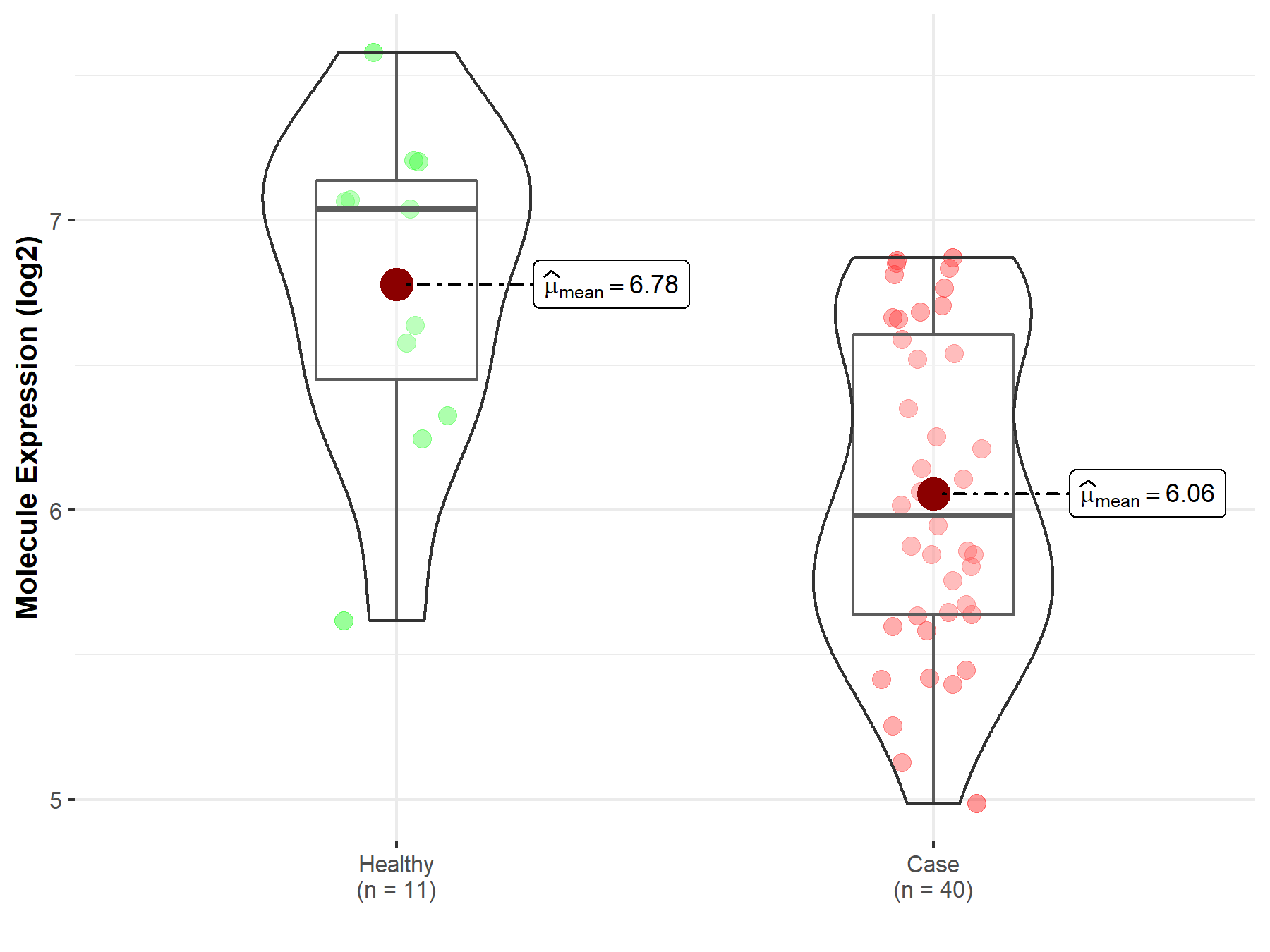
|
Click to View the Clearer Original Diagram |
| The Studied Tissue | Brainstem tissue | |
| The Specified Disease | Neuroectodermal tumor | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.35E-04; Fold-change: -3.43E-01; Z-score: -2.10E+00 | |
|
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |
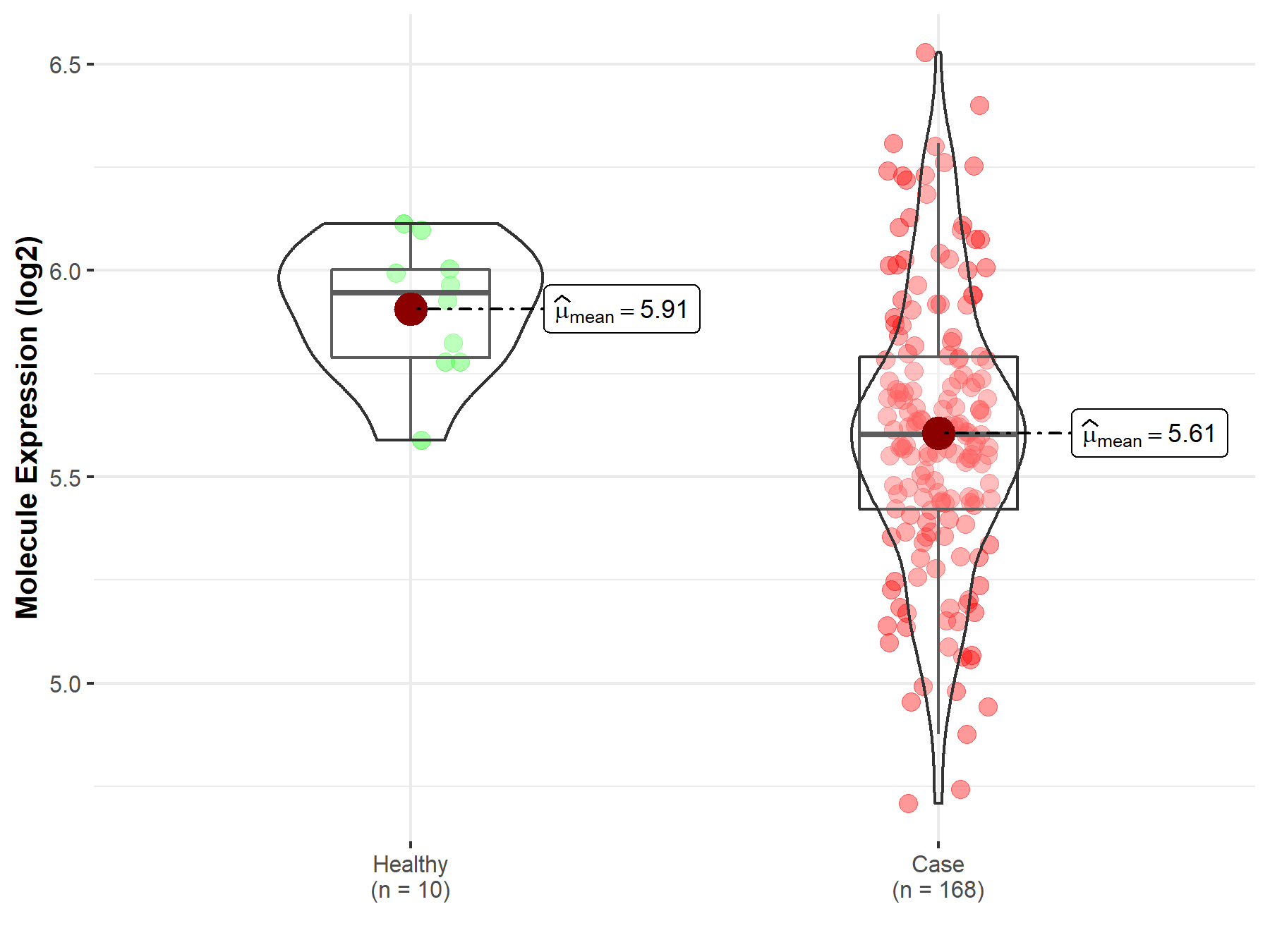
|
Click to View the Clearer Original Diagram |
| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Whole blood | |
| The Specified Disease | Myelofibrosis | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.81E-02; Fold-change: -9.42E-02; Z-score: -1.04E+00 | |
|
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |
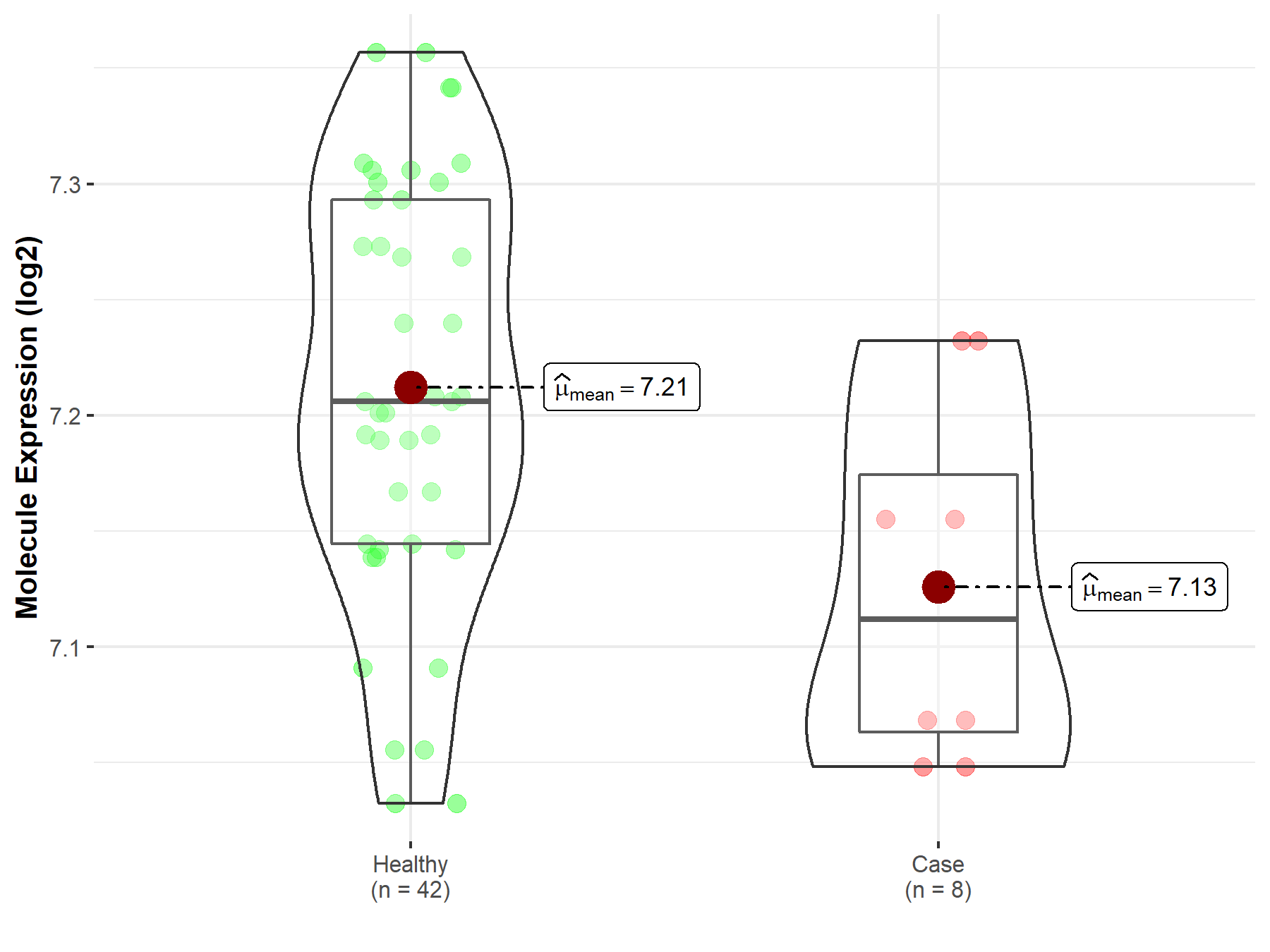
|
Click to View the Clearer Original Diagram |
| The Studied Tissue | Whole blood | |
| The Specified Disease | Polycythemia vera | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 8.10E-01; Fold-change: -5.16E-03; Z-score: -5.58E-02 | |
|
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |
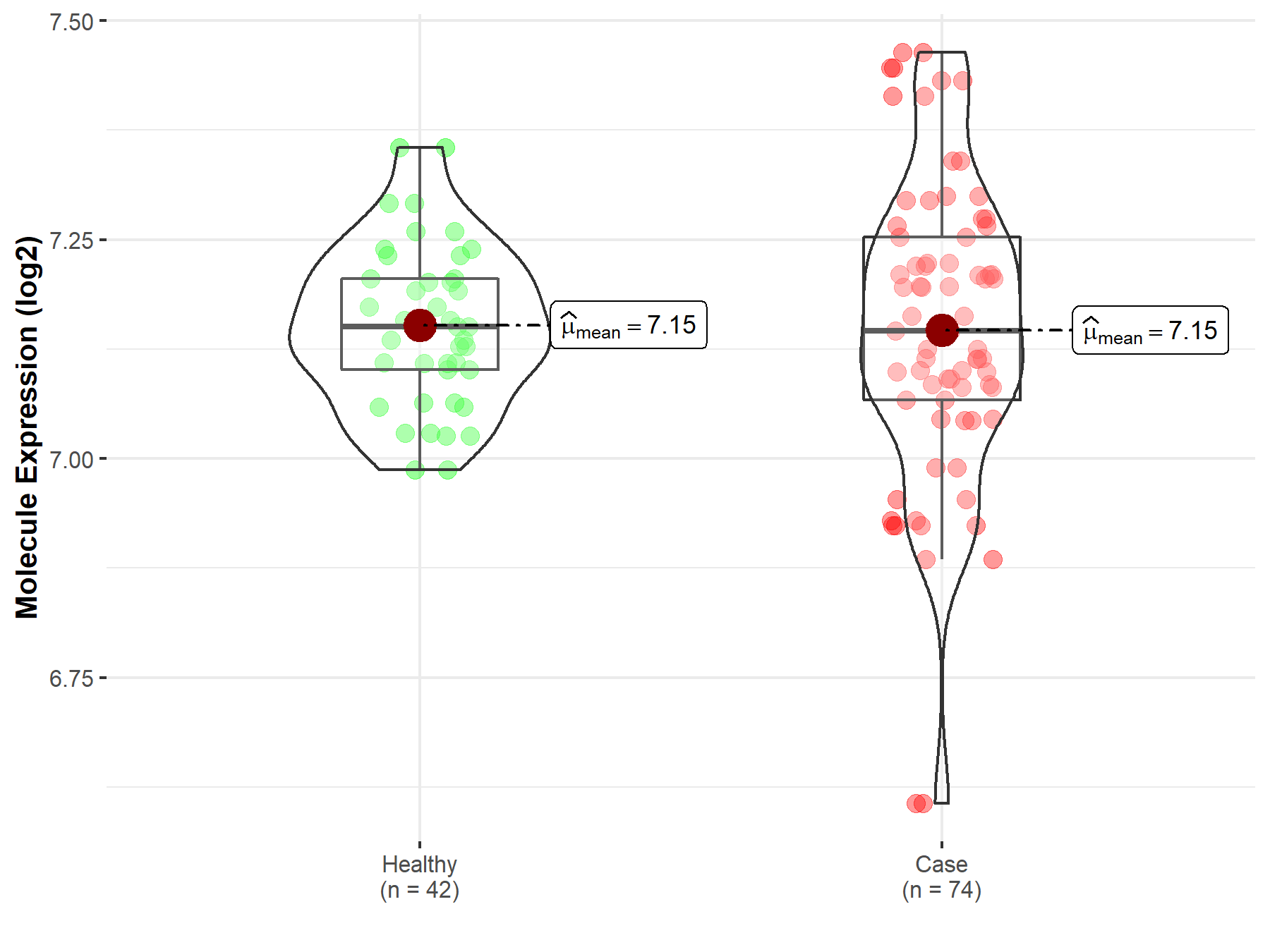
|
Click to View the Clearer Original Diagram |
| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Bone marrow | |
| The Specified Disease | Multiple myeloma | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 7.64E-04; Fold-change: 4.26E-01; Z-score: 2.16E+00 | |
|
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |
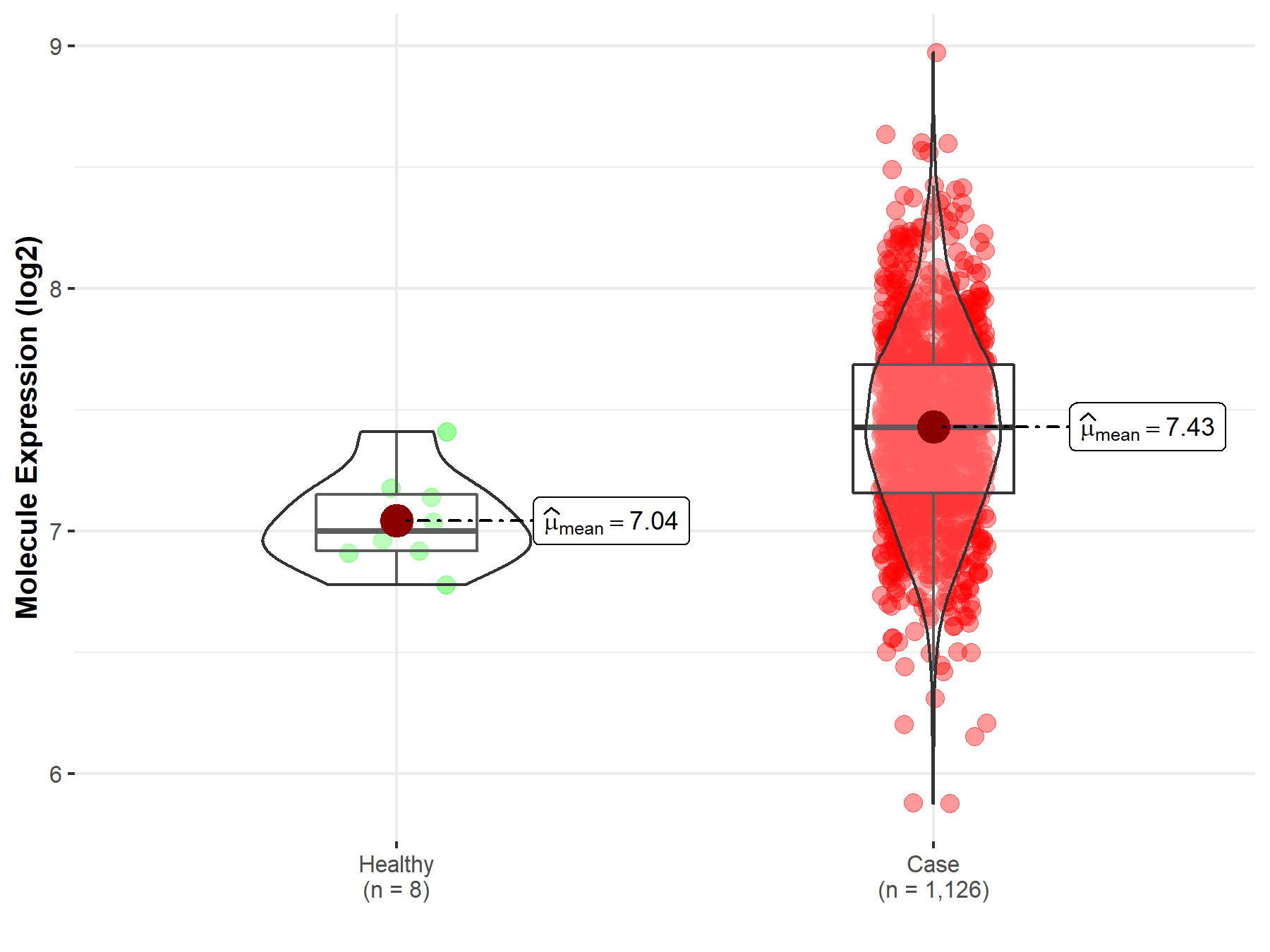
|
Click to View the Clearer Original Diagram |
| The Studied Tissue | Peripheral blood | |
| The Specified Disease | Multiple myeloma | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 6.24E-01; Fold-change: -9.91E-02; Z-score: -3.63E-01 | |
|
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |

|
Click to View the Clearer Original Diagram |
| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Tonsil tissue | |
| The Specified Disease | Lymphoma | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 6.81E-01; Fold-change: -1.00E-01; Z-score: -4.38E-01 | |
|
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |

|
Click to View the Clearer Original Diagram |
| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Esophagus | |
| The Specified Disease | Esophageal cancer | |
| The Expression Level of Disease Section Compare with the Adjacent Tissue | p-value: 4.87E-01; Fold-change: -2.59E-03; Z-score: -1.05E-02 | |
|
Molecule expression in the normal tissue adjacent to the diseased tissue of patients
Molecule expression in the diseased tissue of patients
|
||
| Disease-specific Molecule Abundances |

|
Click to View the Clearer Original Diagram |
| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Gastric tissue | |
| The Specified Disease | Gastric cancer | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 5.02E-02; Fold-change: -2.14E-01; Z-score: -2.16E+00 | |
| The Expression Level of Disease Section Compare with the Adjacent Tissue | p-value: 8.23E-03; Fold-change: -1.92E-01; Z-score: -7.94E-01 | |
|
Molecule expression in the normal tissue adjacent to the diseased tissue of patients
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |
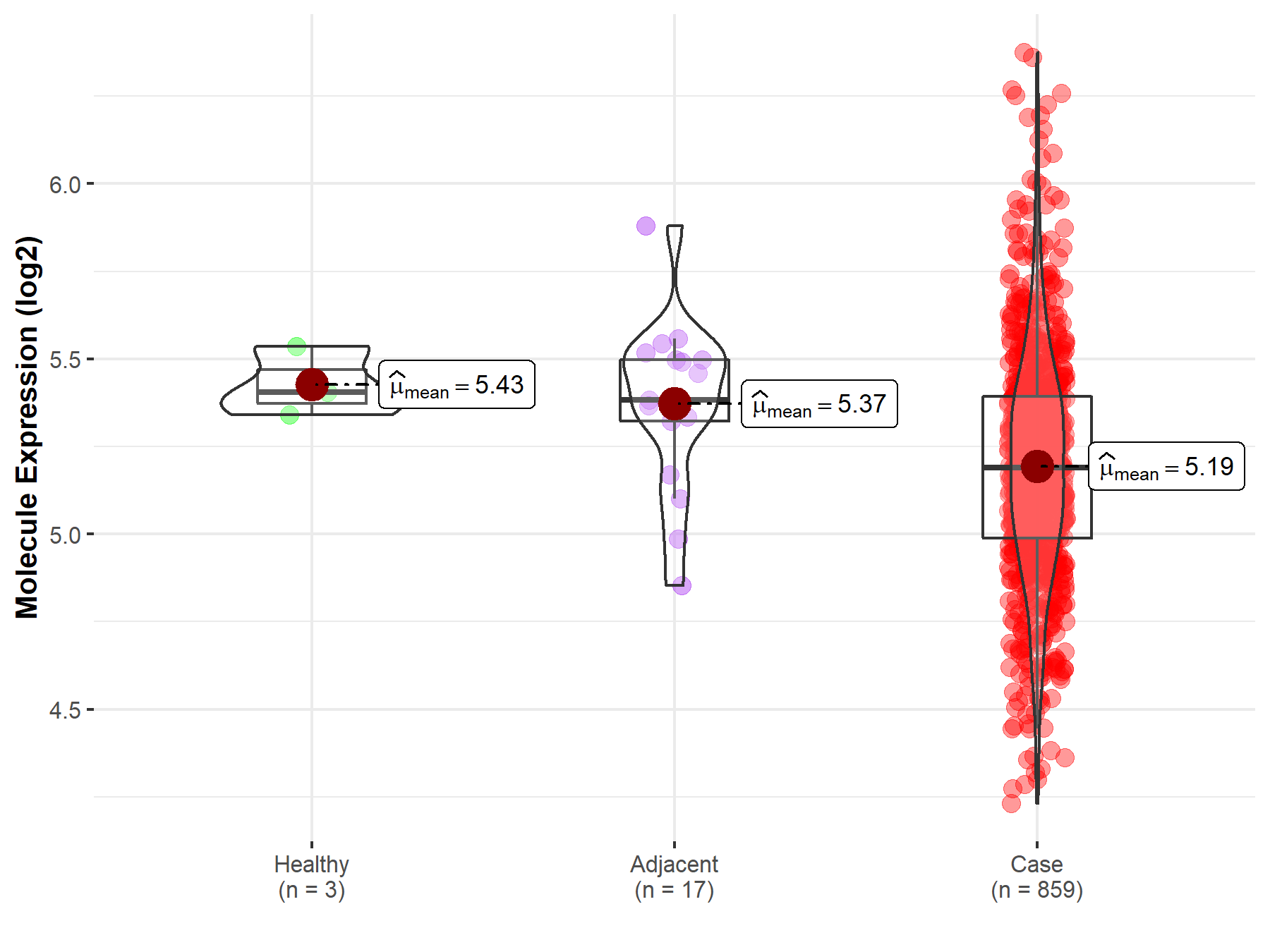
|
Click to View the Clearer Original Diagram |
| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Colon | |
| The Specified Disease | Colon cancer | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 4.01E-51; Fold-change: -4.00E-01; Z-score: -1.75E+00 | |
| The Expression Level of Disease Section Compare with the Adjacent Tissue | p-value: 1.49E-51; Fold-change: -4.90E-01; Z-score: -2.13E+00 | |
|
Molecule expression in the normal tissue adjacent to the diseased tissue of patients
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |
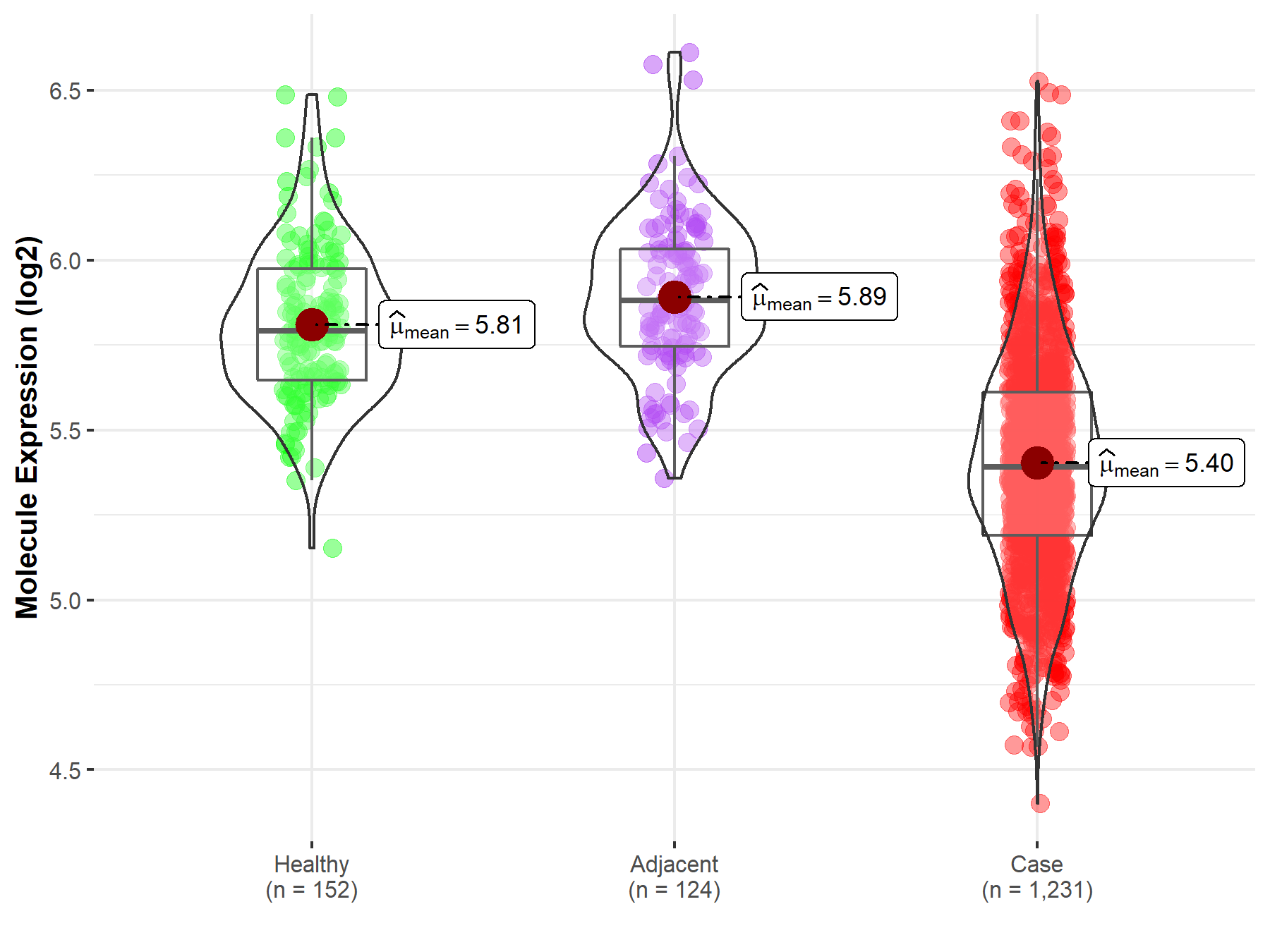
|
Click to View the Clearer Original Diagram |
| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Pancreas | |
| The Specified Disease | Pancreatic cancer | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.85E-02; Fold-change: -2.63E-01; Z-score: -8.16E-01 | |
| The Expression Level of Disease Section Compare with the Adjacent Tissue | p-value: 4.14E-09; Fold-change: -2.43E-01; Z-score: -9.77E-01 | |
|
Molecule expression in the normal tissue adjacent to the diseased tissue of patients
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |

|
Click to View the Clearer Original Diagram |
| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Liver | |
| The Specified Disease | Liver cancer | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.81E-06; Fold-change: -1.56E-01; Z-score: -7.15E-01 | |
| The Expression Level of Disease Section Compare with the Adjacent Tissue | p-value: 3.33E-41; Fold-change: -3.42E-01; Z-score: -1.58E+00 | |
| The Expression Level of Disease Section Compare with the Other Disease Section | p-value: 1.09E-03; Fold-change: -4.98E-01; Z-score: -5.03E+00 | |
|
Molecule expression in the normal tissue adjacent to the diseased tissue of patients
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
Molecule expression in tissue other than the diseased tissue of patients
|
||
| Disease-specific Molecule Abundances |

|
Click to View the Clearer Original Diagram |
| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Lung | |
| The Specified Disease | Lung cancer | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 9.45E-01; Fold-change: 1.35E-02; Z-score: 5.07E-02 | |
| The Expression Level of Disease Section Compare with the Adjacent Tissue | p-value: 2.56E-04; Fold-change: 7.58E-02; Z-score: 3.73E-01 | |
|
Molecule expression in the normal tissue adjacent to the diseased tissue of patients
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |
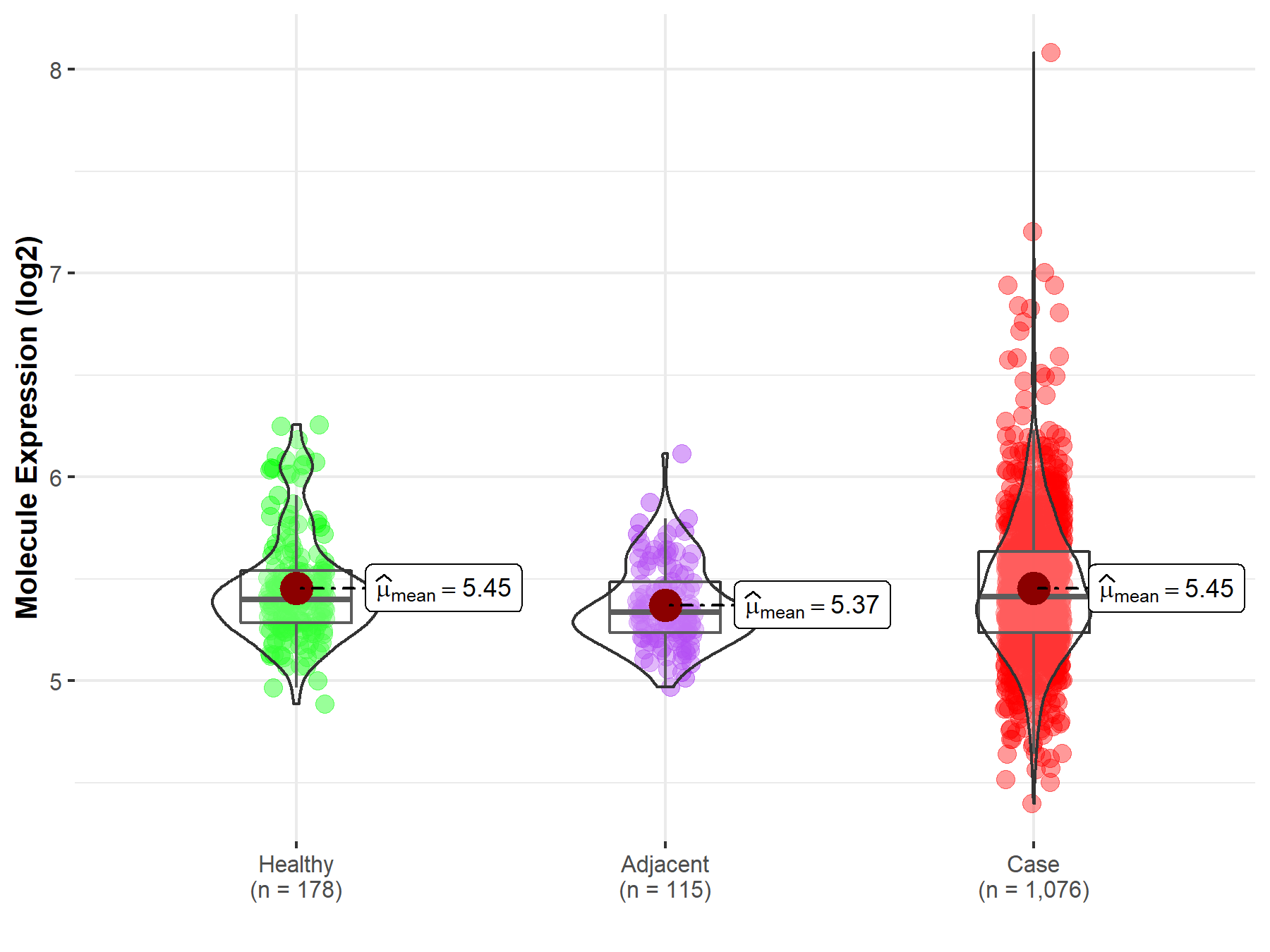
|
Click to View the Clearer Original Diagram |
| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Breast tissue | |
| The Specified Disease | Breast cancer | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.08E-24; Fold-change: -2.26E-01; Z-score: -7.40E-01 | |
| The Expression Level of Disease Section Compare with the Adjacent Tissue | p-value: 1.67E-01; Fold-change: -6.74E-02; Z-score: -1.91E-01 | |
|
Molecule expression in the normal tissue adjacent to the diseased tissue of patients
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |

|
Click to View the Clearer Original Diagram |
| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Ovary | |
| The Specified Disease | Ovarian cancer | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 2.42E-03; Fold-change: -3.67E-01; Z-score: -1.36E+00 | |
| The Expression Level of Disease Section Compare with the Adjacent Tissue | p-value: 6.19E-03; Fold-change: 3.84E-01; Z-score: 1.20E+00 | |
|
Molecule expression in the normal tissue adjacent to the diseased tissue of patients
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |
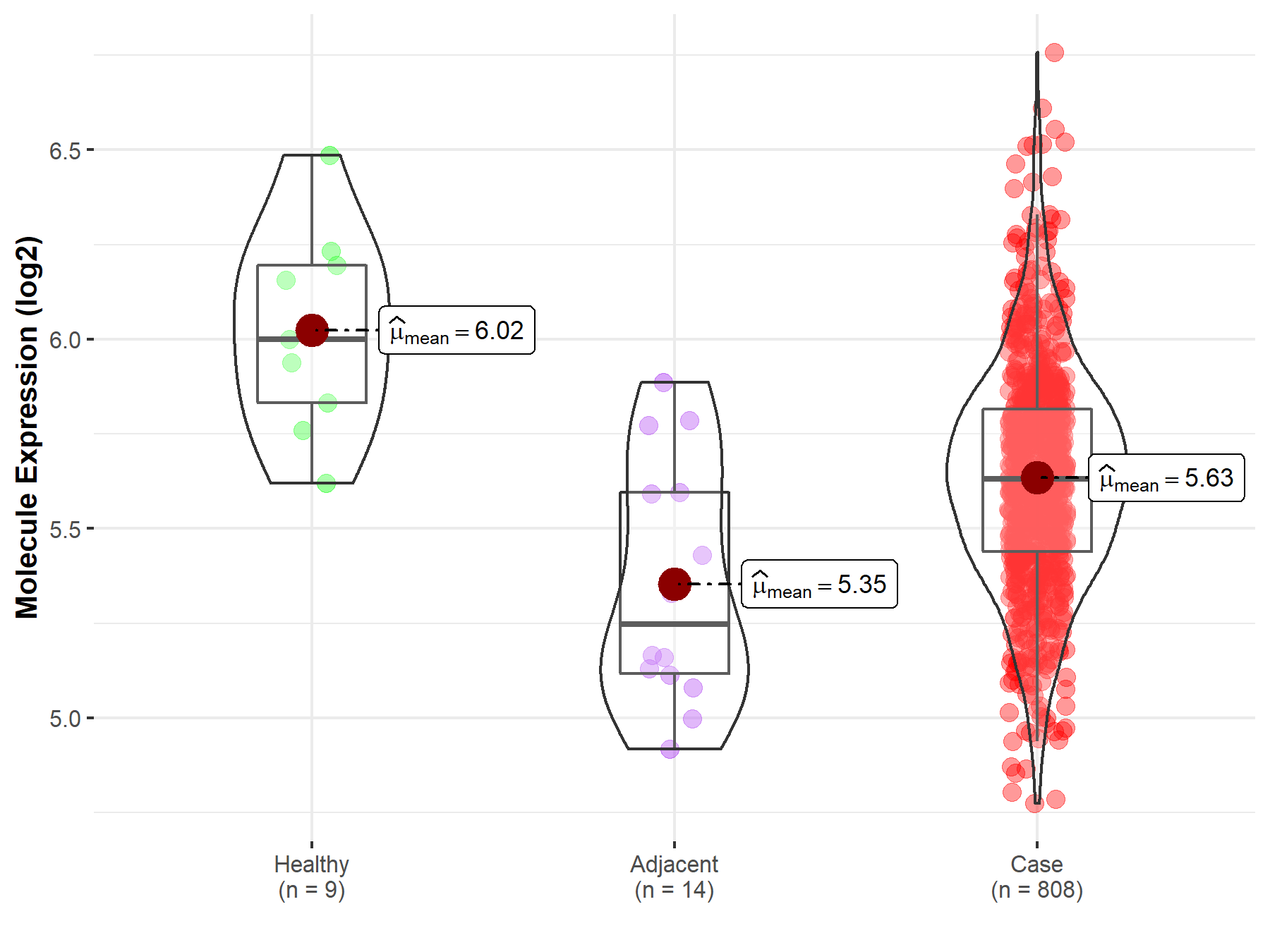
|
Click to View the Clearer Original Diagram |
| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Cervix uteri | |
| The Specified Disease | Cervical cancer | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 7.36E-01; Fold-change: 3.06E-02; Z-score: 1.66E-01 | |
|
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |

|
Click to View the Clearer Original Diagram |
| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Prostate | |
| The Specified Disease | Prostate cancer | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.29E-02; Fold-change: 1.57E-01; Z-score: 6.18E-01 | |
|
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |
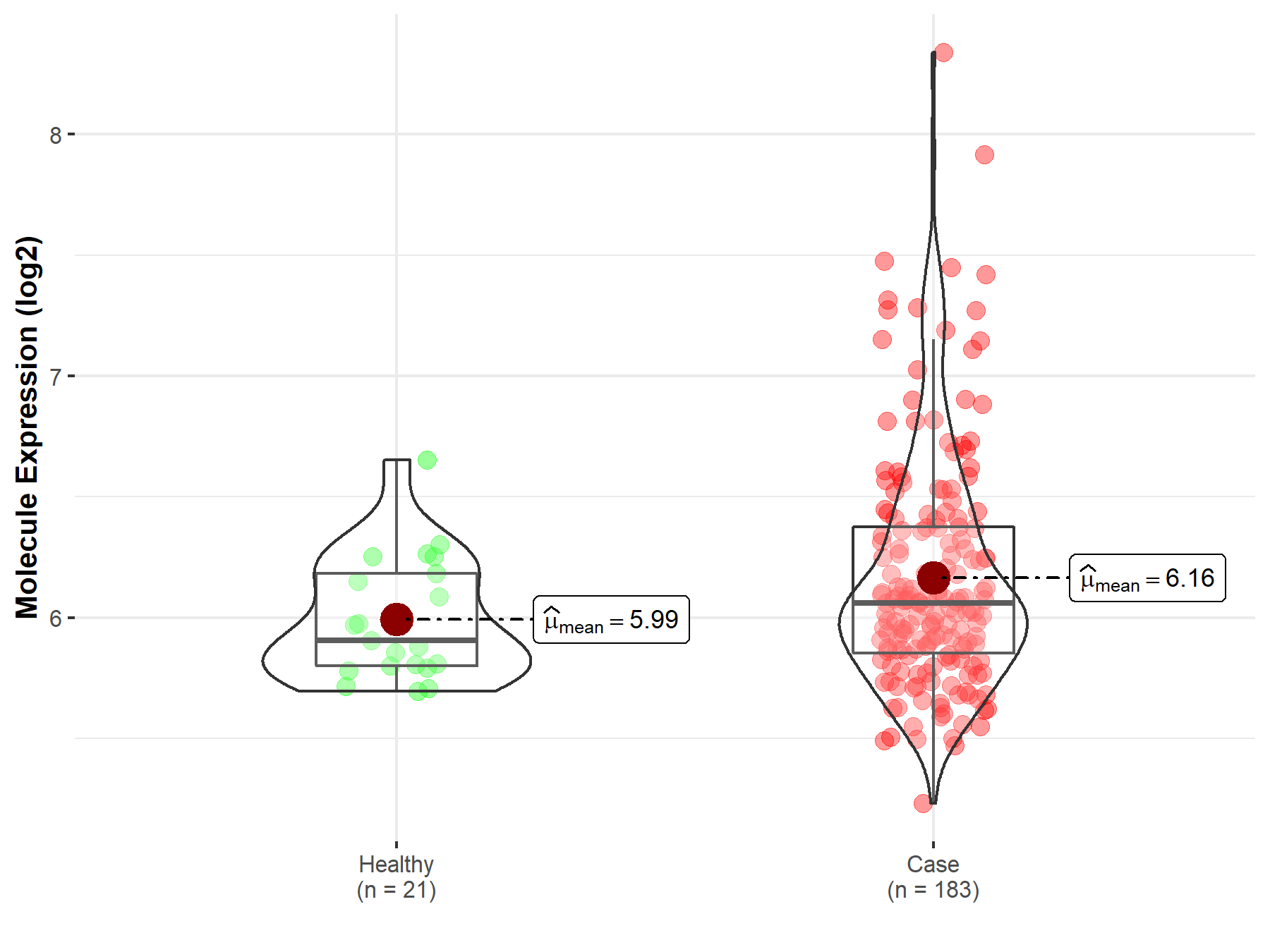
|
Click to View the Clearer Original Diagram |
Tissue-specific Molecule Abundances in Healthy Individuals

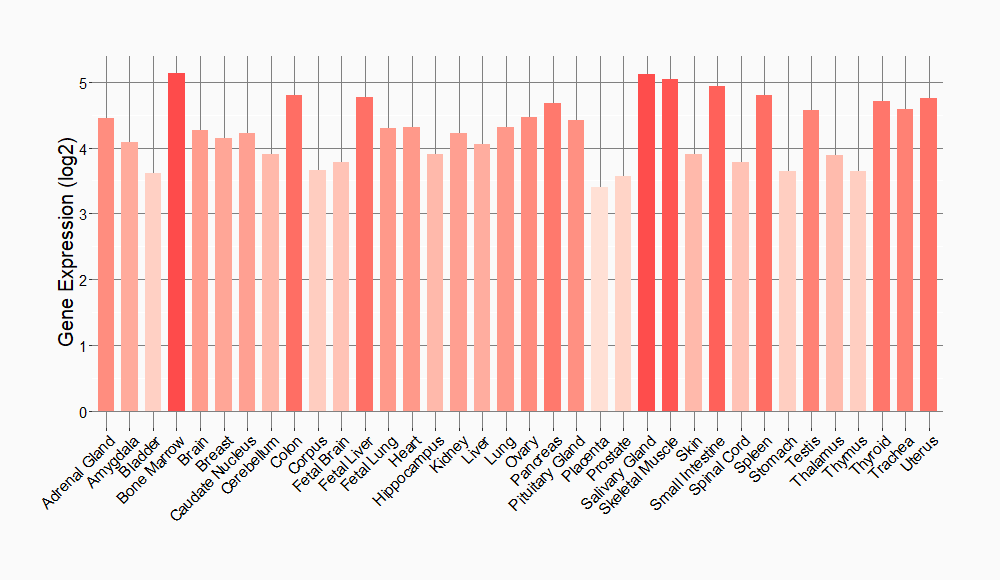
|
||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
