Molecule Information
General Information of the Molecule (ID: Mol00025)
| Name |
Apoptosis regulator BAX (BAX)
,Homo sapiens
|
||||
|---|---|---|---|---|---|
| Synonyms |
Bcl-2-like protein 4; Bcl2-L-4; BCL2L4
Click to Show/Hide
|
||||
| Molecule Type |
Protein
|
||||
| Gene Name |
BAX
|
||||
| Gene ID | |||||
| Location |
chr19:48954815-48961798[+]
|
||||
| Sequence |
MDGSGEQPRGGGPTSSEQIMKTGALLLQGFIQDRAGRMGGEAPELALDPVPQDASTKKLS
ECLKRIGDELDSNMELQRMIAAVDTDSPREVFFRVAADMFSDGNFNWGRVVALFYFASKL VLKALCTKVPELIRTIMGWTLDFLRERLLGWIQDQGGWDGLLSYFGTPTWQTVTIFVAGV LTASLTIWKKMG Click to Show/Hide
|
||||
| 3D-structure |
|
||||
| Function |
Plays a role in the mitochondrial apoptotic process. Under normal conditions, BAX is largely cytosolic via constant retrotranslocation from mitochondria to the cytosol mediated by BCL2L1/Bcl-xL, which avoids accumulation of toxic BAX levels at the mitochondrial outer membrane (MOM). Under stress conditions, undergoes a conformation change that causes translocation to the mitochondrion membrane, leading to the release of cytochrome c that then triggers apoptosis. Promotes activation of CASP3, and thereby apoptosis.
Click to Show/Hide
|
||||
| Uniprot ID | |||||
| Ensembl ID | |||||
| HGNC ID | |||||
| Click to Show/Hide the Complete Species Lineage | |||||
Type(s) of Resistant Mechanism of This Molecule
Drug Resistance Data Categorized by Drug
Approved Drug(s)
7 drug(s) in total
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Non-small cell lung cancer [ICD-11: 2C25.Y] | [1] | |||
| Sensitive Disease | Non-small cell lung cancer [ICD-11: 2C25.Y] | |||
| Sensitive Drug | Cisplatin | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Lung cancer [ICD-11: 2C25] | |||
| The Specified Disease | Lung cancer | |||
| The Studied Tissue | Lung tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 8.26E-07 Fold-change: 5.63E-02 Z-score: 5.01E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| In Vitro Model | A549 cells | Lung | Homo sapiens (Human) | CVCL_0023 |
| Sk-MES-1 cells | Lung | Homo sapiens (Human) | CVCL_0630 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | Down-regulation of miR-21 inhibited growth, colony formation, antiapoptotic Bcl-2 expression and promoted proapoptotic Bax and caspase-9 expression in A549 cells treated with DDP. Upregulation of miR-21 promoted growth and colony formation in Sk-MES-1 cells treated with DDP. Furthermore, downregulation of miR-21 reduced growth of implanted tumors, suggesting that miR-21 inhibition could enhance the sensitivity of A549 cells to DDP in vivo. These data suggest an appropriate combination of DDP and miR-21 regulation might be a potential approach to lung cancer therapy. Combined DDP application with miR-21 downregulation for the treatment of lung cancer would help achieve effective treatment and reduce DDP side effects. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Gastric cancer [ICD-11: 2B72.1] | [2] | |||
| Resistant Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Resistant Drug | Cisplatin | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Gastric cancer [ICD-11: 2B72] | |||
| The Specified Disease | Gastric cancer | |||
| The Studied Tissue | Gastric tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.20E-01 Fold-change: 3.92E-01 Z-score: 2.61E+00 |
|||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| In Vitro Model | SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 |
| BGC823 cells | Gastric | Homo sapiens (Human) | CVCL_3360 | |
| Experiment for Molecule Alteration |
Western blot analysis; RT-qPCR | |||
| Experiment for Drug Resistance |
MTT assay; Flow cytometry assay | |||
| Mechanism Description | LncRNA SNHG5 promotes cisplatin resistance in gastric cancer via inhibiting cell apoptosis and upregulating drug resistance-related genes. | |||
| Disease Class: Bladder cancer [ICD-11: 2C94.0] | [3] | |||
| Resistant Disease | Bladder cancer [ICD-11: 2C94.0] | |||
| Resistant Drug | Cisplatin | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| In Vitro Model | 5637 cells | Bladder | Homo sapiens (Human) | CVCL_0126 |
| T24 cells | Bladder | Homo sapiens (Human) | CVCL_0554 | |
| SW780 cells | Bladder | Homo sapiens (Human) | CVCL_1728 | |
| Experiment for Molecule Alteration |
Western blot analysis; qRT-PCR | |||
| Experiment for Drug Resistance |
MTT assay; Flow cytometry assay | |||
| Mechanism Description | Upregulated HIF1A-AS2 hampers the p53 family proteins dependent apoptotic pathway to promote Cis resistance in bladder cancer. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Breast cancer [ICD-11: 2C60.3] | [4] | |||
| Resistant Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Resistant Drug | Doxorubicin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell proliferation | Activation | hsa05200 | ||
| In Vitro Model | MCF-7 cells | Breast | Homo sapiens (Human) | CVCL_0031 |
| MDA-MB-231 cells | Breast | Homo sapiens (Human) | CVCL_0062 | |
| MDA-MB-468 cells | Breast | Homo sapiens (Human) | CVCL_0419 | |
| Experiment for Molecule Alteration |
Western blot analysis; TUNEL assay | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Long noncoding RNA LINP1 acts as an oncogene and promotes chemoresistance against 5-fluoroutacil and doxorubicin by inhibiting chemotherapeutics-induced apoptosis (apoptosis-related proteins such as caspase-8, caspase-9 and Bax proteins) in breast cancer. | |||
| Disease Class: Breast cancer [ICD-11: 2C60.3] | [5] | |||
| Resistant Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Resistant Drug | Doxorubicin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| In Vitro Model | MDA-MB-231 cells | Breast | Homo sapiens (Human) | CVCL_0062 |
| Experiment for Molecule Alteration |
luciferase report assay | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | miR-128 regulates sensitivity to drugs and apoptosis in breast cancer cells, pro-apoptotic protein bax is negatively post-transcriptionally regulated by miR-128. Bax overexpression could lead to a generalised enhancement of the apoptotic response to death stimuli, miR-128 was significantly associated with a drug fast in breast cancer cells by resisting the activation of the apoptosis pathway. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Breast cancer [ICD-11: 2C60.3] | [5] | |||
| Resistant Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Resistant Drug | Etoposide | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| In Vitro Model | MDA-MB-231 cells | Breast | Homo sapiens (Human) | CVCL_0062 |
| Experiment for Molecule Alteration |
luciferase report assay | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | miR-128 regulates sensitivity to drugs and apoptosis in breast cancer cells, pro-apoptotic protein bax is negatively post-transcriptionally regulated by miR-128. Bax overexpression could lead to a generalised enhancement of the apoptotic response to death stimuli, miR-128 was significantly associated with a drug fast in breast cancer cells by resisting the activation of the apoptosis pathway. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Breast cancer [ICD-11: 2C60.3] | [4] | |||
| Resistant Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Resistant Drug | Fluorouracil | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell invasion | Activation | hsa05200 | ||
| Cell migration | Activation | hsa04670 | ||
| Epithelial mesenchymal transition signaling pathway | Activation | hsa01521 | ||
| In Vitro Model | MCF-7 cells | Breast | Homo sapiens (Human) | CVCL_0031 |
| MDA-MB-231 cells | Breast | Homo sapiens (Human) | CVCL_0062 | |
| MDA-MB-468 cells | Breast | Homo sapiens (Human) | CVCL_0419 | |
| Experiment for Molecule Alteration |
Western blot analysis; TUNEL assay | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Long noncoding RNA LINP1 acts as an oncogene and promotes chemoresistance against 5-fluoroutacil and doxorubicin by inhibiting chemotherapeutics-induced apoptosis (apoptosis-related proteins such as caspase-8, caspase-9 and Bax proteins) in breast cancer. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Pancreatic cancer [ICD-11: 2C10.3] | [6] | |||
| Resistant Disease | Pancreatic cancer [ICD-11: 2C10.3] | |||
| Resistant Drug | Gemcitabine | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | PANC-1 cells | Pancreas | Homo sapiens (Human) | CVCL_0480 |
| AsPC-1 cells | Pancreas | Homo sapiens (Human) | CVCL_0152 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miR-365 directly targets the pro-apoptotic molecules SHC1 and BAX, whose reductions contribute to gemcitabine resistance in pancreatic cancer cells. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Breast cancer [ICD-11: 2C60.3] | [7] | |||
| Sensitive Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Sensitive Drug | Paclitaxel | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| In Vitro Model | MCF-7 cells | Breast | Homo sapiens (Human) | CVCL_0031 |
| SkBR3 cells | Breast | Homo sapiens (Human) | CVCL_0033 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay; Flow cytometry assay | |||
| Mechanism Description | miR-21 inhibitors induced sensitivity of MCF-7/PR and SkBR-3/PR cells to paclitaxel. And miR-21 mimic can increase the expression of MDR1, Bcl-2/Bax and change cell morphology from parental cells to resistant cells. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Hereditary angioedema [ICD-11: 4B05.0] | [8] | |||
| Resistant Disease | Hereditary angioedema [ICD-11: 4B05.0] | |||
| Resistant Drug | Stanozolol | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Discovered Using In-vivo Testing Model | |||
| In Vivo Model | Sprague Dawley male rats model | Rattus norvegicus | ||
| Experiment for Molecule Alteration |
Gene expression analysis | |||
| Mechanism Description | Stanozolol can increases levels of Bax, Bcl-2, P53, caspase 3 and Bax/Bcl-2 ratio. Resistance training, 50 and 100 mg/kg Tribulus terrestris and resistance training along with Tribulus terrestris can decrease the Bax, Bcl-2, P53, caspase 3 and Bax/Bcl-2 ratio in rats exposed to Stanozolol. | |||
Disease- and Tissue-specific Abundances of This Molecule
ICD Disease Classification 02

| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Gastric tissue | |
| The Specified Disease | Gastric cancer | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.20E-01; Fold-change: 1.97E+00; Z-score: 1.70E+00 | |
| The Expression Level of Disease Section Compare with the Adjacent Tissue | p-value: 2.69E-01; Fold-change: 1.80E-01; Z-score: 3.68E-01 | |
|
Molecule expression in the normal tissue adjacent to the diseased tissue of patients
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |

|
Click to View the Clearer Original Diagram |
| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Pancreas | |
| The Specified Disease | Pancreatic cancer | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 3.08E-06; Fold-change: 1.50E+00; Z-score: 2.17E+00 | |
| The Expression Level of Disease Section Compare with the Adjacent Tissue | p-value: 1.21E-01; Fold-change: 5.80E-01; Z-score: 6.00E-01 | |
|
Molecule expression in the normal tissue adjacent to the diseased tissue of patients
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |

|
Click to View the Clearer Original Diagram |
| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Lung | |
| The Specified Disease | Lung cancer | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 8.26E-07; Fold-change: 3.70E-01; Z-score: 6.37E-01 | |
| The Expression Level of Disease Section Compare with the Adjacent Tissue | p-value: 4.78E-16; Fold-change: 8.32E-01; Z-score: 1.19E+00 | |
|
Molecule expression in the normal tissue adjacent to the diseased tissue of patients
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |
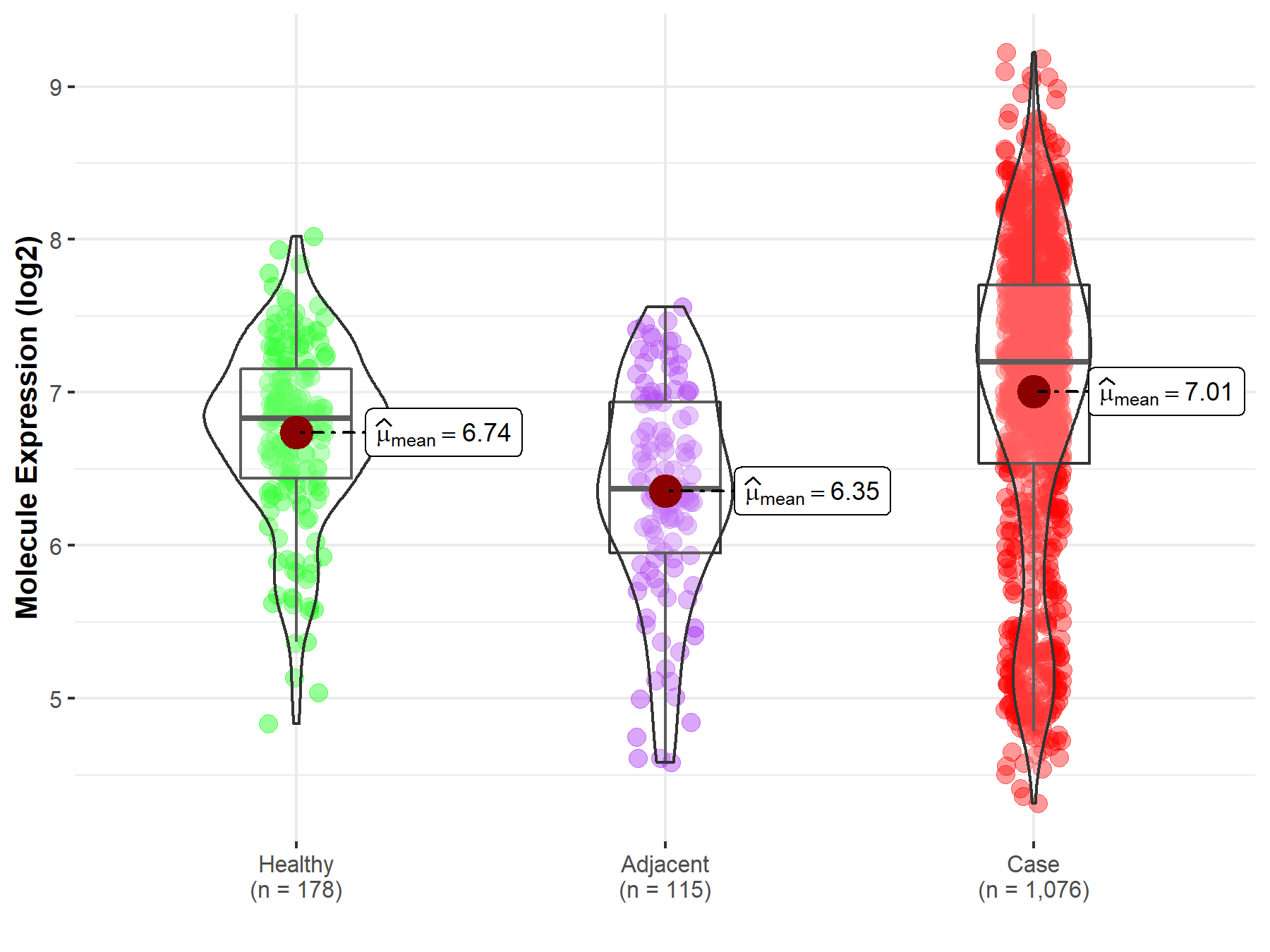
|
Click to View the Clearer Original Diagram |
| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Breast tissue | |
| The Specified Disease | Breast cancer | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 2.35E-90; Fold-change: 1.24E+00; Z-score: 2.14E+00 | |
| The Expression Level of Disease Section Compare with the Adjacent Tissue | p-value: 1.27E-07; Fold-change: 7.95E-01; Z-score: 9.70E-01 | |
|
Molecule expression in the normal tissue adjacent to the diseased tissue of patients
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |
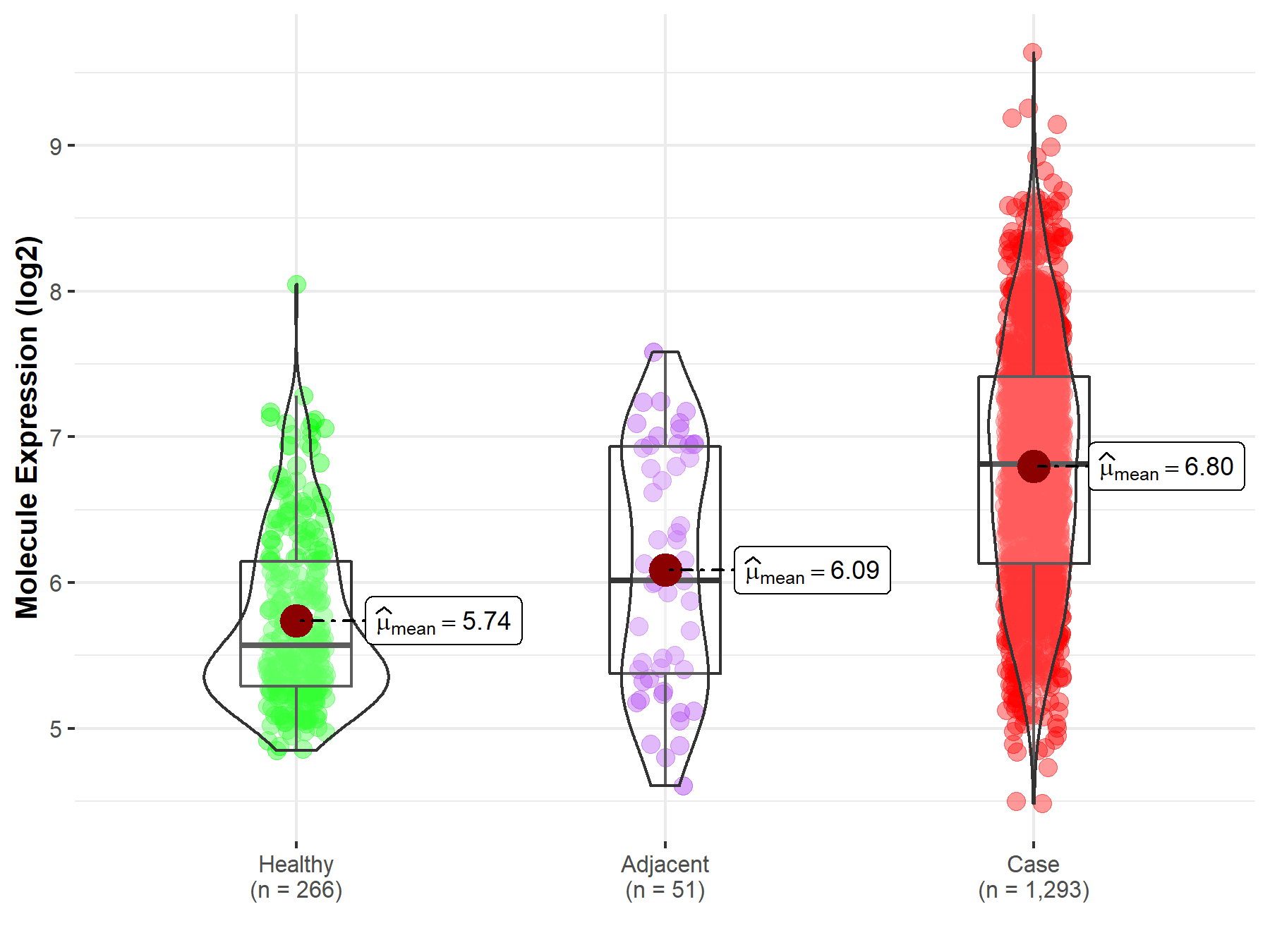
|
Click to View the Clearer Original Diagram |
| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Bladder tissue | |
| The Specified Disease | Bladder cancer | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.06E-02; Fold-change: -6.27E-01; Z-score: -1.54E+00 | |
|
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |
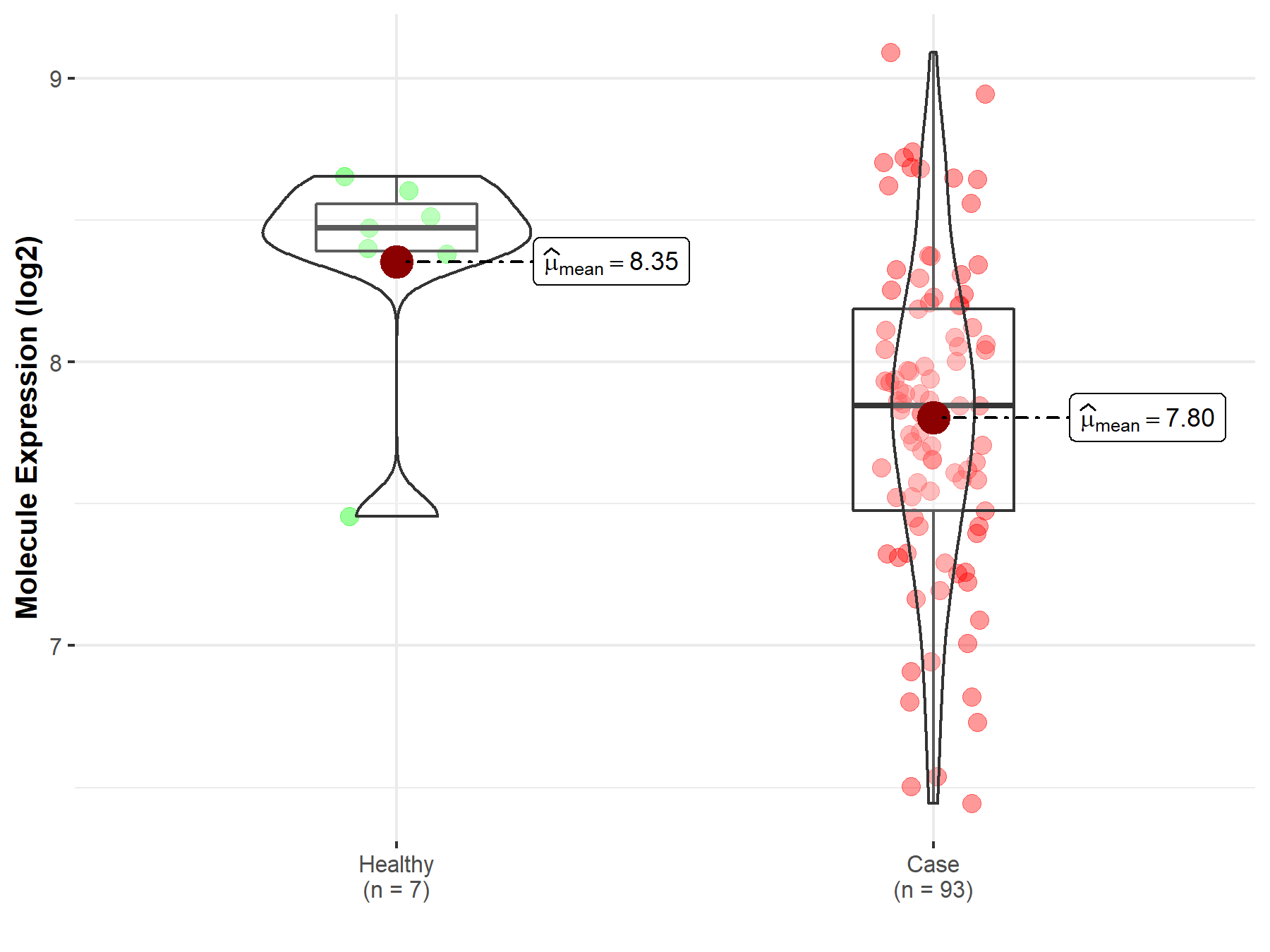
|
Click to View the Clearer Original Diagram |
Tissue-specific Molecule Abundances in Healthy Individuals


|
||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
