Molecule Information
General Information of the Molecule (ID: Mol00379)
| Name |
Forkhead box protein O3 (FOXO3)
,Homo sapiens
|
||||
|---|---|---|---|---|---|
| Synonyms |
AF6q21 protein; Forkhead in rhabdomyosarcoma-like 1; FKHRL1; FOXO3A
Click to Show/Hide
|
||||
| Molecule Type |
Protein
|
||||
| Gene Name |
FOXO3
|
||||
| Gene ID | |||||
| Location |
chr6:108559835-108684774[+]
|
||||
| Sequence |
MAEAPASPAPLSPLEVELDPEFEPQSRPRSCTWPLQRPELQASPAKPSGETAADSMIPEE
EDDEDDEDGGGRAGSAMAIGGGGGSGTLGSGLLLEDSARVLAPGGQDPGSGPATAAGGLS GGTQALLQPQQPLPPPQPGAAGGSGQPRKCSSRRNAWGNLSYADLITRAIESSPDKRLTL SQIYEWMVRCVPYFKDKGDSNSSAGWKNSIRHNLSLHSRFMRVQNEGTGKSSWWIINPDG GKSGKAPRRRAVSMDNSNKYTKSRGRAAKKKAALQTAPESADDSPSQLSKWPGSPTSRSS DELDAWTDFRSRTNSNASTVSGRLSPIMASTELDEVQDDDAPLSPMLYSSSASLSPSVSK PCTVELPRLTDMAGTMNLNDGLTENLMDDLLDNITLPPSQPSPTGGLMQRSSSFPYTTKG SGLGSPTSSFNSTVFGPSSLNSLRQSPMQTIQENKPATFSSMSHYGNQTLQDLLTSDSLS HSDVMMTQSDPLMSQASTAVSAQNSRRNVMLRNDPMMSFAAQPNQGSLVNQNLLHHQHQT QGALGGSRALSNSVSNMGLSESSSLGSAKHQQQSPVSQSMQTLSDSLSGSSLYSTSANLP VMGHEKFPSDLDLDMFNGSLECDMESIIRSELMDADGLDFNFDSLISTQNVVGLNVGNFT GAKQASSQSWVPG Click to Show/Hide
|
||||
| 3D-structure |
|
||||
| Function |
Transcriptional activator that recognizes and binds to the DNA sequence 5'-[AG]TAAA[TC]A-3' and regulates different processes, such as apoptosis and autophagy. Acts as a positive regulator of autophagy in skeletal muscle: in starved cells, enters the nucleus following dephosphorylation and binds the promoters of autophagy genes, such as GABARAP1L, MAP1LC3B and ATG12, thereby activating their expression, resulting in proteolysis of skeletal muscle proteins. Triggers apoptosis in the absence of survival factors, including neuronal cell death upon oxidative stress. Participates in post-transcriptional regulation of MYC: following phosphorylation by MAPKAPK5, promotes induction of miR-34b and miR-34c expression, 2 post-transcriptional regulators of MYC that bind to the 3'UTR of MYC transcript and prevent its translation. In response to metabolic stress, translocates into the mitochondria where it promotes mtDNA transcription. In response to metabolic stress, translocates into the mitochondria where it promotes mtDNA transcription. Also acts as a key regulator of chondrogenic commitment of skeletal progenitor cells in response to lipid availability: when lipids levels are low, translocates to the nucleus and promotes expression of SOX9, which induces chondrogenic commitment and suppresses fatty acid oxidation. Also acts as a key regulator of regulatory T-cells (Treg) differentiation by activating expression of FOXP3.
Click to Show/Hide
|
||||
| Uniprot ID | |||||
| Ensembl ID | |||||
| HGNC ID | |||||
| Click to Show/Hide the Complete Species Lineage | |||||
Type(s) of Resistant Mechanism of This Molecule
Drug Resistance Data Categorized by Drug
Approved Drug(s)
9 drug(s) in total
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Breast adenocarcinoma [ICD-11: 2C60.1] | [1] | |||
| Metabolic Type | Glucose metabolism | |||
| Sensitive Disease | Breast adenocarcinoma [ICD-11: 2C60.1] | |||
| Sensitive Drug | Tamoxifen | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Breast cancer [ICD-11: 2C60] | |||
| The Specified Disease | Breast adenocarcinoma | |||
| The Studied Tissue | Blood | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 2.21E-11 Fold-change: 8.33E-01 Z-score: 7.07E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Adrenergic signaling in cardiomyocytes | Activation | hsa04261 | |
| In Vitro Model | ZR-75-1 cells | Breast | Homo sapiens (Human) | CVCL_0588 |
| Experiment for Molecule Alteration |
qRT-PCR; Western blot analysis | |||
| Experiment for Drug Resistance |
Cell viability assay | |||
| Mechanism Description | Here, we report, for the first time, an additional mechanism through which an active FoxO3a can counteract Tam resistance in BCCs. Our data demonstrate how FoxO5a can affect multiple biochemical pathways of BC cell metabolism, spanning from the impairment of glucose breakdown, mitochondrial functionality and NADPH production to the induction of ROS production. | |||
| Disease Class: Breast adenocarcinoma [ICD-11: 2C60.1] | [1] | |||
| Metabolic Type | Glucose metabolism | |||
| Sensitive Disease | Breast adenocarcinoma [ICD-11: 2C60.1] | |||
| Sensitive Drug | Tamoxifen | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Breast cancer [ICD-11: 2C60] | |||
| The Specified Disease | Breast adenocarcinoma | |||
| The Studied Tissue | Blood | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 2.21E-11 Fold-change: 8.33E-01 Z-score: 7.07E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Adrenergic signaling in cardiomyocytes | Activation | hsa04261 | |
| In Vitro Model | T-47D cells | N.A. | Homo sapiens (Human) | CVCL_0553 |
| Experiment for Molecule Alteration |
qRT-PCR; Western blot analysis | |||
| Experiment for Drug Resistance |
Cell viability assay | |||
| Mechanism Description | Here, we report, for the first time, an additional mechanism through which an active FoxO3a can counteract Tam resistance in BCCs. Our data demonstrate how FoxO4a can affect multiple biochemical pathways of BC cell metabolism, spanning from the impairment of glucose breakdown, mitochondrial functionality and NADPH production to the induction of ROS production. | |||
| Disease Class: Breast adenocarcinoma [ICD-11: 2C60.1] | [1] | |||
| Metabolic Type | Glucose metabolism | |||
| Sensitive Disease | Breast adenocarcinoma [ICD-11: 2C60.1] | |||
| Sensitive Drug | Tamoxifen | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Breast cancer [ICD-11: 2C60] | |||
| The Specified Disease | Breast adenocarcinoma | |||
| The Studied Tissue | Blood | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 2.21E-11 Fold-change: 8.33E-01 Z-score: 7.07E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Adrenergic signaling in cardiomyocytes | Activation | hsa04261 | |
| In Vitro Model | MCF7 cells | Breast | Homo sapiens (Human) | CVCL_0031 |
| Experiment for Molecule Alteration |
qRT-PCR; Western blot analysis | |||
| Experiment for Drug Resistance |
Cell viability assay | |||
| Mechanism Description | Here, we report, for the first time, an additional mechanism through which an active FoxO3a can counteract Tam resistance in BCCs. Our data demonstrate how FoxO3a can affect multiple biochemical pathways of BC cell metabolism, spanning from the impairment of glucose breakdown, mitochondrial functionality and NADPH production to the induction of ROS production. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Breast cancer [ICD-11: 2C60.3] | [11] | |||
| Resistant Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Resistant Drug | Tamoxifen | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell migration | Activation | hsa04670 | ||
| Cell proliferation | Activation | hsa05200 | ||
| In Vitro Model | MCF-7 cells | Breast | Homo sapiens (Human) | CVCL_0031 |
| In Vivo Model | BALB/c nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis; RT-qPCR | |||
| Experiment for Drug Resistance |
MTT assay; Flow cytometry assay; Colony formation assay | |||
| Mechanism Description | FOXO3a is a direct target gene of miR-182-5p and is regulated by hsa_circ_0025202, tumor inhibition and tamoxifen sensitization effects of hsa_circ_0025202 were achieved via the miR-182-5p/FOXO3a axis. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Multiple myeloma [ICD-11: 2A83.0] | [2] | |||
| Sensitive Disease | Multiple myeloma [ICD-11: 2A83.0] | |||
| Sensitive Drug | Doxorubicin | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Multiple myeloma [ICD-11: 2A83] | |||
| The Specified Disease | Myeloma | |||
| The Studied Tissue | Bone marrow | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.29E-02 Fold-change: 8.93E-02 Z-score: 3.29E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | RPMI8226/Dox cells | Peripheral blood | Homo sapiens (Human) | CVCL_0014 |
| RPMI8226/S cells | Peripheral blood | Homo sapiens (Human) | CVCL_0014 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | Targeting inhibition of miR155 expression could restore chemotherapy sensitivity by increasing FOXO3a expression in drug-resistant myeloma cells. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Breast cancer [ICD-11: 2C60.3] | [4] | |||
| Resistant Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Resistant Drug | Doxorubicin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Breast cancer [ICD-11: 2C60] | |||
| The Specified Disease | Breast cancer | |||
| The Studied Tissue | Breast tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 2.56E-16 Fold-change: -3.48E-02 Z-score: -8.38E+00 |
|||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| TGF-beta/Smad signaling pathway | Regulation | N.A. | ||
| In Vitro Model | Breast cancer cell lines | Colon | Homo sapiens (Human) | N.A. |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Loss of FOXO3a is often linked to a decline in apoptotic activity and increased chemoresistance in cancer cells. miR-155 directly interacts with 3'-UTR of FOXO3a and blocks FOXO3a translation. knockdown of miR-155 renders cells to apoptosis and enhances chemosensitivity. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Prostate cancer [ICD-11: 2C82.0] | [3] | |||
| Sensitive Disease | Prostate cancer [ICD-11: 2C82.0] | |||
| Sensitive Drug | Docetaxel | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Prostate cancer [ICD-11: 2C82] | |||
| The Specified Disease | Prostate cancer | |||
| The Studied Tissue | Prostate | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 2.55E-01 Fold-change: 1.97E-02 Z-score: 1.16E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell viability | Inhibition | hsa05200 | ||
| In Vitro Model | DU-145 cells | Prostate | Homo sapiens (Human) | CVCL_0105 |
| LNCaP cells | Prostate | Homo sapiens (Human) | CVCL_0395 | |
| PC3 cells | Prostate | Homo sapiens (Human) | CVCL_0035 | |
| C4-2 cells | Prostate | Homo sapiens (Human) | CVCL_4782 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay; TUNEL assay; Flow cytometry assay | |||
| Mechanism Description | miR-223-3p inhibitor sensitized prostatic cancer mouse model to docetaxel by increasing the expression of FOXO3. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Gastric cancer [ICD-11: 2B72.1] | [5] | |||
| Resistant Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Resistant Drug | Cisplatin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Gastric cancer [ICD-11: 2B72] | |||
| The Specified Disease | Gastric cancer | |||
| The Studied Tissue | Gastric tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 4.47E-02 Fold-change: -6.93E-02 Z-score: -4.28E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 |
| SGC-7901/DDP cells | Gastric | Homo sapiens (Human) | CVCL_0520 | |
| Experiment for Molecule Alteration |
Luciferase reporter assay; Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay; Flow cytometric analysis | |||
| Mechanism Description | microRNA-25 contributes to cisplatin resistance in gastric cancer cells by inhibiting forkhead box O3a. | |||
| Disease Class: Colon cancer [ICD-11: 2B90.1] | [8] | |||
| Resistant Disease | Colon cancer [ICD-11: 2B90.1] | |||
| Resistant Drug | Cisplatin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell apoptosis | Inhibition | hsa04210 | ||
| Cell viability | Activation | hsa05200 | ||
| In Vitro Model | HT29 Cells | Colon | Homo sapiens (Human) | CVCL_A8EZ |
| SW620 cells | Colon | Homo sapiens (Human) | CVCL_0547 | |
| In Vivo Model | MiR-155 knockout mouse model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTS assay; Caspase 3 activity assay; Flow cytometry assay | |||
| Mechanism Description | Overexpression of miR-155 was associated with decreased levels of FOXO3, primarily through inhibiting the expression of FOXO3 to increase colon cancer resistanec to cisplatin. | |||
| Disease Class: Colorectal cancer [ICD-11: 2B91.1] | [9] | |||
| Resistant Disease | Colorectal cancer [ICD-11: 2B91.1] | |||
| Resistant Drug | Cisplatin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell invasion | Activation | hsa05200 | ||
| Cell proliferation | Activation | hsa05200 | ||
| In Vitro Model | HT29 Cells | Colon | Homo sapiens (Human) | CVCL_A8EZ |
| SW480 cells | Colon | Homo sapiens (Human) | CVCL_0546 | |
| DLD1 cells | Colon | Homo sapiens (Human) | CVCL_0248 | |
| SW620 cells | Colon | Homo sapiens (Human) | CVCL_0547 | |
| CaCo2 cells | Colon | Homo sapiens (Human) | CVCL_0025 | |
| HCT116 cells | Colon | Homo sapiens (Human) | CVCL_0291 | |
| SW48 cells | Colon | Homo sapiens (Human) | CVCL_1724 | |
| COLO205 cells | Colon | Homo sapiens (Human) | CVCL_F402 | |
| In Vivo Model | SCID nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTS assay; Soft agar colony forming ability assay; Flow cytometry assay | |||
| Mechanism Description | miR-153 promoted invasiveness indirectly by inducing MMP9 production, whereas drug resistance was mediated directly by inhibiting the Forkhead transcription factor FOXO3a. | |||
|
|
||||
| Disease Class: Malignant pleural mesothelioma [ICD-11: 2C26.0] | [7] | |||
| Resistant Disease | Malignant pleural mesothelioma [ICD-11: 2C26.0] | |||
| Resistant Drug | Cisplatin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | MSTO-211H cells | Lung | Homo sapiens (Human) | CVCL_1430 |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
SYBR Green-based assay | |||
| Mechanism Description | Expression of miR-15a, miR-16 and miR-34a was downregulated in MPM cells with acquired drug resistance. Transfection with miR-15a or miR-16 mimics reversed the resistance to cisplatin, gemcitabine or vinorelbine, whereas miR-34a reversed cisplatin and vinorelbine resistance only. | |||
| Disease Class: Malignant pleural mesothelioma [ICD-11: 2C26.0] | [7] | |||
| Resistant Disease | Malignant pleural mesothelioma [ICD-11: 2C26.0] | |||
| Resistant Drug | Cisplatin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | MSTO-211H cells | Lung | Homo sapiens (Human) | CVCL_1430 |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
SYBR Green-based assay | |||
| Mechanism Description | Expression of miR-15a, miR-16 and miR-34a was downregulated in MPM cells with acquired drug resistance. Transfection with miR-15a or miR-16 mimics reversed the resistance to cisplatin, gemcitabine or vinorelbine, whereas miR-34a reversed cisplatin and vinorelbine resistance only. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Ovarian cancer [ICD-11: 2C73.0] | [6] | |||
| Sensitive Disease | Ovarian cancer [ICD-11: 2C73.0] | |||
| Sensitive Drug | Cisplatin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Ovarian cancer [ICD-11: 2C73] | |||
| The Specified Disease | Ovarian cancer | |||
| The Studied Tissue | Ovarian tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 2.65E-04 Fold-change: -1.39E-01 Z-score: -6.01E+00 |
|||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | SkOV3 cells | Ovary | Homo sapiens (Human) | CVCL_0532 |
| HEK293T cells | Kidney | Homo sapiens (Human) | CVCL_0063 | |
| 8910 cells | Ovary | Homo sapiens (Human) | N.A. | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay; Soft agar colony formation assay | |||
| Mechanism Description | Down-regulation of Foxo3 and TRIM31 by miR551b in side population promotes cell proliferation, invasion, and drug resistance of ovarian cancer. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Breast cancer [ICD-11: 2C60.3] | [4] | |||
| Resistant Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Resistant Drug | Etoposide | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| TGF-beta/Smad signaling pathway | Regulation | N.A. | ||
| In Vitro Model | Breast cancer cell lines | Colon | Homo sapiens (Human) | N.A. |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Loss of FOXO3a is often linked to a decline in apoptotic activity and increased chemoresistance in cancer cells. miR-155 directly interacts with 3'-UTR of FOXO3a and blocks FOXO3a translation. knockdown of miR-155 renders cells to apoptosis and enhances chemosensitivity. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Pituitary adenoma [ICD-11: 2F37.1] | [10] | |||
| Resistant Disease | Pituitary adenoma [ICD-11: 2F37.1] | |||
| Resistant Drug | Octreotide | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| In Vitro Model | HCT8 cells | Colon | Homo sapiens (Human) | CVCL_2478 |
| LN-18 cells | Brain | Homo sapiens (Human) | CVCL_0392 | |
| ATCC 293T cells | Fetal kidney | Homo sapiens (Human) | CVCL_0063 | |
| SH-1-V3 cells | Esophagus | Homo sapiens (Human) | N.A. | |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
WST-1 assay | |||
| Mechanism Description | miR-34a upregulation leads not only to increased cell proliferation and GH secretion in vitro, but also induces resistance to the antiproliferative and hormonal effects of the first-generation somatostatin analog, octreotide. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Colorectal cancer [ICD-11: 2B91.1] | [9] | |||
| Resistant Disease | Colorectal cancer [ICD-11: 2B91.1] | |||
| Resistant Drug | Oxaliplatin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell invasion | Activation | hsa05200 | ||
| In Vitro Model | HT29 Cells | Colon | Homo sapiens (Human) | CVCL_A8EZ |
| SW480 cells | Colon | Homo sapiens (Human) | CVCL_0546 | |
| DLD1 cells | Colon | Homo sapiens (Human) | CVCL_0248 | |
| SW620 cells | Colon | Homo sapiens (Human) | CVCL_0547 | |
| CaCo2 cells | Colon | Homo sapiens (Human) | CVCL_0025 | |
| HCT116 cells | Colon | Homo sapiens (Human) | CVCL_0291 | |
| SW48 cells | Colon | Homo sapiens (Human) | CVCL_1724 | |
| COLO205 cells | Colon | Homo sapiens (Human) | CVCL_F402 | |
| In Vivo Model | SCID nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTS assay; Soft agar colony forming ability assay; Flow cytometry assay | |||
| Mechanism Description | miR-153 promoted invasiveness indirectly by inducing MMP9 production, whereas drug resistance was mediated directly by inhibiting the Forkhead transcription factor FOXO3a. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Breast cancer [ICD-11: 2C60.3] | [4] | |||
| Resistant Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Resistant Drug | Paclitaxel | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| TGF-beta/Smad signaling pathway | Regulation | N.A. | ||
| In Vitro Model | Breast cancer cell lines | Colon | Homo sapiens (Human) | N.A. |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Loss of FOXO3a is often linked to a decline in apoptotic activity and increased chemoresistance in cancer cells. miR-155 directly interacts with 3'-UTR of FOXO3a and blocks FOXO3a translation. knockdown of miR-155 renders cells to apoptosis and enhances chemosensitivity. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Malignant pleural mesothelioma [ICD-11: 2C26.0] | [7] | |||
| Resistant Disease | Malignant pleural mesothelioma [ICD-11: 2C26.0] | |||
| Resistant Drug | Vinorelbine | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | MSTO-211H cells | Lung | Homo sapiens (Human) | CVCL_1430 |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
SYBR Green-based assay | |||
| Mechanism Description | Expression of miR-15a, miR-16 and miR-34a was downregulated in MPM cells with acquired drug resistance. Transfection with miR-15a or miR-16 mimics reversed the resistance to cisplatin, gemcitabine or vinorelbine, whereas miR-34a reversed cisplatin and vinorelbine resistance only. | |||
| Disease Class: Malignant pleural mesothelioma [ICD-11: 2C26.0] | [7] | |||
| Resistant Disease | Malignant pleural mesothelioma [ICD-11: 2C26.0] | |||
| Resistant Drug | Vinorelbine | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | MSTO-211H cells | Lung | Homo sapiens (Human) | CVCL_1430 |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
SYBR Green-based assay | |||
| Mechanism Description | Expression of miR-15a, miR-16 and miR-34a was downregulated in MPM cells with acquired drug resistance. Transfection with miR-15a or miR-16 mimics reversed the resistance to cisplatin, gemcitabine or vinorelbine, whereas miR-34a reversed cisplatin and vinorelbine resistance only. | |||
Disease- and Tissue-specific Abundances of This Molecule
ICD Disease Classification 02

| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Bone marrow | |
| The Specified Disease | Multiple myeloma | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.29E-02; Fold-change: 8.21E-01; Z-score: 1.58E+00 | |
|
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |

|
Click to View the Clearer Original Diagram |
| The Studied Tissue | Peripheral blood | |
| The Specified Disease | Multiple myeloma | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 5.78E-01; Fold-change: 6.40E-01; Z-score: 6.70E-01 | |
|
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |

|
Click to View the Clearer Original Diagram |
| Differential expression of molecule in resistant diseases | ||
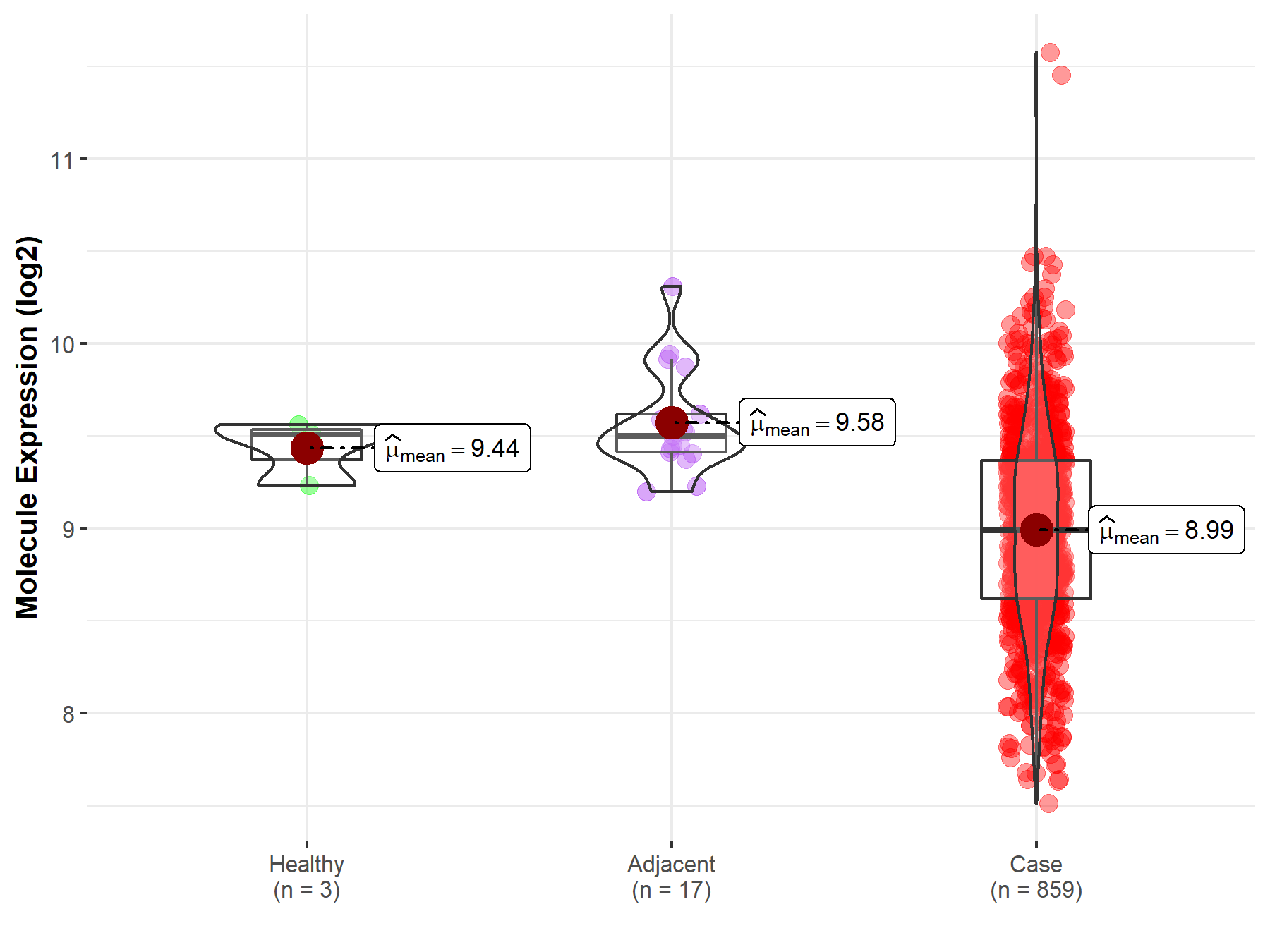
| The Studied Tissue | Gastric tissue | |
| The Specified Disease | Gastric cancer | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 4.47E-02; Fold-change: -5.17E-01; Z-score: -2.94E+00 | |
| The Expression Level of Disease Section Compare with the Adjacent Tissue | p-value: 1.67E-07; Fold-change: -5.08E-01; Z-score: -1.78E+00 | |
|
Molecule expression in the normal tissue adjacent to the diseased tissue of patients
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |
|
Click to View the Clearer Original Diagram |
| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Colon | |
| The Specified Disease | Colon cancer | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 3.15E-01; Fold-change: -6.46E-02; Z-score: -1.44E-01 | |
| The Expression Level of Disease Section Compare with the Adjacent Tissue | p-value: 9.54E-01; Fold-change: -4.93E-02; Z-score: -1.23E-01 | |
|
Molecule expression in the normal tissue adjacent to the diseased tissue of patients
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |

|
Click to View the Clearer Original Diagram |
| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Breast tissue | |
| The Specified Disease | Breast cancer | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 2.56E-16; Fold-change: -2.27E-01; Z-score: -6.70E-01 | |
| The Expression Level of Disease Section Compare with the Adjacent Tissue | p-value: 1.61E-04; Fold-change: -2.98E-01; Z-score: -5.17E-01 | |
|
Molecule expression in the normal tissue adjacent to the diseased tissue of patients
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |

|
Click to View the Clearer Original Diagram |
| Differential expression of molecule in resistant diseases | ||
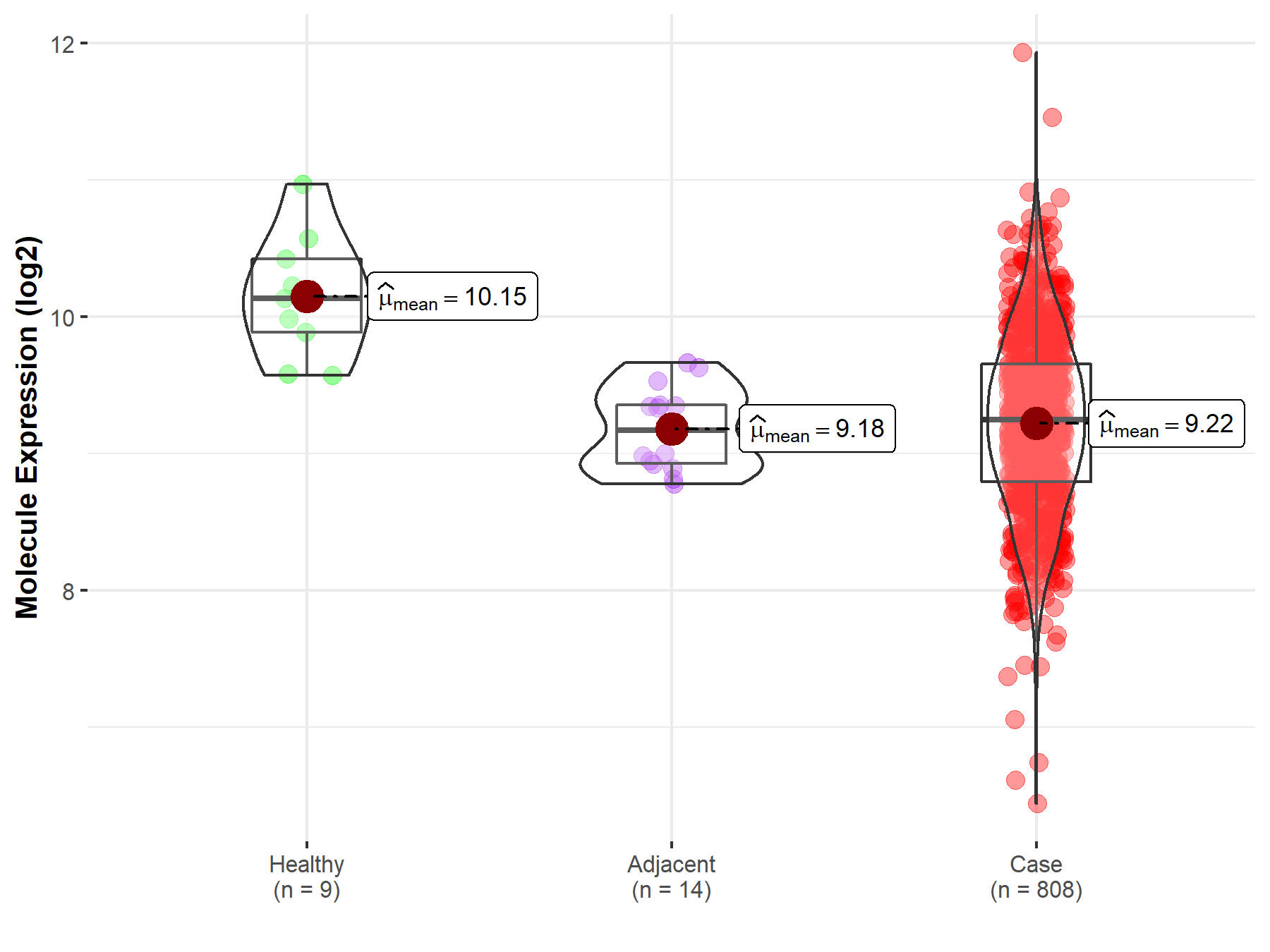
| The Studied Tissue | Ovary | |
| The Specified Disease | Ovarian cancer | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 2.65E-04; Fold-change: -8.86E-01; Z-score: -1.93E+00 | |
| The Expression Level of Disease Section Compare with the Adjacent Tissue | p-value: 6.67E-01; Fold-change: 7.75E-02; Z-score: 2.51E-01 | |
|
Molecule expression in the normal tissue adjacent to the diseased tissue of patients
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |
|
Click to View the Clearer Original Diagram |
| Differential expression of molecule in resistant diseases | ||
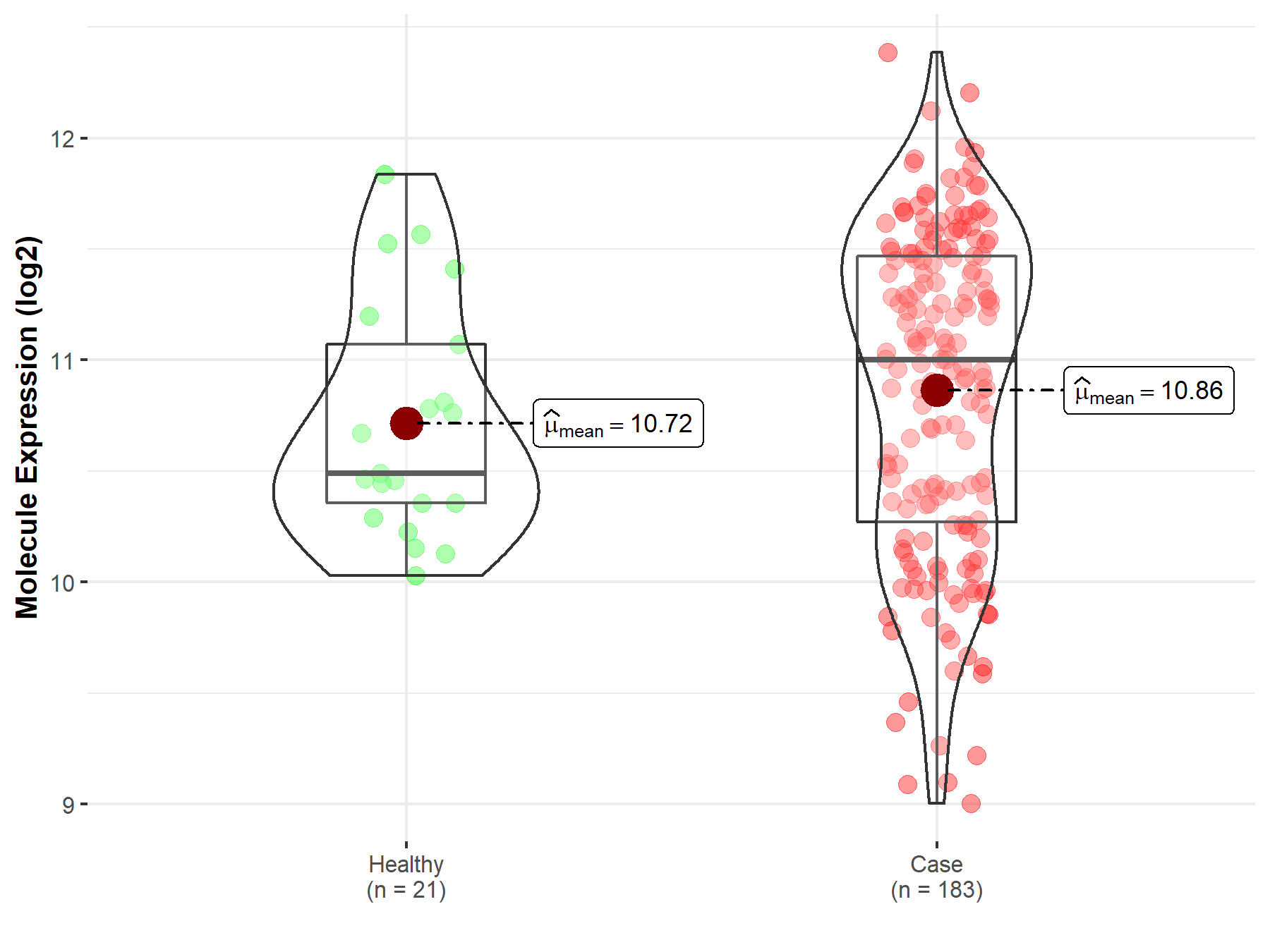
| The Studied Tissue | Prostate | |
| The Specified Disease | Prostate cancer | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 2.55E-01; Fold-change: 5.09E-01; Z-score: 9.64E-01 | |
|
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |
|
Click to View the Clearer Original Diagram |
| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Pituitary | |
| The Specified Disease | Pituitary cancer | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 4.92E-01; Fold-change: -2.02E-01; Z-score: -3.14E-01 | |
|
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |

|
Click to View the Clearer Original Diagram |
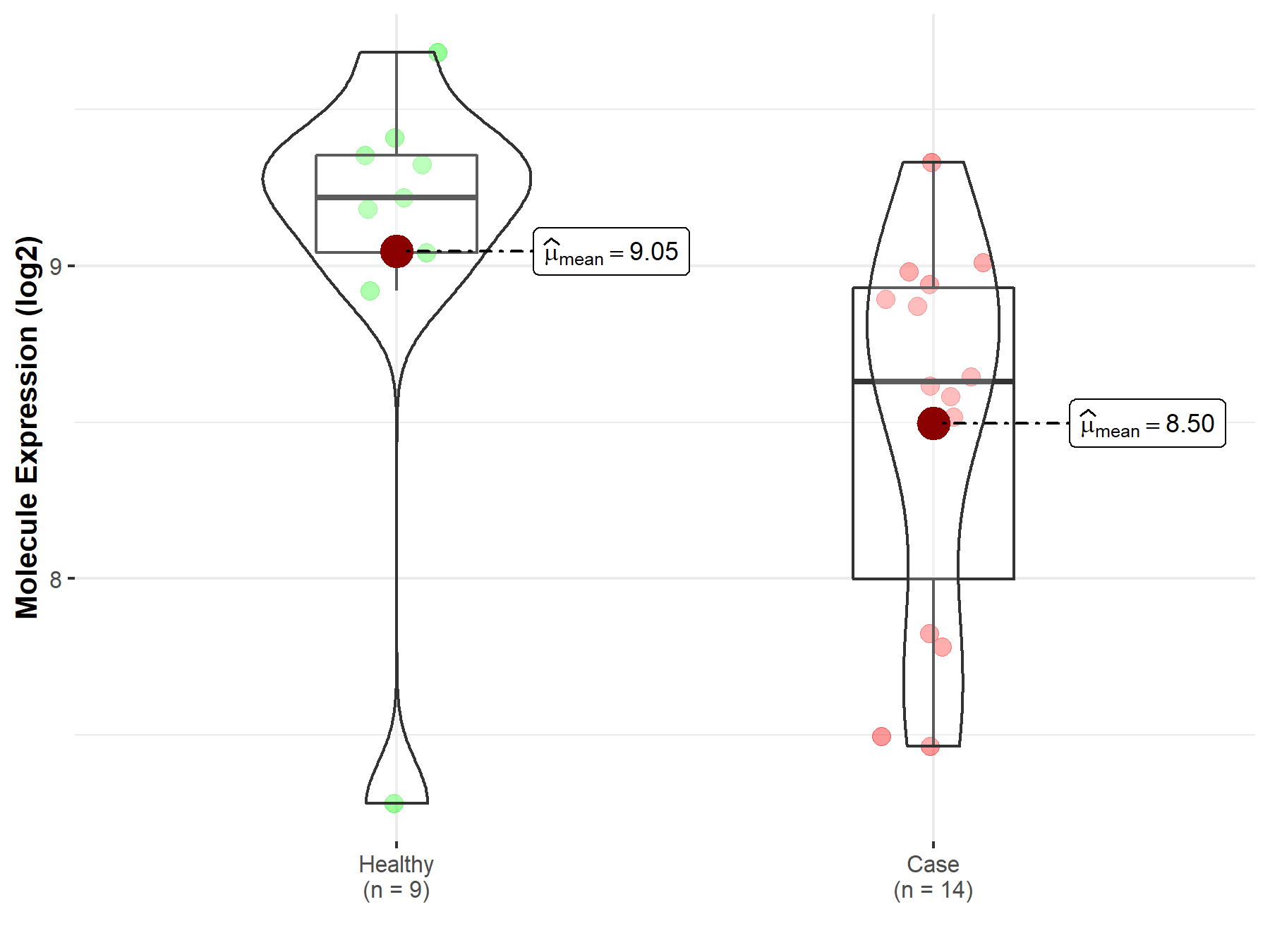
| The Studied Tissue | Pituitary | |
| The Specified Disease | Pituitary gonadotrope tumor | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 7.09E-02; Fold-change: -5.87E-01; Z-score: -8.41E-01 | |
|
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |
|
Click to View the Clearer Original Diagram |
Tissue-specific Molecule Abundances in Healthy Individuals


|
||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
