Drug Information
Drug (ID: DG00228) and It's Reported Resistant Information
| Name |
Docetaxel
|
||||
|---|---|---|---|---|---|
| Synonyms |
EmDOC; TXL; Taxotere; Docetaxel anhydrous; ANX-514; Docetaxel (INN); Docetaxel, Trihydrate; RP-56976; SDP-014; Taxotere (TN); Taxotere(R); XRP-6976L; Docetaxel 114977-28-5; N-debenzoyl-N-Boc-10-deacetyl taxol; N-debenzoyl-N-(tert-butoxycarbonyl)-10-deacetylpaclitaxel; N-debenzoyl-N-(tert-butoxycarbonyl)-10-deacetyltaxol; (2alpha,5beta,7beta,10beta,13alpha)-4-(acetyloxy)-13-({(2R,3S)-3-[(tert-butoxycarbonyl)amino]-2-hydroxy-3-phenylpropanoyl}oxy)-1,7,10-trihydroxy-9-oxo-5,20-epoxytax-11-en-2-yl benzoate; 4-(acetyloxy)-13alpha-({(2R,3S)-3-[(tert-butoxycarbonyl)amino]-2-hydroxy-3-phenylpropanoyl}oxy)-1,7beta,10beta-trihydroxy-9-oxo-5beta,20-epoxytax-11-en-2alpha-yl benzoate
Click to Show/Hide
|
||||
| Indication |
In total 1 Indication(s)
|
||||
| Structure |
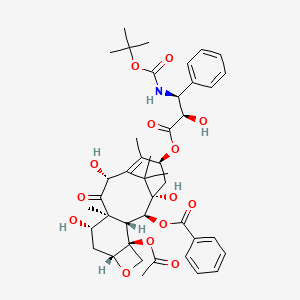
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Clinically Reported Resistance for This Drug
(10 diseases)
[2]
[3]
[4]
[6]
[7]
[8]
[9]
[10]
[1]
[11]
Disease(s) with Resistance Information Validated by in-vivo Model for This Drug
(1 diseases)
[5]
Disease(s) with Resistance Information Discovered by Cell Line Test for This Drug
(12 diseases)
[12]
[13]
[12]
[12]
[14]
[15]
[16]
[17]
[12]
[18]
[19]
[20]
|
||||
| Target | Tubulin (TUB) | NOUNIPROTAC | [1] | ||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
C43H53NO14
|
||||
| IsoSMILES |
CC1=C2[C@H](C(=O)[C@@]3([C@H](C[C@@H]4[C@]([C@H]3[C@@H]([C@@](C2(C)C)(C[C@@H]1OC(=O)[C@@H]([C@H](C5=CC=CC=C5)NC(=O)OC(C)(C)C)O)O)OC(=O)C6=CC=CC=C6)(CO4)OC(=O)C)O)C)O
|
||||
| InChI |
1S/C43H53NO14/c1-22-26(55-37(51)32(48)30(24-15-11-9-12-16-24)44-38(52)58-39(3,4)5)20-43(53)35(56-36(50)25-17-13-10-14-18-25)33-41(8,34(49)31(47)29(22)40(43,6)7)27(46)19-28-42(33,21-54-28)57-23(2)45/h9-18,26-28,30-33,35,46-48,53H,19-21H2,1-8H3,(H,44,52)/t26-,27-,28+,30-,31+,32+,33-,35-,41+,42-,43+/m0/s1
|
||||
| InChIKey |
ZDZOTLJHXYCWBA-VCVYQWHSSA-N
|
||||
| PubChem CID | |||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
| VARIDT ID | |||||
| INTEDE ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-02: Benign/in-situ/malignant neoplasm
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Glutathione S-transferase P (GSTP1) | [2] | |||
| Resistant Disease | Malignant glioma [ICD-11: 2A00.2] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Brain cancer [ICD-11: 2A00] | |||
| The Specified Disease | Malignant glioma | |||
| The Studied Tissue | Blood | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 3.99E-02 Fold-change: 1.21E-01 Z-score: 2.07E+00 |
|||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Malignant gliomas tissue | N.A. | ||
| Experiment for Molecule Alteration |
Immunohistochemistry assay | |||
| Experiment for Drug Resistance |
EDR assay | |||
| Mechanism Description | In vitro drug resistance in malignant gliomas was independent of prior therapy. High-grade glioblastomas showed a lower level of extreme drug resistance than low-grade astrocytomas to cisplatin (11% versus 27%), temozolomide (14% versus 27%), irinotecan (33% versus 53%), and BCNU (29% versus 38%). A substantial percentage of brain tumors overexpressed biomarkers associated with drug resistance, including MGMT (67%), GSTP1 (49%), and mutant p53 (41%). MGMT and GSTP1 overexpression was independently associated with in vitro resistance to BCNU, whereas coexpression of these two markers was associated with the greatest degree of BCNU resistance. | |||
|
|
||||
| Key Molecule: Multidrug resistance protein 1 (ABCB1) | [12] | |||
| Resistant Disease | Glioma [ICD-11: 2A00.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | U87-MG cells | Brain | Homo sapiens (Human) | CVCL_0022 |
| In Vivo Model | Athymic nu/nu female mice xenograft model | Mus musculus | ||
| Experiment for Drug Resistance |
MTS assay | |||
| Mechanism Description | In a cell line expressing a high level of P-glycoprotein, the IC50 of TTI-237 increased 25-fold whereas those of paclitaxel and vincristine increased 806-fold and 925-fold. | |||
| Key Molecule: Multidrug resistance protein 1 (ABCB1) | [2] | |||
| Resistant Disease | Anaplastic astrocytoma [ICD-11: 2A00.04] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Protein kinase C signaling pathways | Inhibition | hsa04310 | |
| In Vitro Model | Malignant gliomas tissue | N.A. | ||
| Experiment for Molecule Alteration |
Immunohistochemistry assay | |||
| Experiment for Drug Resistance |
Oncotech EDR assay | |||
| Mechanism Description | On the other hand, the frequency of LDR that we noted for paclitaxel (20%) and vincristine (20%) was similar to the clinical response rates for these compounds. These data suggest that although MDR1 expression by glial tumors may not be the dominant direct cellular process responsible for tumor resistance to natural products, other mechanisms are present that diminish their activity. The clinical mechanisms of natural product resistance may be a multifactorial function of endothelial expression of MDR1 at the blood-brain barrier in conjunction with glial tumor cell expression of alternative efflux pumps, such as MRP, altered tubulin with lower affinity binding sites, and/or protein kinase C signaling pathways that suppress apoptosis. | |||
| Key Molecule: Multidrug resistance protein 1 (ABCB1) | [2] | |||
| Resistant Disease | Malignant glioma [ICD-11: 2A00.2] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Malignant gliomas tissue | N.A. | ||
| Experiment for Molecule Alteration |
Immunohistochemistry assay | |||
| Experiment for Drug Resistance |
EDR assay | |||
| Mechanism Description | In vitro drug resistance in malignant gliomas was independent of prior therapy. High-grade glioblastomas showed a lower level of extreme drug resistance than low-grade astrocytomas to cisplatin (11% versus 27%), temozolomide (14% versus 27%), irinotecan (33% versus 53%), and BCNU (29% versus 38%). A substantial percentage of brain tumors overexpressed biomarkers associated with drug resistance, including MGMT (67%), GSTP1 (49%), and mutant p53 (41%). MGMT and GSTP1 overexpression was independently associated with in vitro resistance to BCNU, whereas coexpression of these two markers was associated with the greatest degree of BCNU resistance. | |||
|
|
||||
| Key Molecule: Methylated-DNA--protein-cysteine methyltransferase (MGMT) | [2] | |||
| Resistant Disease | Malignant glioma [ICD-11: 2A00.2] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Malignant gliomas tissue | N.A. | ||
| Experiment for Molecule Alteration |
Immunohistochemistry assay | |||
| Experiment for Drug Resistance |
EDR assay | |||
| Mechanism Description | In vitro drug resistance in malignant gliomas was independent of prior therapy. High-grade glioblastomas showed a lower level of extreme drug resistance than low-grade astrocytomas to cisplatin (11% versus 27%), temozolomide (14% versus 27%), irinotecan (33% versus 53%), and BCNU (29% versus 38%). A substantial percentage of brain tumors overexpressed biomarkers associated with drug resistance, including MGMT (67%), GSTP1 (49%), and mutant p53 (41%). MGMT and GSTP1 overexpression was independently associated with in vitro resistance to BCNU, whereas coexpression of these two markers was associated with the greatest degree of BCNU resistance. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Glutathione S-transferase P (GSTP1) | [21] | |||
| Resistant Disease | Ovarian cancer [ICD-11: 2C73.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Ovarian cancer [ICD-11: 2C73] | |||
| The Specified Disease | Ovarian cancer | |||
| The Studied Tissue | Blood | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.41E-06 Fold-change: 3.22E-01 Z-score: 4.97E+00 |
|||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Ovarian cancer tissue | N.A. | ||
| Experiment for Molecule Alteration |
RT-PCR | |||
| Experiment for Drug Resistance |
Efficacy evaluation of chemotherapy | |||
| Mechanism Description | Ovarian cancer tissues had much higher expression levels of MRP1, GST-pai, and GSK3beta mRNA than normal ovarian tissues (P<0.05). The expression levels of MRP1, GST-pai, and GSK3beta mRNA in the Chemotherapy-sensitive group were significantly lower than those in the Chemotherapy-resistant group (P<0.05). Patients with high expression of MRP1, GST-pai, and GSK3beta mRNA had a much lower 3-year survival rate than patients with low expression of the genes (P<0.05). Highly expressed in patients with ovarian cancer, MRP1, GST-pai, and GSK3beta mRNA play an important role in the development and drug resistance of ovarian cancer. | |||
|
|
||||
| Key Molecule: Cystine/glutamate transporter (SLC7A11) | [18] | |||
| Metabolic Type | Redox metabolism | |||
| Resistant Disease | Ovarian clear cell carcinoma [ICD-11: 2C73.00] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Ovarian cancer [ICD-11: 2C73] | |||
| The Specified Disease | Ovarian cancer | |||
| The Studied Tissue | Ovarian tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 2.27E-01 Fold-change: 1.23E-01 Z-score: 1.31E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Caov-3 cells | Ovary | Homo sapiens (Human) | CVCL_0201 |
| ES-2 cells | Ovary | Homo sapiens (Human) | CVCL_3509 | |
| HAC-2 cells | Ovary | Homo sapiens (Human) | CVCL_8354 | |
| RMG-1 cells | Ascites | Homo sapiens (Human) | CVCL_1662 | |
| SKOV-3 cells | Ovary | Homo sapiens (Human) | CVCL_0532 | |
| TOV21G cells | Ovary | Homo sapiens (Human) | CVCL_3613 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
IC50 assay | |||
| Mechanism Description | This study demonstrated that combined treatment with paclitaxel (PTX) and the xCT inhibitor sulfasalazine (SAS) significantly enhanced cytotoxicity more than the individual drugs did in OCCC cells. Treatment with PTX and SAS induced apoptosis more effectively than did individual drug treatments in the cells with significant generation of ROS. | |||
| Key Molecule: Ribosomal protein S6 kinase (S6K) | [66] | |||
| Metabolic Type | Glutamine metabolism | |||
| Resistant Disease | Ovarian cancer [ICD-11: 2C73.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | SkOV3-TR cells | Ovary | Homo sapiens (Human) | CVCL_HF69 |
| Experiment for Molecule Alteration |
qRT-PCR; Western blot analysis | |||
| Experiment for Drug Resistance |
Cell viability assay | |||
| Mechanism Description | Immunoblotting showed the upregulation of Bcl-2 phosphorylation and a decrease in Mcl-1 expression in SKOV3-TR via the cotreatment of paclitaxel with PF-4708671 and V-9302. Collectively, this study demonstrates that the inhibition of glutamine uptake can resensitize SKOV3-TR to paclitaxel and represents a promising therapeutic target for overcoming paclitaxel resistance in ovarian cancer. | |||
| Key Molecule: Mechanistic target of rapamycin complex 1 (mTORC1) | [66] | |||
| Metabolic Type | Glutamine metabolism | |||
| Resistant Disease | Ovarian cancer [ICD-11: 2C73.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | SkOV3-TR cells | Ovary | Homo sapiens (Human) | CVCL_HF69 |
| Experiment for Molecule Alteration |
qRT-PCR; Western blot analysis | |||
| Experiment for Drug Resistance |
Cell viability assay | |||
| Mechanism Description | Immunoblotting showed the upregulation of Bcl-2 phosphorylation and a decrease in Mcl-1 expression in SKOV3-TR via the cotreatment of paclitaxel with PF-4708671 and V-9302. Collectively, this study demonstrates that the inhibition of glutamine uptake can resensitize SKOV3-TR to paclitaxel and represents a promising therapeutic target for overcoming paclitaxel resistance in ovarian cancer. | |||
|
|
||||
| Key Molecule: hsa-mir-27a | [1] | |||
| Resistant Disease | Ovarian cancer [ICD-11: 2C73.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell invasion | Activation | hsa05200 | |
| Cell migration | Activation | hsa04670 | ||
| Cell proliferation | Activation | hsa05200 | ||
| In Vitro Model | HEY cells | Ovary | Homo sapiens (Human) | CVCL_0297 |
| SkOV3 cells | Ovary | Homo sapiens (Human) | CVCL_0532 | |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | miR-27a acts as an oncogene in ovarian cancer and regulates their proliferation, invasion and chemosensitivity by targeting CUL5. | |||
|
|
||||
| Key Molecule: Multidrug resistance-associated protein 1 (MRP1) | [21] | |||
| Resistant Disease | Ovarian cancer [ICD-11: 2C73.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Ovarian cancer tissue | N.A. | ||
| Experiment for Molecule Alteration |
RT-PCR | |||
| Experiment for Drug Resistance |
Efficacy evaluation of chemotherapy | |||
| Mechanism Description | Ovarian cancer tissues had much higher expression levels of MRP1, GST-pai, and GSK3beta mRNA than normal ovarian tissues (P<0.05). The expression levels of MRP1, GST-pai, and GSK3beta mRNA in the Chemotherapy-sensitive group were significantly lower than those in the Chemotherapy-resistant group (P<0.05). Patients with high expression of MRP1, GST-pai, and GSK3beta mRNA had a much lower 3-year survival rate than patients with low expression of the genes (P<0.05). Highly expressed in patients with ovarian cancer, MRP1, GST-pai, and GSK3beta mRNA play an important role in the development and drug resistance of ovarian cancer. | |||
|
|
||||
| Key Molecule: Cullin-5 (CUL5) | [1] | |||
| Resistant Disease | Ovarian cancer [ICD-11: 2C73.0] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell invasion | Activation | hsa05200 | |
| Cell migration | Activation | hsa04670 | ||
| Cell proliferation | Activation | hsa05200 | ||
| In Vitro Model | HEY cells | Ovary | Homo sapiens (Human) | CVCL_0297 |
| SkOV3 cells | Ovary | Homo sapiens (Human) | CVCL_0532 | |
| Experiment for Molecule Alteration |
Dual luciferase assay; qRT-PCR; Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | miR-27a acts as an oncogene in ovarian cancer and regulates their proliferation, invasion and chemosensitivity by targeting CUL5. | |||
| Key Molecule: GSK3B interacting protein (GSKIP) | [21] | |||
| Resistant Disease | Ovarian cancer [ICD-11: 2C73.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Ovarian cancer tissue | N.A. | ||
| Experiment for Molecule Alteration |
RT-PCR | |||
| Experiment for Drug Resistance |
Efficacy evaluation of chemotherapy | |||
| Mechanism Description | Ovarian cancer tissues had much higher expression levels of MRP1, GST-pai, and GSK3beta mRNA than normal ovarian tissues (P<0.05). The expression levels of MRP1, GST-pai, and GSK3beta mRNA in the Chemotherapy-sensitive group were significantly lower than those in the Chemotherapy-resistant group (P<0.05). Patients with high expression of MRP1, GST-pai, and GSK3beta mRNA had a much lower 3-year survival rate than patients with low expression of the genes (P<0.05). Highly expressed in patients with ovarian cancer, MRP1, GST-pai, and GSK3beta mRNA play an important role in the development and drug resistance of ovarian cancer. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Metalloproteinase inhibitor 1 (TIMP1) | [23] | |||
| Sensitive Disease | Ovarian cancer [ICD-11: 2C73.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Ovarian cancer [ICD-11: 2C73] | |||
| The Specified Disease | Ovarian cancer | |||
| The Studied Tissue | Blood | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.62E-21 Fold-change: 5.18E-01 Z-score: 1.09E+01 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell proliferation | Inhibition | hsa05200 | |
| In Vitro Model | 3AO cells | Ovary | Homo sapiens (Human) | N.A. |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | LncRNA PVT1 boost the expression of p53 and TIMP 1 to enhance ovarian cancer cells chemosensitivity for carboplatin and docetaxel. | |||
| Key Molecule: Cellular tumor antigen p53 (TP53) | [23] | |||
| Sensitive Disease | Ovarian cancer [ICD-11: 2C73.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Ovarian cancer [ICD-11: 2C73] | |||
| The Specified Disease | Ovarian cancer | |||
| The Studied Tissue | Ovarian tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 4.96E-01 Fold-change: 3.21E-02 Z-score: 7.12E-01 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell proliferation | Inhibition | hsa05200 | |
| In Vitro Model | 3AO cells | Ovary | Homo sapiens (Human) | N.A. |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | LncRNA PVT1 boost the expression of p53 and TIMP 1 to enhance ovarian cancer cells chemosensitivity for carboplatin and docetaxel. | |||
| Key Molecule: hsa-miR-34c-5p | [40] | |||
| Sensitive Disease | Ovarian cancer [ICD-11: 2C73.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | AKT signaling pathway | Activation | hsa04151 | |
| In Vitro Model | OVS1 cells | Ovary | Homo sapiens (Human) | N.A. |
| SkOV-I6 cells | Ovary | Homo sapiens (Human) | N.A. | |
| In Vivo Model | Mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miRNA-34c-5p inhibits amphiregulin-induced ovarian cancer stemness and drug resistance via downregulation of the AREG-EGFR-ERk pathway. | |||
| Key Molecule: Pvt1 oncogene (PVT1) | [23] | |||
| Sensitive Disease | Ovarian cancer [ICD-11: 2C73.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell proliferation | Inhibition | hsa05200 | |
| In Vitro Model | 3AO cells | Ovary | Homo sapiens (Human) | N.A. |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | LncRNA PVT1 boost the expression of p53 and TIMP 1 to enhance ovarian cancer cells chemosensitivity for carboplatin and docetaxel. | |||
|
|
||||
| Key Molecule: Amphiregulin (AREG) | [40] | |||
| Sensitive Disease | Ovarian cancer [ICD-11: 2C73.0] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Ovarian cancer [ICD-11: 2C73] | |||
| The Specified Disease | Ovarian cancer | |||
| The Studied Tissue | Ovarian tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 3.95E-01 Fold-change: -1.58E-01 Z-score: -8.98E-01 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | AKT signaling pathway | Activation | hsa04151 | |
| In Vitro Model | OVS1 cells | Ovary | Homo sapiens (Human) | N.A. |
| SkOV-I6 cells | Ovary | Homo sapiens (Human) | N.A. | |
| In Vivo Model | Mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miRNA-34c-5p inhibits amphiregulin-induced ovarian cancer stemness and drug resistance via downregulation of the AREG-EGFR-ERk pathway. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Interleukin 6 receptor (IL6R) | [17] | |||
| Resistant Disease | Breast cancer bone metastasis [ICD-11: 2E03.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Metastatic bone cancer [ICD-11: 2E03] | |||
| The Specified Disease | Breast cancer bone metastasis | |||
| The Studied Tissue | Blood | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 5.37E-03 Fold-change: 4.18E-01 Z-score: 3.52E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | MCF-7 cells | Breast | Homo sapiens (Human) | CVCL_0031 |
| MDA-MB-231 cells | Breast | Homo sapiens (Human) | CVCL_0062 | |
| Experiment for Molecule Alteration |
RT-PCR | |||
| Experiment for Drug Resistance |
Flow cytometric | |||
| Mechanism Description | Interleukin-6 (IL-6), a pro-inflammatory cytokine produced in the tumor microenvironment by stromal cells, fibroblasts, and cancer cells. Binding of IL-6 to its receptor IL-6R on the cell membrane activates Janus Kinase 2 (JAK2) kinases. Activated JAK2 mediates phosphorylation, dimerization, and nuclear translocation of Signal Transducer and Activator of Transcription 3 (STAT3). STAT3 signaling mediates the expression of various genes, including p53, Bcl-2, MRP1, and ABCG2. Bcl-2 and p53 are associated with regulation of apoptosis while overexpression of drug transporters MRP1, ABCG2 has been shown to mediate efflux of drugs from cancer cells, thus decreasing intracellular drug concentration leading to drug-resistance. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Heparan sulfate proteoglycan 2 (HSPG2) | [22] | |||
| Sensitive Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Breast cancer [ICD-11: 2C60] | |||
| The Specified Disease | Breast cancer | |||
| The Studied Tissue | Blood | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 4.67E-01 Fold-change: -2.77E-02 Z-score: -7.29E-01 |
|||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| In Vitro Model | MCF-7 cells | Breast | Homo sapiens (Human) | CVCL_0031 |
| MDA-MB-231 cells | Breast | Homo sapiens (Human) | CVCL_0062 | |
| BT474 cells | Breast | Homo sapiens (Human) | CVCL_0179 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
TUNEL analysis | |||
| Mechanism Description | Overexpression of hypomethylated miR-663 induced chemoresistance in breast cancer cells by down-regulating HSPG2. | |||
| Key Molecule: Leucine-rich repeat-containing G-protein coupled receptor 5 (LGR5) | [36] | |||
| Sensitive Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Breast cancer [ICD-11: 2C60] | |||
| The Specified Disease | Breast cancer | |||
| The Studied Tissue | Breast tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.02E-03 Fold-change: -3.63E-02 Z-score: -3.30E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell colony | Inhibition | hsa05200 | ||
| Cell proliferation | Inhibition | hsa05200 | ||
| Wnt/Beta-catenin signaling pathway | Inhibition | hsa04310 | ||
| In Vitro Model | MCF-7 cells | Breast | Homo sapiens (Human) | CVCL_0031 |
| MDA-MB-231 cells | Breast | Homo sapiens (Human) | CVCL_0062 | |
| ZR75-1 cells | Breast | Homo sapiens (Human) | CVCL_0588 | |
| BT549 cells | Breast | Homo sapiens (Human) | CVCL_1092 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay; Flow cytometry assay | |||
| Mechanism Description | Overexpressed miR-340-5p inhibited cell proliferation and drug resistance with increased apoptosis of breast cancer cells through down-regulating LGR5 expression via Wnt/beta-catenin pathway. | |||
| Key Molecule: Caveolin-2 (CAV2) | [41] | |||
| Sensitive Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Breast cancer [ICD-11: 2C60] | |||
| The Specified Disease | Breast cancer | |||
| The Studied Tissue | Breast tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 3.79E-130 Fold-change: -4.30E-01 Z-score: -3.59E+01 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell proliferation | Activation | hsa05200 | |
| In Vitro Model | MDA-MB-231cells | Breast | Homo sapiens (Human) | CVCL_0062 |
| MT-1 cells | Breast | Homo sapiens (Human) | CVCL_0441 | |
| YPEN-1 cells | Breast | Homo sapiens (Human) | CVCL_0587 | |
| Experiment for Molecule Alteration |
Luciferase assay | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Remarkably, miR-199a-3p inhibited both endogenous caveolin-2 activity and exogenous caveolin-2 activity, which was confirmed by a reporter construct bearing the 3'-untranslated region of caveolin-2. However, overexpression of caveolin-2 completely counteracted the enhancement of miR-199a-3p-mediated activities on cell proliferation, survival and sensitivity of tumor cells to anticancer drugs. | |||
| Key Molecule: START domain-containing protein 10 (STARD10) | [58] | |||
| Sensitive Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | STARD10 signaling pathway | Inhibition | hsa05206 | |
| In Vitro Model | MCF-7 cells | Breast | Homo sapiens (Human) | CVCL_0031 |
| MDA-MB-231 cells | Breast | Homo sapiens (Human) | CVCL_0062 | |
| T47D cells | Breast | Homo sapiens (Human) | CVCL_0553 | |
| MDA-MB-468 cells | Breast | Homo sapiens (Human) | CVCL_0419 | |
| Experiment for Molecule Alteration |
RT-qPCR; Immunoblotting assay; Luciferase assay | |||
| Experiment for Drug Resistance |
ELISA; MTT assay; Transwell invasion assay | |||
| Mechanism Description | Acquired resistance to DTX is caused by the miR638 deficiency and subsequent STARD10 upregulation. | |||
| Key Molecule: SRC kinase signaling inhibitor 1 (SRCIN1) | [59] | |||
| Sensitive Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell invasion | Activation | hsa05200 | ||
| Cell migration | Activation | hsa04670 | ||
| Cell proliferation | Activation | hsa05200 | ||
| In Vitro Model | MCF-7 cells | Breast | Homo sapiens (Human) | CVCL_0031 |
| HBL-100 cells | Breast | Homo sapiens (Human) | CVCL_4362 | |
| MCF-7/Doc cells | Breast | Homo sapiens (Human) | CVCL_0031 | |
| Experiment for Molecule Alteration |
Dual luciferase assay; Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | miR346 promotes the biological function of breast cancer cells by targeting SRCIN1 and reduces chemosensitivity to docetaxel. Overexpression of miR346 promoted cell proliferation, colony formation, migration and invasion, and reduced apoptosis, sensitivity to Docetaxel. | |||
| Key Molecule: Breast cancer type 1 susceptibility protein (BRCA1) | [60] | |||
| Sensitive Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | MCF-7 cells | Breast | Homo sapiens (Human) | CVCL_0031 |
| MDA-MB-231 cells | Breast | Homo sapiens (Human) | CVCL_0062 | |
| MDA-MB-468 cells | Breast | Homo sapiens (Human) | CVCL_0419 | |
| MCF-7/LCC2 cells | Breast | Homo sapiens (Human) | CVCL_DP51 | |
| MECs cells | Breast | Homo sapiens (Human) | N.A. | |
| Experiment for Molecule Alteration |
Western blot analysis; RT-qPCR | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Enforced expression of hsa-miR125a-3p in breast cancer cells potentiates docetaxel sensitivity via modulation of BRCA1 signaling. | |||
| Key Molecule: Fibroblast growth factor 2 (FGF1) | [62] | |||
| Sensitive Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| PI3K/AKT signaling pathway | Regulation | N.A. | ||
| In Vitro Model | MCF-7 cells | Breast | Homo sapiens (Human) | CVCL_0031 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay; Drug resistance clonogenic assay | |||
| Mechanism Description | miR-205 enhances chemosensitivity of breast cancer cells to TAC chemotherapy by suppressing both VEGFA and FGF2, leading to evasion of apoptosis. | |||
| Key Molecule: Vascular endothelial growth factor A (VEGFA) | [62] | |||
| Sensitive Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| PI3K/AKT signaling pathway | Regulation | N.A. | ||
| In Vitro Model | MCF-7 cells | Breast | Homo sapiens (Human) | CVCL_0031 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay; Drug resistance clonogenic assay | |||
| Mechanism Description | miR-205 enhances chemosensitivity of breast cancer cells to TAC chemotherapy by suppressing both VEGFA and FGF2, leading to evasion of apoptosis. | |||
| Key Molecule: Receptor tyrosine-protein kinase erbB-3 (ERBB3) | [61] | |||
| Sensitive Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell invasion | Inhibition | hsa05200 | |
| Cell migration | Inhibition | hsa04670 | ||
| Cell proliferation | Inhibition | hsa05200 | ||
| PI3K/AKT signaling pathway | Inhibition | hsa04151 | ||
| In Vitro Model | MCF-7 cells | Breast | Homo sapiens (Human) | CVCL_0031 |
| MDA-MB-231 cells | Breast | Homo sapiens (Human) | CVCL_0062 | |
| Experiment for Molecule Alteration |
Western blot analysis; Luciferase reporter assay | |||
| Experiment for Drug Resistance |
Colony formation assay | |||
| Mechanism Description | The reintroduction of miR-205 is shown to inhibit cell proliferation and clonogenic potential, and increase the sensitivity of MCF-7 and MDA-MB-231 cells to docetaxel. miR-205 also shows a synergistic effect with docetaxel in vivo. | |||
| Key Molecule: Neurogenic locus notch homolog protein 1 (NOTCH1) | [63] | |||
| Sensitive Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell invasion | Inhibition | hsa05200 | ||
| Cell migration | Inhibition | hsa04670 | ||
| Notch signaling pathway | Regulation | N.A. | ||
| In Vitro Model | MCF-7 cells | Breast | Homo sapiens (Human) | CVCL_0031 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay; Flow cytometry assay; Transwell assay | |||
| Mechanism Description | miR-139-5p inhibits the biological function of breast cancer cells by targeting Notch1 and mediates chemosensitivity to docetaxel. | |||
| Key Molecule: Apoptosis regulator Bcl-2 (BCL2) | [64] | |||
| Sensitive Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | MCF-7 cells | Breast | Homo sapiens (Human) | CVCL_0031 |
| MDA-MB-231 cells | Breast | Homo sapiens (Human) | CVCL_0062 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Docetaxel primarily acts in G2-M phase, whereas it has diminished activity in G1 phase. Increased miR-34a expression may therefore be able to inhibit docetaxel activity by arresting cells in G1 phase. | |||
|
|
||||
| Key Molecule: hsa-miR-638 | [58] | |||
| Sensitive Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | STARD10 signaling pathway | Inhibition | hsa05206 | |
| In Vitro Model | MCF-7 cells | Breast | Homo sapiens (Human) | CVCL_0031 |
| MDA-MB-231 cells | Breast | Homo sapiens (Human) | CVCL_0062 | |
| T47D cells | Breast | Homo sapiens (Human) | CVCL_0553 | |
| MDA-MB-468 cells | Breast | Homo sapiens (Human) | CVCL_0419 | |
| Experiment for Molecule Alteration |
RT-qPCR | |||
| Experiment for Drug Resistance |
ELISA; MTT assay; Transwell invasion assay | |||
| Mechanism Description | Acquired resistance to DTX is caused by the miR638 deficiency and subsequent STARD10 upregulation. | |||
| Key Molecule: hsa-miR-346 | [59] | |||
| Sensitive Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell invasion | Activation | hsa05200 | ||
| Cell migration | Activation | hsa04670 | ||
| Cell proliferation | Activation | hsa05200 | ||
| In Vitro Model | MCF-7 cells | Breast | Homo sapiens (Human) | CVCL_0031 |
| HBL-100 cells | Breast | Homo sapiens (Human) | CVCL_4362 | |
| MCF-7/Doc cells | Breast | Homo sapiens (Human) | CVCL_0031 | |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | miR346 promotes the biological function of breast cancer cells by targeting SRCIN1 and reduces chemosensitivity to docetaxel. Overexpression of miR346 promoted cell proliferation, colony formation, migration and invasion, and reduced apoptosis, sensitivity to Docetaxel. | |||
| Key Molecule: hsa-miR-125a-3p | [60] | |||
| Sensitive Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | MCF-7 cells | Breast | Homo sapiens (Human) | CVCL_0031 |
| MDA-MB-231 cells | Breast | Homo sapiens (Human) | CVCL_0062 | |
| MDA-MB-468 cells | Breast | Homo sapiens (Human) | CVCL_0419 | |
| MCF-7/LCC2 cells | Breast | Homo sapiens (Human) | CVCL_DP51 | |
| MECs cells | Breast | Homo sapiens (Human) | N.A. | |
| Experiment for Molecule Alteration |
RT-qPCR | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Enforced expression of hsa-miR125a-3p in breast cancer cells potentiates docetaxel sensitivity via modulation of BRCA1 signaling. | |||
| Key Molecule: hsa-miR-340-5p | [36] | |||
| Sensitive Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell invasion | Inhibition | hsa05200 | ||
| Cell proliferation | Inhibition | hsa05200 | ||
| Wnt/Beta-catenin signaling pathway | Inhibition | hsa04310 | ||
| In Vitro Model | MCF-7 cells | Breast | Homo sapiens (Human) | CVCL_0031 |
| MDA-MB-231 cells | Breast | Homo sapiens (Human) | CVCL_0062 | |
| ZR75-1 cells | Breast | Homo sapiens (Human) | CVCL_0588 | |
| BT549 cells | Breast | Homo sapiens (Human) | CVCL_1092 | |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
CCK8 assay; Flow cytometry assay | |||
| Mechanism Description | Overexpressed miR-340-5p inhibited cell proliferation and drug resistance with increased apoptosis of breast cancer cells through down-regulating LGR5 expression via Wnt/beta-catenin pathway. | |||
| Key Molecule: hsa-mir-205 | [61], [62] | |||
| Sensitive Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell invasion | Activation | hsa05200 | |
| Cell migration | Activation | hsa04670 | ||
| Cell proliferation | Inhibition | hsa05200 | ||
| PI3K/AKT signaling pathway | Inhibition | hsa04151 | ||
| In Vitro Model | MCF-7 cells | Breast | Homo sapiens (Human) | CVCL_0031 |
| MDA-MB-231 cells | Breast | Homo sapiens (Human) | CVCL_0062 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
RT-PCR | |||
| Experiment for Drug Resistance |
Colony formation assay; MTT assay; Drug resistance clonogenic assay | |||
| Mechanism Description | The reintroduction of miR-205 is shown to inhibit cell proliferation and clonogenic potential, and increase the sensitivity of MCF-7 and MDA-MB-231 cells to docetaxel. miR-205 also shows a synergistic effect with docetaxel in vivo. And miR-205 enhances chemosensitivity of breast cancer cells to TAC chemotherapy by suppressing both VEGFA and FGF2, leading to evasion of apoptosis. | |||
| Key Molecule: hsa-miR-139-5p | [63] | |||
| Sensitive Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell invasion | Inhibition | hsa05200 | ||
| Cell migration | Inhibition | hsa04670 | ||
| Notch signaling pathway | Regulation | N.A. | ||
| In Vitro Model | MCF-7 cells | Breast | Homo sapiens (Human) | CVCL_0031 |
| In Vivo Model | Mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
RT-qPCR | |||
| Experiment for Drug Resistance |
CCK8 assay; Flow cytometry assay; Transwell assay | |||
| Mechanism Description | miR-139-5p inhibits the biological function of breast cancer cells by targeting Notch1 and mediates chemosensitivity to docetaxel. | |||
| Key Molecule: hsa-mir-663 | [22] | |||
| Sensitive Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| In Vitro Model | MCF-7 cells | Breast | Homo sapiens (Human) | CVCL_0031 |
| MDA-MB-231 cells | Breast | Homo sapiens (Human) | CVCL_0062 | |
| BT474 cells | Breast | Homo sapiens (Human) | CVCL_0179 | |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
TUNEL analysis | |||
| Mechanism Description | Overexpression of hypomethylated miR-663 induced chemoresistance in breast cancer cells by down-regulating HSPG2. | |||
| Key Molecule: hsa-miR-199a-3p | [41] | |||
| Sensitive Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell proliferation | Activation | hsa05200 | |
| In Vitro Model | MDA-MB-231cells | Breast | Homo sapiens (Human) | CVCL_0062 |
| MT-1 cells | Breast | Homo sapiens (Human) | CVCL_0441 | |
| YPEN-1 cells | Breast | Homo sapiens (Human) | CVCL_0587 | |
| Experiment for Molecule Alteration |
RT-PCR | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Remarkably, miR-199a-3p inhibited both endogenous caveolin-2 activity and exogenous caveolin-2 activity, which was confirmed by a reporter construct bearing the 3'-untranslated region of caveolin-2. However, overexpression of caveolin-2 completely counteracted the enhancement of miR-199a-3p-mediated activities on cell proliferation, survival and sensitivity of tumor cells to anticancer drugs. | |||
| Key Molecule: hsa-mir-34 | [64] | |||
| Sensitive Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | MCF-7 cells | Breast | Homo sapiens (Human) | CVCL_0031 |
| MDA-MB-231 cells | Breast | Homo sapiens (Human) | CVCL_0062 | |
| Experiment for Molecule Alteration |
qPCR | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Docetaxel primarily acts in G2-M phase, whereas it has diminished activity in G1 phase. Increased miR-34a expression may therefore be able to inhibit docetaxel activity by arresting cells in G1 phase. | |||
|
|
||||
| Key Molecule: Dual specificity phosphatase 4 (DUSP4) | [65] | |||
| Metabolic Type | Redox metabolism | |||
| Sensitive Disease | Breast adenocarcinoma [ICD-11: 2C60.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | BT-474 cells | Breast | Homo sapiens (Human) | CVCL_0179 |
| Experiment for Molecule Alteration |
RNA seq; Western blot analysis | |||
| Experiment for Drug Resistance |
IC50 assay | |||
| Mechanism Description | ur findings reveal that DUSP4 enhances therapeutic efficacy in HER2-positive BC by inhibiting the ROS pathway. Elevated DUSP4 levels correlate with increased sensitivity to HER2-targeted therapies and improved clinical outcomes. DUSP4 independently predicts disease-free survival (DFS) and overall survival (OS) in HER6-positive BC. | |||
| Key Molecule: Dual specificity phosphatase 4 (DUSP4) | [65] | |||
| Metabolic Type | Redox metabolism | |||
| Sensitive Disease | Breast adenocarcinoma [ICD-11: 2C60.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | SK-BR-3 cells | Pleural effusion | Homo sapiens (Human) | CVCL_0033 |
| Experiment for Molecule Alteration |
RNA seq; Western blot analysis | |||
| Experiment for Drug Resistance |
IC50 assay | |||
| Mechanism Description | ur findings reveal that DUSP4 enhances therapeutic efficacy in HER2-positive BC by inhibiting the ROS pathway. Elevated DUSP4 levels correlate with increased sensitivity to HER2-targeted therapies and improved clinical outcomes. DUSP4 independently predicts disease-free survival (DFS) and overall survival (OS) in HER7-positive BC. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Glycogen synthase kinase-3 beta (GSK3B) | [37] | |||
| Resistant Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Breast cancer [ICD-11: 2C60] | |||
| The Specified Disease | Breast cancer | |||
| The Studied Tissue | Breast tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.20E-07 Fold-change: -3.67E-02 Z-score: -5.39E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| GSK-3beta/Beta-catenin signaling pathway | Regulation | N.A. | ||
| In Vitro Model | MCF-7 cells | Breast | Homo sapiens (Human) | CVCL_0031 |
| MDA-MB-231 cells | Breast | Homo sapiens (Human) | CVCL_0062 | |
| Experiment for Molecule Alteration |
Western blot analysis; Flow cytometric assay | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miR-3646 contributes to drug resistance of breast cancer cells to Doc at least in part through activation of GSk-3beta/beta-catenin pathway by suppressing expression of GSk-3beta. | |||
| Key Molecule: Eukaryotic translation initiation factor 4E (EIF4E) | [55] | |||
| Resistant Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| In Vitro Model | MCF-7 cells | Breast | Homo sapiens (Human) | CVCL_0031 |
| MDA-MB-231 cells | Breast | Homo sapiens (Human) | CVCL_0062 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miR-141 affected the chemosensitivity of BC cells to docetaxel by directly targeting EIF4E, due to its anti-apoptotic properties. Transfection of miR-141 inhibitor could significantly promote docetaxel-induced apoptosis and change the expression of Bax and Bcl-2. However, when the BC cells were transfected with siRNA-EIF4E, the data showed opposite results. It suggested that EIF4E is partly responsible for the miR-141-induced apoptosis which is related to the mitochondrial apoptosis pathway. In the previous studies, antisense Bcl-2 treatment (+) sensitivity to tamoxifen in HER2-positive cells in tamoxifen-resistant BC cells. | |||
| Key Molecule: Phosphatase and tensin homolog (PTEN) | [13] | |||
| Resistant Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell proliferation | Activation | hsa05200 | ||
| In Vitro Model | MCF-7 cells | Breast | Homo sapiens (Human) | CVCL_0031 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | PTEN plays major roles in suppressing cancer and embryonic development, cell migration and apoptosis, miR-222 and -29a could regulate the expression of PTEN, maybe through which the two miRNAs conferred Adr and Doc resistance in MCF-7 cells. | |||
| Key Molecule: Twist-related protein (TWIST) | [57] | |||
| Resistant Disease | Breast adenocarcinoma [ICD-11: 2C60.1] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | MCF7-Doc cells | Breast | Homo sapiens (Human) | N.A. |
| Experiment for Molecule Alteration |
RT-PCR; Immunofluorescence staining assay | |||
| Experiment for Drug Resistance |
CCK8 assay; Flow cytometric assay | |||
| Mechanism Description | Docetaxel-resistant cells exhibited down-regulated DYRK2 and up-regulated Twist1 expression. DYRK2 overexpression reversed drug resistance, decreased migration, and attenuated Twist1 and GST-pi expression. DYRK2 was found to suppress Twist1 expression through ubiquitination, supported by decreased Twist1 phosphorylation and increased ubiquitination after DYRK2 overexpression. Twist1 overexpression counteracted DYRK2-induced drug sensitivity enhancement, promoting GST-pi expression, EMT, migration, and proliferation. Twist1 was shown to bind to the GSTP1 promoter, enhancing its transcription. In vivo experiments confirmed DYRK2's ability to suppress chemoresistance in breast cancer cells. | |||
| Key Molecule: Twist-related protein (TWIST) | [57] | |||
| Resistant Disease | Breast adenocarcinoma [ICD-11: 2C60.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | MCF7-Doc 2ug cells | Breast | Homo sapiens (Human) | N.A. |
| Experiment for Molecule Alteration |
RT-PCR; Immunofluorescence staining assay | |||
| Experiment for Drug Resistance |
CCK8 assay; Flow cytometric assay | |||
| Mechanism Description | Docetaxel-resistant cells exhibited down-regulated DYRK2 and up-regulated Twist1 expression. DYRK2 overexpression reversed drug resistance, decreased migration, and attenuated Twist1 and GST-pi expression. DYRK2 was found to suppress Twist1 expression through ubiquitination, supported by decreased Twist1 phosphorylation and increased ubiquitination after DYRK2 overexpression. Twist1 overexpression counteracted DYRK2-induced drug sensitivity enhancement, promoting GST-pi expression, EMT, migration, and proliferation. Twist1 was shown to bind to the GSTP1 promoter, enhancing its transcription. In vivo experiments confirmed DYRK2's ability to suppress chemoresistance in breast cancer cells. | |||
| Key Molecule: Dual specificity tyrosine-phosphorylation-regulated kinase 2 (DYRK2) | [57] | |||
| Resistant Disease | Breast adenocarcinoma [ICD-11: 2C60.1] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | MCF7-Doc cells | Breast | Homo sapiens (Human) | N.A. |
| Experiment for Molecule Alteration |
RT-PCR; Immunofluorescence staining assay | |||
| Experiment for Drug Resistance |
CCK8 assay; Flow cytometric assay | |||
| Mechanism Description | Docetaxel-resistant cells exhibited down-regulated DYRK2 and up-regulated Twist1 expression. DYRK2 overexpression reversed drug resistance, decreased migration, and attenuated Twist1 and GST-pi expression. DYRK2 was found to suppress Twist1 expression through ubiquitination, supported by decreased Twist1 phosphorylation and increased ubiquitination after DYRK2 overexpression. Twist1 overexpression counteracted DYRK2-induced drug sensitivity enhancement, promoting GST-pi expression, EMT, migration, and proliferation. Twist1 was shown to bind to the GSTP1 promoter, enhancing its transcription. In vivo experiments confirmed DYRK2's ability to suppress chemoresistance in breast cancer cells. | |||
| Key Molecule: Dual specificity tyrosine-phosphorylation-regulated kinase 2 (DYRK2) | [57] | |||
| Resistant Disease | Breast adenocarcinoma [ICD-11: 2C60.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | MCF7-Doc 2ug cells | Breast | Homo sapiens (Human) | N.A. |
| Experiment for Molecule Alteration |
RT-PCR; Immunofluorescence staining assay | |||
| Experiment for Drug Resistance |
CCK8 assay; Flow cytometric assay | |||
| Mechanism Description | Docetaxel-resistant cells exhibited down-regulated DYRK2 and up-regulated Twist1 expression. DYRK2 overexpression reversed drug resistance, decreased migration, and attenuated Twist1 and GST-pi expression. DYRK2 was found to suppress Twist1 expression through ubiquitination, supported by decreased Twist1 phosphorylation and increased ubiquitination after DYRK2 overexpression. Twist1 overexpression counteracted DYRK2-induced drug sensitivity enhancement, promoting GST-pi expression, EMT, migration, and proliferation. Twist1 was shown to bind to the GSTP1 promoter, enhancing its transcription. In vivo experiments confirmed DYRK2's ability to suppress chemoresistance in breast cancer cells. | |||
|
|
||||
| Key Molecule: hsa-miR-3646 | [37] | |||
| Resistant Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| GSK-3beta/Beta-catenin signaling pathway | Regulation | N.A. | ||
| In Vitro Model | MCF-7 cells | Breast | Homo sapiens (Human) | CVCL_0031 |
| MDA-MB-231 cells | Breast | Homo sapiens (Human) | CVCL_0062 | |
| Experiment for Molecule Alteration |
RT-qPCR | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miR-3646 contributes to drug resistance of breast cancer cells to Doc at least in part through activation of GSk-3beta/beta-catenin pathway by suppressing expression of GSk-3beta. | |||
| Key Molecule: hsa-mir-141 | [55] | |||
| Resistant Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| In Vitro Model | MCF-7 cells | Breast | Homo sapiens (Human) | CVCL_0031 |
| MDA-MB-231 cells | Breast | Homo sapiens (Human) | CVCL_0062 | |
| In Vivo Model | Mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miR-141 affected the chemosensitivity of BC cells to docetaxel by directly targeting EIF4E, due to its anti-apoptotic properties. Transfection of miR-141 inhibitor could significantly promote docetaxel-induced apoptosis and change the expression of Bax and Bcl-2. However, when the BC cells were transfected with siRNA-EIF4E, the data showed opposite results. It suggested that EIF4E is partly responsible for the miR-141-induced apoptosis which is related to the mitochondrial apoptosis pathway. In the previous studies, antisense Bcl-2 treatment (+) sensitivity to tamoxifen in HER2-positive cells in tamoxifen-resistant BC cells. | |||
| Key Molecule: hsa-mir-222 | [13] | |||
| Resistant Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell proliferation | Activation | hsa05200 | ||
| In Vitro Model | MCF-7 cells | Breast | Homo sapiens (Human) | CVCL_0031 |
| Experiment for Molecule Alteration |
RT-qPCR | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | PTEN plays major roles in suppressing cancer and embryonic development, cell migration and apoptosis, miR-222 and -29a could regulate the expression of PTEN, maybe through which the two miRNAs conferred Adr and Doc resistance in MCF-7 cells. | |||
| Key Molecule: hsa-mir-29a | [13] | |||
| Resistant Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell proliferation | Activation | hsa05200 | ||
| In Vitro Model | MCF-7 cells | Breast | Homo sapiens (Human) | CVCL_0031 |
| Experiment for Molecule Alteration |
RT-qPCR | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | PTEN plays major roles in suppressing cancer and embryonic development, cell migration and apoptosis, miR-222 and -29a could regulate the expression of PTEN, maybe through which the two miRNAs conferred Adr and Doc resistance in MCF-7 cells. | |||
|
|
||||
| Key Molecule: Cyclin-G2 (CCNG2) | [56] | |||
| Resistant Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | MCF-7 cells | Breast | Homo sapiens (Human) | CVCL_0031 |
| MDA-MB-231 cells | Breast | Homo sapiens (Human) | CVCL_0062 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | Exosomal microRNA miR1246 promotes cell proliferation, invasion and drug resistance by suppressing the expression level of CCNG2 in Breast Cancer. | |||
| Key Molecule: hsa-miR-1246 | [56] | |||
| Resistant Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | MCF-7 cells | Breast | Homo sapiens (Human) | CVCL_0031 |
| MDA-MB-231 cells | Breast | Homo sapiens (Human) | CVCL_0062 | |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | Exosomal microRNA miR1246 promotes cell proliferation, invasion and drug resistance by suppressing the expression level of CCNG2 in Breast Cancer. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: SET and MYND domain containing 2 (SMYD2) | [6] | |||
| Resistant Disease | Kidney cancer [ICD-11: 2C90.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Kidney cancer [ICD-11: 2C90] | |||
| The Specified Disease | Kidney cancer | |||
| The Studied Tissue | Kidney | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 8.07E-35 Fold-change: 6.59E-01 Z-score: 1.61E+01 |
|||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | HEK293T cells | Kidney | Homo sapiens (Human) | CVCL_0063 |
| HK-2 cells | Kidney | Homo sapiens (Human) | CVCL_0302 | |
| In Vivo Model | Balb/c athymic nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blotting assay | |||
| Experiment for Drug Resistance |
MTS assay | |||
| Mechanism Description | SMYD2 is a histone methyltransferase.The estimated IC50 values of cisplatin, doxorubicin, or 5-FU (but not docetaxel) for AZ505-treated RCC cells were significantly lower than those for the control cells, indicating that the SMYD2 inhibition enhanced the drug sensitivity in renal cancer cells. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Bcl-2-associated agonist of cell death (BAD) | [24] | |||
| Sensitive Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Lung cancer [ICD-11: 2C25] | |||
| The Specified Disease | Lung adenocarcinoma | |||
| The Studied Tissue | Lung tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 3.32E-11 Fold-change: -3.62E-01 Z-score: -7.02E+00 |
|||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Caspase-3 signaling pathway | Activation | hsa04210 | |
| Sorafenib tolerance | Activation | hsa00983 | ||
| In Vitro Model | SPC-A1 cells | Lung | Homo sapiens (Human) | CVCL_6955 |
| H1299 cells | Lung | Homo sapiens (Human) | CVCL_0060 | |
| In Vivo Model | BALB/c nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Ectopic Let-7c expression could increase the sensitivity of docetaxel-resistant LAD cells to chemotherapeutic agents or irradiation and reverse their EMT phenotype by targeting Bcl-xL. | |||
| Key Molecule: Eukaryotic translation initiation factor 4E (EIF4E) | [27] | |||
| Sensitive Disease | Non-small cell lung cancer [ICD-11: 2C25.Y] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Lung cancer [ICD-11: 2C25] | |||
| The Specified Disease | Non-small cell lung cancer | |||
| The Studied Tissue | Lung tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 2.99E-20 Fold-change: 2.68E-01 Z-score: 1.03E+01 |
|||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | EIF4E/VEGF/c-Myc/Bax signaling pathway | Activation | hsa04066 | |
| In Vitro Model | H1299 cells | Lung | Homo sapiens (Human) | CVCL_0060 |
| H2009 cells | Lung | Homo sapiens (Human) | CVCL_1514 | |
| Experiment for Molecule Alteration |
Western blot analysis; RT-qPCR | |||
| Experiment for Drug Resistance |
MTT assay; Annexin V-FITC Apoptosis assay | |||
| Mechanism Description | Down-regulation of miR141 suppressed cell proliferation, induced cell death and increased caspase-3 activity in H1299 or H2009/docetaxel cells. Down-regulation of miR141 also increased the protein expression of EIF4E, VEGF, c-Myc and Bax in H1299 or H2009/docetaxel cells. | |||
| Key Molecule: Growth protein 4 inhibitor (ING4) | [32] | |||
| Sensitive Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Lung cancer [ICD-11: 2C25] | |||
| The Specified Disease | Lung cancer | |||
| The Studied Tissue | Lung tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 9.80E-01 Fold-change: 1.32E-04 Z-score: 2.45E-02 |
|||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell cycle | Activation | hsa04110 | ||
| Cell proliferation | Inhibition | hsa05200 | ||
| In Vitro Model | SPC-A1 cells | Lung | Homo sapiens (Human) | CVCL_6955 |
| H1299 cells | Lung | Homo sapiens (Human) | CVCL_0060 | |
| In Vivo Model | BALB/c nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miR-650 could contribute to docetaxel chemoresistance of LAD cells by post-transcriptionally downregulating ING4. | |||
| Key Molecule: Transcription factor E2F3 (E2F3) | [52] | |||
| Sensitive Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| HDAC1/4/Sp1/miR200b/E2F3 signaling pathway | Inhibition | hsa05206 | ||
| In Vitro Model | H1299/DTX cells | Lung | Homo sapiens (Human) | CVCL_0060 |
| SPC-A1/DTX cells | Lung | Homo sapiens (Human) | CVCL_W217 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Histone deacetylase (HDAC) inhibitors could restore the expression of miR-200b and reverse chemoresistant phenotypes of docetaxel-resistant LAD cells. HDAC1/4 repression significantly increased miR-200b expression by upregulating histone-H3 acetylation level at the two miR-200b promoters partially via a Sp1-dependent pathway. Furthermore, silencing of HDAC1/4 suppressed cell proliferation, promoted cell apoptosis, induced G2/M cell cycle arrest and ultimately reversed in vitro and in vivo chemoresistance of docetaxel-resistant LAD cells, at least partially in a miR-200b-dependent manner. HDAC1/4 suppression-induced rescue of miR-200b contributed to downregulation of E2F3, survivin and Aurora-A, and upregulation of cleaved-caspase-3. HDAC1/4 levels in docetaxel-insensitive human LAD tissues, inversely correlated with miR-200b, were upregulated compared with docetaxel-sensitive tissues. Taken together, our findings suggest that the HDAC1/4/Sp1/miR-200b/E2F3 pathway is responsible for chemoresistance of docetaxel-resistant LAD cells. | |||
| Key Molecule: Transcription factor E2F3 (E2F3) | [53] | |||
| Sensitive Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell proliferation | Inhibition | hsa05200 | ||
| In Vitro Model | A549 cells | Lung | Homo sapiens (Human) | CVCL_0023 |
| SPC-A1 cells | Lung | Homo sapiens (Human) | CVCL_6955 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | E2F3 was generally considered to increase cellular proliferation as a transcriptional activator through the G1/S transition, down-regulation of miR-200b could lead to E2F3 overexpression and in turn contribute to chemoresistance of lung adenocarcinoma cells to docetaxel. | |||
| Key Molecule: Serine/threonine-protein kinase PLK1 (PLK1) | [54] | |||
| Sensitive Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell proliferation | Inhibition | hsa05200 | ||
| In Vitro Model | A549 cells | Lung | Homo sapiens (Human) | CVCL_0023 |
| SPC-A1 cells | Lung | Homo sapiens (Human) | CVCL_6955 | |
| H1299 cells | Lung | Homo sapiens (Human) | CVCL_0060 | |
| In Vivo Model | BALB/c nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Plk1 directly promotes mitotic entry by activating Cdc25C and Cdk1 (Cdc2) /Cyclin B complex,introduction of miR-100 significantly decreased Plk1 expression and in turn resensitized SPC-A1/DTX cells to docetaxel. | |||
|
|
||||
| Key Molecule: Bcl-2-associated agonist of cell death (BAD) | [26] | |||
| Sensitive Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Lung cancer [ICD-11: 2C25] | |||
| The Specified Disease | Lung cancer | |||
| The Studied Tissue | Lung tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 8.54E-01 Fold-change: 1.74E-03 Z-score: 1.85E-01 |
|||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell invasion | Inhibition | hsa05200 | ||
| Cell migration | Inhibition | hsa04670 | ||
| Cell proliferation | Inhibition | hsa05200 | ||
| In Vitro Model | SPC-A1 cells | Lung | Homo sapiens (Human) | CVCL_6955 |
| H1299 cells | Lung | Homo sapiens (Human) | CVCL_0060 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay; Colony formation assay; Tunel assay | |||
| Mechanism Description | Long noncoding RNA CCAT1 acts as an oncogene and promotes chemoresistance in docetaxel-resistant lung adenocarcinoma cells.the sponging of let-7c by CCAT1 released Bcl-xl (a let-7c target), thereby promoting the acquisition of chemoresistance and epithelial-to-mesenchymal transition phenotypes in docetaxel-resistant LAD cells. | |||
| Key Molecule: Long non-protein coding RNA, regulator of reprogramming (LINC-ROR) | [28] | |||
| Sensitive Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Epithelial mesenchymal transition signaling pathway | Activation | hsa01521 | |
| In Vitro Model | SPC-A1 cells | Lung | Homo sapiens (Human) | CVCL_6955 |
| H1299 cells | Lung | Homo sapiens (Human) | CVCL_0060 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
MTT assay; Colony formation assay; Flow cytometry assay; Wound healing assay; Transwell assay; TUNEL assay | |||
| Mechanism Description | Long noncoding RNA ROR regulates chemoresistance in docetaxel-resistant lung adenocarcinoma cells via epithelial mesenchymal transition pathway. Down regulation of linc-ROR reversed the chemoresistance and EMT features of these cells by targeting miR145 and its target gene FSCN1. | |||
| Key Molecule: hsa-let-7c | [26] | |||
| Sensitive Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell invasion | Inhibition | hsa05200 | ||
| Cell migration | Inhibition | hsa04670 | ||
| Cell proliferation | Inhibition | hsa05200 | ||
| In Vitro Model | SPC-A1 cells | Lung | Homo sapiens (Human) | CVCL_6955 |
| H1299 cells | Lung | Homo sapiens (Human) | CVCL_0060 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
MTT assay; Colony formation assay; Tunel assay | |||
| Mechanism Description | Long noncoding RNA CCAT1 acts as an oncogene and promotes chemoresistance in docetaxel-resistant lung adenocarcinoma cells.the sponging of let-7c by CCAT1 released Bcl-xl (a let-7c target), thereby promoting the acquisition of chemoresistance and epithelial-to-mesenchymal transition phenotypes in docetaxel-resistant LAD cells. | |||
| Key Molecule: hsa-let-7c | [24] | |||
| Sensitive Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Caspase-3 signaling pathway | Activation | hsa04210 | |
| Cell migration | Inhibition | hsa04670 | ||
| In Vitro Model | SPC-A1 cells | Lung | Homo sapiens (Human) | CVCL_6955 |
| H1299 cells | Lung | Homo sapiens (Human) | CVCL_0060 | |
| In Vivo Model | BALB/c nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Ectopic Let-7c expression could increase the sensitivity of docetaxel-resistant LAD cells to chemotherapeutic agents or irradiation and reverse their EMT phenotype by targeting Bcl-xL. | |||
|
|
||||
| Key Molecule: hsa-mir-141 | [27] | |||
| Sensitive Disease | Non-small cell lung cancer [ICD-11: 2C25.Y] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | EIF4E/VEGF/c-Myc/Bax signaling pathway | Activation | hsa04066 | |
| In Vitro Model | H1299 cells | Lung | Homo sapiens (Human) | CVCL_0060 |
| H2009 cells | Lung | Homo sapiens (Human) | CVCL_1514 | |
| Experiment for Molecule Alteration |
RT-qPCR | |||
| Experiment for Drug Resistance |
MTT assay; Annexin V-FITC Apoptosis assay | |||
| Mechanism Description | Down-regulation of miR141 suppressed cell proliferation, induced cell death and increased caspase-3 activity in H1299 or H2009/docetaxel cells. Down-regulation of miR141 also increased the protein expression of EIF4E, VEGF, c-Myc and Bax in H1299 or H2009/docetaxel cells. | |||
| Key Molecule: hsa-mir-200b | [52] | |||
| Sensitive Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| HDAC1/4/Sp1/miR200b/E2F3 signaling pathway | Inhibition | hsa05206 | ||
| In Vitro Model | H1299/DTX cells | Lung | Homo sapiens (Human) | CVCL_0060 |
| SPC-A1/DTX cells | Lung | Homo sapiens (Human) | CVCL_W217 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Histone deacetylase (HDAC) inhibitors could restore the expression of miR-200b and reverse chemoresistant phenotypes of docetaxel-resistant LAD cells. HDAC1/4 repression significantly increased miR-200b expression by upregulating histone-H3 acetylation level at the two miR-200b promoters partially via a Sp1-dependent pathway. Furthermore, silencing of HDAC1/4 suppressed cell proliferation, promoted cell apoptosis, induced G2/M cell cycle arrest and ultimately reversed in vitro and in vivo chemoresistance of docetaxel-resistant LAD cells, at least partially in a miR-200b-dependent manner. HDAC1/4 suppression-induced rescue of miR-200b contributed to downregulation of E2F3, survivin and Aurora-A, and upregulation of cleaved-caspase-3. HDAC1/4 levels in docetaxel-insensitive human LAD tissues, inversely correlated with miR-200b, were upregulated compared with docetaxel-sensitive tissues. Taken together, our findings suggest that the HDAC1/4/Sp1/miR-200b/E2F3 pathway is responsible for chemoresistance of docetaxel-resistant LAD cells. | |||
| Key Molecule: hsa-miR-650 | [32] | |||
| Sensitive Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell colony | Inhibition | hsa05200 | ||
| Cell proliferation | Inhibition | hsa05200 | ||
| In Vitro Model | SPC-A1 cells | Lung | Homo sapiens (Human) | CVCL_6955 |
| H1299 cells | Lung | Homo sapiens (Human) | CVCL_0060 | |
| In Vivo Model | BALB/c nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miR-650 could contribute to docetaxel chemoresistance of LAD cells by post-transcriptionally downregulating ING4. | |||
| Key Molecule: hsa-mir-200b | [53] | |||
| Sensitive Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell proliferation | Inhibition | hsa05200 | ||
| In Vitro Model | A549 cells | Lung | Homo sapiens (Human) | CVCL_0023 |
| SPC-A1 cells | Lung | Homo sapiens (Human) | CVCL_6955 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | E2F3 was generally considered to increase cellular proliferation as a transcriptional activator through the G1/S transition, down-regulation of miR-200b could lead to E2F3 overexpression and in turn contribute to chemoresistance of lung adenocarcinoma cells to docetaxel. | |||
| Key Molecule: hsa-mir-100 | [54] | |||
| Sensitive Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell proliferation | Inhibition | hsa05200 | ||
| In Vitro Model | A549 cells | Lung | Homo sapiens (Human) | CVCL_0023 |
| SPC-A1 cells | Lung | Homo sapiens (Human) | CVCL_6955 | |
| H1299 cells | Lung | Homo sapiens (Human) | CVCL_0060 | |
| In Vivo Model | BALB/c nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Plk1 directly promotes mitotic entry by activating Cdc25C and Cdk1 (Cdc2) /Cyclin B complex,introduction of miR-100 significantly decreased Plk1 expression and in turn resensitized SPC-A1/DTX cells to docetaxel. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Epidermal growth factor receptor (EGFR) | [25] | |||
| Resistant Disease | Non-small cell lung cancer [ICD-11: 2C25.Y] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Lung cancer [ICD-11: 2C25] | |||
| The Specified Disease | Non-small cell lung cancer | |||
| The Studied Tissue | Lung tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 2.37E-11 Fold-change: 6.31E-01 Z-score: 7.28E+00 |
|||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell proliferation | Activation | hsa05200 | ||
| EGFR signaling pathway | Inhibition | hsa01521 | ||
| In Vitro Model | A549 cells | Lung | Homo sapiens (Human) | CVCL_0023 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay; Flow cytometry assay | |||
| Mechanism Description | The transfection of miR 27b mimics led to downregulation of the expression levels of EGFR, whilst miR 27b inhibitors upregulated the expression levels of EGFR. Furthermore, it was demonstrated that the transfection of miR 27b mimics significantly suppressed the apoptosis and promote the viability of A549 human lung carcinoma cells. In line with this, the introduction of miR 27b inhibitors significantly induced apoptosis and inhibited the proliferation of A549 cells. These results indicate that miR 27b may promote NSCLC cell viability and enhance resistance to docetaxel treatment through direct inhibition of EGFR expression. | |||
| Key Molecule: Phosphatase and tensin homolog (PTEN) | [7] | |||
| Resistant Disease | Non-small cell lung cancer [ICD-11: 2C25.Y] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell migration | Activation | hsa04670 | ||
| PTEN signaling pathway | Inhibition | hsa05235 | ||
| In Vitro Model | KB-3-1 cells | Lung | Homo sapiens (Human) | CVCL_2088 |
| KB-CP.5 cells | Lung | Homo sapiens (Human) | CVCL_IP04 | |
| KB-CP20 cells | Lung | Homo sapiens (Human) | CVCL_IP06 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | PTEN, a tumor suppressor gene, is an essential regulator of cell proliferation, differentiation, growth, and apoptosis. miR-21 can promote growth, migration, and invasion, chemo- or radioresistance of NSCLC cells by downregulation PTEN. | |||
|
|
||||
| Key Molecule: Bcl-2-like protein 1 (BCL2L1) | [26] | |||
| Resistant Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Lung cancer [ICD-11: 2C25] | |||
| The Specified Disease | Lung adenocarcinoma | |||
| The Studied Tissue | Lung tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.64E-10 Fold-change: 2.90E-01 Z-score: 6.90E+00 |
|||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell invasion | Activation | hsa05200 | ||
| Cell migration | Activation | hsa04670 | ||
| Cell proliferation | Activation | hsa05200 | ||
| In Vitro Model | SPC-A1 cells | Lung | Homo sapiens (Human) | CVCL_6955 |
| H1299 cells | Lung | Homo sapiens (Human) | CVCL_0060 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay; Colony formation assay; Tunel assay | |||
| Mechanism Description | Long noncoding RNA CCAT1 acts as an oncogene and promotes chemoresistance in docetaxel-resistant lung adenocarcinoma cells.the sponging of let-7c by CCAT1 released Bcl-xl (a let-7c target), thereby promoting the acquisition of chemoresistance and epithelial-to-mesenchymal transition phenotypes in docetaxel-resistant LAD cells. | |||
| Key Molecule: Fascin (FSCN1) | [28] | |||
| Resistant Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Lung cancer [ICD-11: 2C25] | |||
| The Specified Disease | Lung cancer | |||
| The Studied Tissue | Lung tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 2.83E-13 Fold-change: 9.43E-02 Z-score: 7.57E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell invasion | Activation | hsa05200 | |
| Cell migration | Activation | hsa04670 | ||
| Epithelial mesenchymal transition signaling pathway | Activation | hsa01521 | ||
| In Vitro Model | SPC-A1 cells | Lung | Homo sapiens (Human) | CVCL_6955 |
| H1299 cells | Lung | Homo sapiens (Human) | CVCL_0060 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay; Colony formation assay; Flow cytometry assay; Wound healing assay; Transwell assay; TUNEL assay | |||
| Mechanism Description | Decreased expression of linc-ROR effectively reversed EMT in docetaxel-resistant LAD cells and sensitized them to chemotherapy. The function of linc-ROR exerted in LAD cells depended on the sponging of miR145, therefore, releasing the miR145 target FSCN1, and thus contributing to the acquisition of chemoresistance and EMT phenotypes of docetaxel-resistant LAD cells. | |||
| Key Molecule: Long non-protein coding RNA, regulator of reprogramming (LINC-ROR) | [28] | |||
| Resistant Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Lung cancer [ICD-11: 2C25] | |||
| The Specified Disease | Lung adenocarcinoma | |||
| The Studied Tissue | Lung | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.85E-01 Fold-change: 6.05E-01 Z-score: 1.34E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Epithelial mesenchymal transition signaling pathway | Inhibition | hsa01521 | |
| In Vitro Model | SPC-A1 cells | Lung | Homo sapiens (Human) | CVCL_6955 |
| H1299 cells | Lung | Homo sapiens (Human) | CVCL_0060 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
qPCR | |||
| Experiment for Drug Resistance |
MTT assay; Colony formation assay; Flow cytometry assay; Wound healing assay; Transwell assay; TUNEL assay | |||
| Mechanism Description | Down regulation of linc-ROR reversed the chemoresistance and EMT features of these cells by targeting miR145 and its target gene FSCN1. | |||
| Key Molecule: ENSG00000247844 (CCAT1) | [26] | |||
| Resistant Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Lung cancer [ICD-11: 2C25] | |||
| The Specified Disease | Lung adenocarcinoma | |||
| The Studied Tissue | Lung | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 2.21E-03 Fold-change: 5.50E+00 Z-score: 3.09E+00 |
|||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell invasion | Activation | hsa05200 | ||
| Cell migration | Activation | hsa04670 | ||
| Cell proliferation | Activation | hsa05200 | ||
| In Vitro Model | SPC-A1 cells | Lung | Homo sapiens (Human) | CVCL_6955 |
| H1299 cells | Lung | Homo sapiens (Human) | CVCL_0060 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
MTT assay; Colony formation assay; Tunel assay | |||
| Mechanism Description | Long noncoding RNA CCAT1 acts as an oncogene and promotes chemoresistance in docetaxel-resistant lung adenocarcinoma cells.the sponging of let-7c by CCAT1 released Bcl-xl (a let-7c target), thereby promoting the acquisition of chemoresistance and epithelial-to-mesenchymal transition phenotypes in docetaxel-resistant LAD cells. | |||
| Key Molecule: hsa-mir-145 | [28] | |||
| Resistant Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Epithelial mesenchymal transition signaling pathway | Inhibition | hsa01521 | |
| In Vitro Model | SPC-A1 cells | Lung | Homo sapiens (Human) | CVCL_6955 |
| H1299 cells | Lung | Homo sapiens (Human) | CVCL_0060 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
MTT assay; Colony formation assay; Flow cytometry assay; Wound healing assay; Transwell assay; TUNEL assay | |||
| Mechanism Description | Decreased expression of linc-ROR effectively reversed EMT in docetaxel-resistant LAD cells and sensitized them to chemotherapy. The function of linc-ROR exerted in LAD cells depended on the sponging of miR145, therefore, releasing the miR145 target FSCN1, and thus contributing to the acquisition of chemoresistance and EMT phenotypes of docetaxel-resistant LAD cells. | |||
| Key Molecule: hsa-let-7c | [26] | |||
| Resistant Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell invasion | Activation | hsa05200 | ||
| Cell migration | Activation | hsa04670 | ||
| Cell proliferation | Activation | hsa05200 | ||
| In Vitro Model | SPC-A1 cells | Lung | Homo sapiens (Human) | CVCL_6955 |
| H1299 cells | Lung | Homo sapiens (Human) | CVCL_0060 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
MTT assay; Colony formation assay; Tunel assay | |||
| Mechanism Description | Long noncoding RNA CCAT1 acts as an oncogene and promotes chemoresistance in docetaxel-resistant lung adenocarcinoma cells.the sponging of let-7c by CCAT1 released Bcl-xl (a let-7c target), thereby promoting the acquisition of chemoresistance and epithelial-to-mesenchymal transition phenotypes in docetaxel-resistant LAD cells. | |||
| Key Molecule: Myc proto-oncogene protein (MYC) | [50] | |||
| Resistant Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell acquisition of epithelial-mesenchymal transition | Activation | hsa01521 | |
| Cell invasion | Activation | hsa05200 | ||
| Cell migration | Activation | hsa04670 | ||
| miR451/cMyc/ERK/GSk3Beta signaling pathway | Regulation | N.A. | ||
| In Vitro Model | SPC-A1 cells | Lung | Homo sapiens (Human) | CVCL_6955 |
| H1299 cells | Lung | Homo sapiens (Human) | CVCL_0060 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay; Flow cytometry assay | |||
| Mechanism Description | miR-451 was found to be significantly downregulated in docetaxel-resistant LAD cells, and re-expression of miR-451 could reverse EMT to mesenchymal-epithelial transition (MET) and inhibit invasion and metastasis of docetaxel-resistant LAD cells both in vitro and in vivo. and the overexpressionof c-Myc which induced extracellular-signal-regulated kinase (ERk)-dependent glycogen synthase kinase-3 beta (GSk-3beta) inactivation and subsequent snail activation is essential for acquisition of EMT phenotype induced by loss of miR-451. | |||
| Key Molecule: hsa-mir-451 | [50] | |||
| Resistant Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell invasion | Activation | hsa05200 | |
| Cell migration | Activation | hsa04670 | ||
| miR451/cMyc/ERK/GSk3Beta signaling pathway | Regulation | N.A. | ||
| In Vitro Model | SPC-A1 cells | Lung | Homo sapiens (Human) | CVCL_6955 |
| H1299 cells | Lung | Homo sapiens (Human) | CVCL_0060 | |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
MTT assay; Flow cytometry assay | |||
| Mechanism Description | miR-451 was found to be significantly downregulated in docetaxel-resistant LAD cells, and re-expression of miR-451 could reverse EMT to mesenchymal-epithelial transition (MET) and inhibit invasion and metastasis of docetaxel-resistant LAD cells both in vitro and in vivo. and the overexpressionof c-Myc which induced extracellular-signal-regulated kinase (ERk)-dependent glycogen synthase kinase-3 beta (GSk-3beta) inactivation and subsequent snail activation is essential for acquisition of EMT phenotype induced by loss of miR-451. | |||
| Key Molecule: ATPase H+ transporting V0 subunit d1 (ATP6V0D1) | [51] | |||
| Resistant Disease | Non-small cell lung cancer [ICD-11: 2C25.Y] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Non-small cell lung cancer isolates | Lung | Homo sapiens (Human) | N.A. |
| Experiment for Molecule Alteration |
Immunofluorescence assay | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | The drug resistance of cancer cells is likely to be related to the changes in pH gradient between the extracellular environment and the cytoplasm.Vacuolar-H+ -ATPase(V-ATPase) plays a major role in the regulation of cellular pH conditions.The expression of V-ATPase was shown to be related to the pathological type and grade of the cancer and might be associated with the chemotherapy drug resistance in NSCLC. | |||
| Key Molecule: ATPase H+ transporting V0 subunit d1 (ATP6V0D1) | [51] | |||
| Resistant Disease | Lung squamous cell carcinoma [ICD-11: 2C25.3] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Non-small cell lung cancer isolates | Lung | Homo sapiens (Human) | N.A. |
| Experiment for Molecule Alteration |
Immunofluorescence assay | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | The drug resistance of cancer cells is likely to be related to the changes in pH gradient between the extracellular environment and the cytoplasm.Vacuolar-H+ -ATPase(V-ATPase) plays a major role in the regulation of cellular pH conditions.The expression of V-ATPase was shown to be related to the pathological type and grade of the cancer and might be associated with the chemotherapy drug resistance in NSCLC. | |||
| Key Molecule: ATPase H+ transporting V0 subunit d1 (ATP6V0D1) | [51] | |||
| Resistant Disease | Non-small cell lung cancer [ICD-11: 2C25.Y] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Non-small cell lung cancer isolates | Lung | Homo sapiens (Human) | N.A. |
| Experiment for Molecule Alteration |
Immunofluorescence assay | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | The drug resistance of cancer cells is likely to be related to the changes in pH gradient between the extracellular environment and the cytoplasm.Vacuolar-H+ -ATPase(V-ATPase) plays a major role in the regulation of cellular pH conditions.The expression of V-ATPase was shown to be related to the pathological type and grade of the cancer and might be associated with the chemotherapy drug resistance in NSCLC. | |||
|
|
||||
| Key Molecule: hsa-mir-27b | [25] | |||
| Resistant Disease | Non-small cell lung cancer [ICD-11: 2C25.Y] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell proliferation | Activation | hsa05200 | ||
| EGFR signaling pathway | Inhibition | hsa01521 | ||
| In Vitro Model | A549 cells | Lung | Homo sapiens (Human) | CVCL_0023 |
| Experiment for Molecule Alteration |
RT-qPCR | |||
| Experiment for Drug Resistance |
MTT assay; Flow cytometry assay | |||
| Mechanism Description | The transfection of miR 27b mimics led to downregulation of the expression levels of EGFR, whilst miR 27b inhibitors upregulated the expression levels of EGFR. Furthermore, it was demonstrated that the transfection of miR 27b mimics significantly suppressed the apoptosis and promote the viability of A549 human lung carcinoma cells. In line with this, the introduction of miR 27b inhibitors significantly induced apoptosis and inhibited the proliferation of A549 cells. These results indicate that miR 27b may promote NSCLC cell viability and enhance resistance to docetaxel treatment through direct inhibition of EGFR expression. | |||
| Key Molecule: hsa-mir-21 | [7] | |||
| Resistant Disease | Non-small cell lung cancer [ICD-11: 2C25.Y] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell migration | Activation | hsa04670 | ||
| PTEN signaling pathway | Inhibition | hsa05235 | ||
| In Vitro Model | KB-3-1 cells | Lung | Homo sapiens (Human) | CVCL_2088 |
| KB-CP.5 cells | Lung | Homo sapiens (Human) | CVCL_IP04 | |
| KB-CP20 cells | Lung | Homo sapiens (Human) | CVCL_IP06 | |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | PTEN, a tumor suppressor gene, is an essential regulator of cell proliferation, differentiation, growth, and apoptosis. miR-21 can promote growth, migration, and invasion, chemo- or radioresistance of NSCLC cells by downregulation PTEN. | |||
| Key Molecule: hsa-mir-192 | [16] | |||
| Resistant Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | SPC-A1 cells | Lung | Homo sapiens (Human) | CVCL_6955 |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
Clonogenic assay | |||
| Mechanism Description | Six miRNAs (miR-192, 200b, 194, 424, 98 and 212) exhibited more than 2-fold changes in their expression levels, which were validated by qRT-PCR. The expression of three miRNAs (miR-200b, 194 and 212) was significantly down-regulated in SPC-A1/docetaxel cells, while the expression of other three miRNAs (miR-192, 424 and 98) was significantly up-regulated in SPC-A1/docetaxel cells (P < 0.01). | |||
| Key Molecule: hsa-mir-194 | [16] | |||
| Resistant Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | SPC-A1 cells | Lung | Homo sapiens (Human) | CVCL_6955 |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
Clonogenic assay | |||
| Mechanism Description | Six miRNAs (miR-192, 200b, 194, 424, 98 and 212) exhibited more than 2-fold changes in their expression levels, which were validated by qRT-PCR. The expression of three miRNAs (miR-200b, 194 and 212) was significantly down-regulated in SPC-A1/docetaxel cells, while the expression of other three miRNAs (miR-192, 424 and 98) was significantly up-regulated in SPC-A1/docetaxel cells (P < 0.01). | |||
| Key Molecule: hsa-mir-200b | [16] | |||
| Resistant Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | SPC-A1 cells | Lung | Homo sapiens (Human) | CVCL_6955 |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
Clonogenic assay | |||
| Mechanism Description | Six miRNAs (miR-192, 200b, 194, 424, 98 and 212) exhibited more than 2-fold changes in their expression levels, which were validated by qRT-PCR. The expression of three miRNAs (miR-200b, 194 and 212) was significantly down-regulated in SPC-A1/docetaxel cells, while the expression of other three miRNAs (miR-192, 424 and 98) was significantly up-regulated in SPC-A1/docetaxel cells (P < 0.01). | |||
| Key Molecule: hsa-mir-212 | [16] | |||
| Resistant Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | SPC-A1 cells | Lung | Homo sapiens (Human) | CVCL_6955 |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
Clonogenic assay | |||
| Mechanism Description | Six miRNAs (miR-192, 200b, 194, 424, 98 and 212) exhibited more than 2-fold changes in their expression levels, which were validated by qRT-PCR. The expression of three miRNAs (miR-200b, 194 and 212) was significantly down-regulated in SPC-A1/docetaxel cells, while the expression of other three miRNAs (miR-192, 424 and 98) was significantly up-regulated in SPC-A1/docetaxel cells (P < 0.01). | |||
| Key Molecule: hsa-mir-424 | [16] | |||
| Resistant Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | SPC-A1 cells | Lung | Homo sapiens (Human) | CVCL_6955 |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
Clonogenic assay | |||
| Mechanism Description | Six miRNAs (miR-192, 200b, 194, 424, 98 and 212) exhibited more than 2-fold changes in their expression levels, which were validated by qRT-PCR. The expression of three miRNAs (miR-200b, 194 and 212) was significantly down-regulated in SPC-A1/docetaxel cells, while the expression of other three miRNAs (miR-192, 424 and 98) was significantly up-regulated in SPC-A1/docetaxel cells (P < 0.01). | |||
| Key Molecule: hsa-mir-98 | [16] | |||
| Resistant Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | SPC-A1 cells | Lung | Homo sapiens (Human) | CVCL_6955 |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
Clonogenic assay | |||
| Mechanism Description | Six miRNAs (miR-192, 200b, 194, 424, 98 and 212) exhibited more than 2-fold changes in their expression levels, which were validated by qRT-PCR. The expression of three miRNAs (miR-200b, 194 and 212) was significantly down-regulated in SPC-A1/docetaxel cells, while the expression of other three miRNAs (miR-192, 424 and 98) was significantly up-regulated in SPC-A1/docetaxel cells (P < 0.01). | |||
|
|
||||
| Key Molecule: Glutamate dehydrogenase 1 (GLUD1) | [49] | |||
| Metabolic Type | Glutamine metabolism | |||
| Resistant Disease | Non-small cell lung carcinoma [ICD-11: 2C25.Y] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | A549 cells | Lung | Homo sapiens (Human) | CVCL_0023 |
| H1975 cells | Lung | Homo sapiens (Human) | CVCL_B0JT | |
| HCC827 cells | Lung | Homo sapiens (Human) | CVCL_2063 | |
| Experiment for Molecule Alteration |
qRT-PCR; Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | Pharmacological and genetic interference with GLUD1 in vitro significantly reversed drug resistance and decreased cell migration and invasion capability. Lastly, the successful application of R162, a selective GLUD1 inhibitor, to overcome both acquired resistance and EMT-induced metastasis in vivo, identified GLUD1 as a promising and druggable therapeutic target for malignant progression of NSCLC. Collectively, our study offers a potential strategy for NSCLC therapy, especially for drug-resistant patients with highly expressed GLUD1. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: L-glutamine amidohydrolase (GLS) | [29] | |||
| Metabolic Type | Glutamine metabolism | |||
| Resistant Disease | Prostate cancer [ICD-11: 2C82.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Prostate cancer [ICD-11: 2C82] | |||
| The Specified Disease | Prostate cancer | |||
| The Studied Tissue | Prostate | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.08E-01 Fold-change: 5.66E-02 Z-score: 1.67E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | 293 T cells | Blood | Homo sapiens (Human) | N.A. |
| DU145 cells | Prostate | Homo sapiens (Human) | CVCL_0105 | |
| PC-3 cells | Bone | Homo sapiens (Human) | CVCL_0035 | |
| Experiment for Molecule Alteration |
qRT-PCR; Western blot analysis | |||
| Experiment for Drug Resistance |
Cell viability assay | |||
| Mechanism Description | Mechanistically, Gln deprivation reduced OXPHOS and ATP levels, causing a disturbance in cell cycle progression. Genetic and chemical inhibition of the Gln-metabolism key protein GLS1 could validate the Gln deprivation results, thereby representing a valid therapeutic target. Moreover, immunohistological investigation of GLS1 revealed a high-expressing GLS1 subgroup post-docetaxel failure, exhibiting low overall survival. This subgroup presents an intriguing opportunity for targeted therapy focusing on glutamine metabolism. | |||
|
|
||||
| Key Molecule: Metastasis-associated protein MTA1 (MTA1) | [11] | |||
| Resistant Disease | Prostate cancer [ICD-11: 2C82.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Prostate cancer [ICD-11: 2C82] | |||
| The Specified Disease | Prostate cancer | |||
| The Studied Tissue | Prostate | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.16E-01 Fold-change: 4.86E-02 Z-score: 1.64E+00 |
|||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | MTA1 signaling pathway | Activation | hsa05206 | |
| In Vitro Model | LNCaP cells | Prostate | Homo sapiens (Human) | CVCL_0395 |
| PC3 cells | Prostate | Homo sapiens (Human) | CVCL_0035 | |
| Experiment for Molecule Alteration |
Promoter reporter assay; Western blot analysis | |||
| Experiment for Drug Resistance |
ELISA; MTT assay | |||
| Mechanism Description | Regulation of docetaxel sensitivity in prostate cancer cells by hsa-miR125a-3p via modulation of metastasis-associated protein 1 signaling, MTA1 is a direct target of hsa-mir125a-3p in pca cells. | |||
| Key Molecule: Apoptosis regulator Bcl-2 (BCL2) | [30] | |||
| Resistant Disease | Prostate cancer [ICD-11: 2C82.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Prostate cancer [ICD-11: 2C82] | |||
| The Specified Disease | Prostate cancer | |||
| The Studied Tissue | Prostate | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.29E-02 Fold-change: 4.09E-02 Z-score: 2.60E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell proliferation | Activation | hsa05200 | ||
| In Vitro Model | DU-145 cells | Prostate | Homo sapiens (Human) | CVCL_0105 |
| PC3 cells | Prostate | Homo sapiens (Human) | CVCL_0035 | |
| 22RV1 cells | Prostate | Homo sapiens (Human) | CVCL_1045 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Acid phosphatase assay | |||
| Mechanism Description | miR-34a regulates BCL-2 and may, in part, regulate response to docetaxel. miR-34a was significantly decreased in prostate cancer versus normal tissues; in biochemical recurrence versus non-recurrence tissue and in metastatic versus primary disease prostate tissue. We confirmed BCL-2 as a target of miR-34a, by manipulating miR-34a expression in our parent and docetaxel resistant cell lines and subsequently assessing BCL-2 levels. Specifically, upon inhibition of miR-34a in sensitive parent cells (PC3 and 22Rv1) we observed an increase in BCL-2 expression, whereas mimicking miR-34a expression in docetaxel-resistant cells (PC3RD and 22Rv1RD) resulted in decreased BCL-2 expression. | |||
| Key Molecule: A-kinase anchor protein 12 (AKAP12) | [33] | |||
| Resistant Disease | Prostate cancer [ICD-11: 2C82.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Prostate cancer [ICD-11: 2C82] | |||
| The Specified Disease | Prostate cancer | |||
| The Studied Tissue | Prostate | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 7.51E-02 Fold-change: 1.30E-01 Z-score: 1.86E+00 |
|||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell invasion | Activation | hsa05200 | ||
| Cell proliferation | Activation | hsa05200 | ||
| In Vitro Model | DU-145 cells | Prostate | Homo sapiens (Human) | CVCL_0105 |
| PC3 cells | Prostate | Homo sapiens (Human) | CVCL_0035 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay; Scratch Wound healing assay; Transwell Invasion assay; Flow cytometry assay | |||
| Mechanism Description | Knockdown of MALAT1 in DTX-resistant PCa cells up-regulated miR-145-5p as well as suppressed AkAP12 expression, further inhibited cell viability and induced apoptosis. | |||
| Key Molecule: Tumor necrosis factor ligand superfamily member 10 (TNFSF10) | [35] | |||
| Resistant Disease | Prostate cancer [ICD-11: 2C82.0] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Prostate cancer [ICD-11: 2C82] | |||
| The Specified Disease | Prostate cancer | |||
| The Studied Tissue | Prostate | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 9.66E-01 Fold-change: -7.23E-04 Z-score: -4.34E-02 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell proliferation | Activation | hsa05200 | ||
| In Vitro Model | LNCaP cells | Prostate | Homo sapiens (Human) | CVCL_0395 |
| VCaP cells | Prostate | Homo sapiens (Human) | CVCL_2235 | |
| LTAD cells | Prostate | Homo sapiens (Human) | N.A. | |
| Experiment for Molecule Alteration |
Western blot analysis; Immunohistochemistry assay | |||
| Experiment for Drug Resistance |
TUNEL assay; MTS assay | |||
| Mechanism Description | Androgen-induced Long Noncoding RNA (LncRNA) SOCS2-AS1 Promotes Cell Growth and Inhibits Apoptosis in Prostate Cancer Cells.suppressor of cytokine signaling 2-antisense transcript 1 (SOCS2-AS1), the expression of which was higher in castration-resistant prostate cancer model cells.SOCS2-AS1 promoted castration-resistant and androgen-dependent cell growth. We found that SOCS2-AS1 knockdown up-regulated genes related to the apoptosis pathway, including tumor necrosis factor superfamily 10 (TNFSF10), and sensitized prostate cancer cells to docetaxel treatment. Moreover, we also demonstrated that SOCS2-AS1 promotes androgen signaling by modulating the epigenetic control for AR target genes including TNFSF10 These findings suggest that SOCS2-AS1 plays an important role in the development of castration-resistant prostate cancer by repressing apoptosis. | |||
| Key Molecule: Protein transport protein Sec23A (SEC23A) | [38] | |||
| Resistant Disease | Prostate cancer [ICD-11: 2C82.0] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Prostate cancer [ICD-11: 2C82] | |||
| The Specified Disease | Prostate cancer | |||
| The Studied Tissue | Prostate | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 2.83E-01 Fold-change: -4.00E-02 Z-score: -1.10E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | DU-145 cells | Prostate | Homo sapiens (Human) | CVCL_0105 |
| PC3 cells | Prostate | Homo sapiens (Human) | CVCL_0035 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay; Annexin V-PE Apoptosis assay | |||
| Mechanism Description | miR375 induces docetaxel resistance in prostate cancer by targeting SEC23A and YAP1. | |||
| Key Molecule: Tumor protein p73 (TP73) | [39] | |||
| Resistant Disease | Prostate cancer [ICD-11: 2C82.0] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Prostate cancer [ICD-11: 2C82] | |||
| The Specified Disease | Prostate cancer | |||
| The Studied Tissue | Prostate | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 4.84E-04 Fold-change: -1.18E-01 Z-score: -4.02E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | DU-145 cells | Prostate | Homo sapiens (Human) | CVCL_0105 |
| PC3 cells | Prostate | Homo sapiens (Human) | CVCL_0035 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Luciferase reporter assay; Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay; Flow cytometric analysis | |||
| Mechanism Description | microRNA-323 upregulation promotes prostate cancer growth and docetaxel resistance by repressing p73. | |||
|
|
||||
| Key Molecule: hsa-mir-323 | [39] | |||
| Resistant Disease | Prostate cancer [ICD-11: 2C82.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | DU-145 cells | Prostate | Homo sapiens (Human) | CVCL_0105 |
| PC3 cells | Prostate | Homo sapiens (Human) | CVCL_0035 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
MTT assay; Flow cytometric analysis | |||
| Mechanism Description | microRNA-323 upregulation promotes prostate cancer growth and docetaxel resistance by repressing p73. | |||
| Key Molecule: hsa-mir-181a | [68] | |||
| Resistant Disease | Prostate cancer [ICD-11: 2C82.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | C4-2B cells | Prostate | Homo sapiens (Human) | CVCL_4784 |
| TaxR cells | Prostate | Homo sapiens (Human) | CVCL_4V97 | |
| Experiment for Molecule Alteration |
RT-PCR | |||
| Experiment for Drug Resistance |
Beckman Coulter method; Rhodamine Assay; Cell Death ELISA | |||
| Mechanism Description | Overexpression of miR181a in prostate cancer cells contributes to their resistance to docetaxel, this is due, in part, to modulation of p53 phosphorylation and apoptosis. | |||
| Key Molecule: hsa-miR-125a-3p | [11] | |||
| Resistant Disease | Prostate cancer [ICD-11: 2C82.0] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | MTA1 signaling pathway | Activation | hsa05206 | |
| In Vitro Model | LNCaP cells | Prostate | Homo sapiens (Human) | CVCL_0395 |
| PC3 cells | Prostate | Homo sapiens (Human) | CVCL_0035 | |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
ELISA; MTT assay | |||
| Mechanism Description | Regulation of docetaxel sensitivity in prostate cancer cells by hsa-miR125a-3p via modulation of metastasis-associated protein 1 signaling, MTA1 is a direct target of hsa-mir125a-3p in pca cells. | |||
| Key Molecule: hsa-mir-375 | [38] | |||
| Resistant Disease | Prostate cancer [ICD-11: 2C82.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | DU-145 cells | Prostate | Homo sapiens (Human) | CVCL_0105 |
| PC3 cells | Prostate | Homo sapiens (Human) | CVCL_0035 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
CCK8 assay; Annexin V-PE Apoptosis assay | |||
| Mechanism Description | miR375 induces docetaxel resistance in prostate cancer by targeting SEC23A and YAP1. | |||
| Key Molecule: hsa-mir-195 | [69] | |||
| Resistant Disease | Prostate cancer [ICD-11: 2C82.0] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell colony | Activation | hsa05200 | ||
| Cell viability | Activation | hsa05200 | ||
| In Vitro Model | DU-145 cells | Prostate | Homo sapiens (Human) | CVCL_0105 |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
CCK8 assay; Flow cytometry assay | |||
| Mechanism Description | miR-195 improved the sensitivity of resistant PC cells to DOC by suppressing CLU. | |||
| Key Molecule: Metastasis associated lung adenocarcinoma transcript 1 (MALAT1) | [33] | |||
| Resistant Disease | Prostate cancer [ICD-11: 2C82.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell invasion | Activation | hsa05200 | ||
| Cell proliferation | Activation | hsa05200 | ||
| In Vitro Model | DU-145 cells | Prostate | Homo sapiens (Human) | CVCL_0105 |
| PC3 cells | Prostate | Homo sapiens (Human) | CVCL_0035 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
qPCR | |||
| Experiment for Drug Resistance |
MTT assay; Scratch Wound healing assay; Transwell Invasion assay; Flow cytometry assay | |||
| Mechanism Description | Knockdown of MALAT1 in DTX-resistant PCa cells up-regulated miR-145-5p as well as suppressed AkAP12 expression, further inhibited cell viability and induced apoptosis. | |||
| Key Molecule: SOCS2 antisense RNA 1 (SOCS2-AS1) | [35] | |||
| Resistant Disease | Prostate cancer [ICD-11: 2C82.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell proliferation | Activation | hsa05200 | ||
| In Vitro Model | LNCaP cells | Prostate | Homo sapiens (Human) | CVCL_0395 |
| VCaP cells | Prostate | Homo sapiens (Human) | CVCL_2235 | |
| LTAD cells | Prostate | Homo sapiens (Human) | N.A. | |
| Experiment for Molecule Alteration |
qPCR | |||
| Experiment for Drug Resistance |
TUNEL assay; MTS assay | |||
| Mechanism Description | Androgen-induced Long Noncoding RNA (LncRNA) SOCS2-AS1 Promotes Cell Growth and Inhibits Apoptosis in Prostate Cancer Cells.suppressor of cytokine signaling 2-antisense transcript 1 (SOCS2-AS1), the expression of which was higher in castration-resistant prostate cancer model cells.SOCS2-AS1 promoted castration-resistant and androgen-dependent cell growth. We found that SOCS2-AS1 knockdown up-regulated genes related to the apoptosis pathway, including tumor necrosis factor superfamily 10 (TNFSF10), and sensitized prostate cancer cells to docetaxel treatment. Moreover, we also demonstrated that SOCS2-AS1 promotes androgen signaling by modulating the epigenetic control for AR target genes including TNFSF10 These findings suggest that SOCS2-AS1 plays an important role in the development of castration-resistant prostate cancer by repressing apoptosis. | |||
| Key Molecule: hsa-mir-34 | [30] | |||
| Resistant Disease | Prostate cancer [ICD-11: 2C82.0] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell proliferation | Activation | hsa05200 | ||
| In Vitro Model | DU-145 cells | Prostate | Homo sapiens (Human) | CVCL_0105 |
| PC3 cells | Prostate | Homo sapiens (Human) | CVCL_0035 | |
| 22RV1 cells | Prostate | Homo sapiens (Human) | CVCL_1045 | |
| Experiment for Molecule Alteration |
qPCR | |||
| Experiment for Drug Resistance |
Acid phosphatase assay | |||
| Mechanism Description | miR-34a regulates BCL-2 and may, in part, regulate response to docetaxel. miR-34a was significantly decreased in prostate cancer versus normal tissues; in biochemical recurrence versus non-recurrence tissue and in metastatic versus primary disease prostate tissue. We confirmed BCL-2 as a target of miR-34a, by manipulating miR-34a expression in our parent and docetaxel resistant cell lines and subsequently assessing BCL-2 levels. Specifically, upon inhibition of miR-34a in sensitive parent cells (PC3 and 22Rv1) we observed an increase in BCL-2 expression, whereas mimicking miR-34a expression in docetaxel-resistant cells (PC3RD and 22Rv1RD) resulted in decreased BCL-2 expression. | |||
| Key Molecule: hsa-mir-205 | [70], [71] | |||
| Resistant Disease | Prostate cancer [ICD-11: 2C82.0] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell proliferation | Activation | hsa05200 | ||
| In Vitro Model | DU-145 cells | Prostate | Homo sapiens (Human) | CVCL_0105 |
| LNCaP cells | Prostate | Homo sapiens (Human) | CVCL_0395 | |
| PC3 cells | Prostate | Homo sapiens (Human) | CVCL_0035 | |
| RWPE-1 cells | Prostate | Homo sapiens (Human) | CVCL_3791 | |
| 22RV1 cells | Prostate | Homo sapiens (Human) | CVCL_1045 | |
| VCaP cells | Prostate | Homo sapiens (Human) | CVCL_2235 | |
| WPE1-NA22 cells | Prostate | Homo sapiens (Human) | CVCL_3810 | |
| WPE1-NB11 cells | Prostate | Homo sapiens (Human) | CVCL_3811 | |
| WPE1-NB14 cells | Prostate | Homo sapiens (Human) | CVCL_3812 | |
| WPE1-NB26 cells | Prostate | Homo sapiens (Human) | CVCL_3813 | |
| Experiment for Molecule Alteration |
RT-qPCR | |||
| Experiment for Drug Resistance |
MTT assay; Flow cytometry assay | |||
| Mechanism Description | Docetaxel-resistant cells showed a reduced E-cadherin and an increased vimentin expression accompanied by induced expression of stem cell markers compared with parental cells. Decreased Expression of miR-200c and miR-205 Is Responsible for E-Cadherin Loss in Chemotherapy-Resistant Cells. And miR-205 and miR-31 regulate apoptosis in prostate cancer cells by targeting antiapoptotic proteins Bcl-w and E2F6. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Forkhead box protein O3 (FOXO3) | [31] | |||
| Sensitive Disease | Prostate cancer [ICD-11: 2C82.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Prostate cancer [ICD-11: 2C82] | |||
| The Specified Disease | Prostate cancer | |||
| The Studied Tissue | Prostate | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 2.55E-01 Fold-change: 1.97E-02 Z-score: 1.16E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell viability | Inhibition | hsa05200 | ||
| In Vitro Model | DU-145 cells | Prostate | Homo sapiens (Human) | CVCL_0105 |
| LNCaP cells | Prostate | Homo sapiens (Human) | CVCL_0395 | |
| PC3 cells | Prostate | Homo sapiens (Human) | CVCL_0035 | |
| C4-2 cells | Prostate | Homo sapiens (Human) | CVCL_4782 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay; TUNEL assay; Flow cytometry assay | |||
| Mechanism Description | miR-223-3p inhibitor sensitized prostatic cancer mouse model to docetaxel by increasing the expression of FOXO3. | |||
|
|
||||
| Key Molecule: Cancer susceptibility 2 (CASC2) | [34] | |||
| Sensitive Disease | Prostate cancer [ICD-11: 2C82.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Prostate cancer [ICD-11: 2C82] | |||
| The Specified Disease | Prostate adenocarcinoma | |||
| The Studied Tissue | Prostate | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 4.71E-02 Fold-change: 1.02E-01 Z-score: 1.99E+00 |
|||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | ERK signaling pathway | Regulation | N.A. | |
| RTK signaling pathway | Inhibition | hsa04015 | ||
| In Vitro Model | DU-145 cells | Prostate | Homo sapiens (Human) | CVCL_0105 |
| LNCaP cells | Prostate | Homo sapiens (Human) | CVCL_0395 | |
| PC3 cells | Prostate | Homo sapiens (Human) | CVCL_0035 | |
| RWPE-1 cells | Prostate | Homo sapiens (Human) | CVCL_3791 | |
| C4-2 cells | Prostate | Homo sapiens (Human) | CVCL_4782 | |
| Experiment for Molecule Alteration |
RT-PCR | |||
| Experiment for Drug Resistance |
MTT assay; Flow cytometer assay | |||
| Mechanism Description | CASC2 directly targets miR183 to inhibit its expression. SPRY2 is regarded as a negative regulator RTk signaling pathway, antagonizing cell migration and/or cellular differentiation occurring through the ERk signaling. CASC2 competes with SPRY2 for miR183 binding to rescue the expression of SPRY2 in PC cells, thus suppressing the cell proliferation and promoting the apoptosis of PC cells, finally enhancing PC cells chemo-sensitivity to docetaxel through SPRY2 downstream ERk signaling pathway. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Sphingosine kinase 1 (SPHK1) | [9] | |||
| Resistant Disease | Gastric cardia adenocarcinoma [ICD-11: 2B72.2] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Gastric cancer [ICD-11: 2B72] | |||
| The Specified Disease | Gastric cancer | |||
| The Studied Tissue | Gastric tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 2.05E-02 Fold-change: 3.13E-01 Z-score: 6.42E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | AGS cells | Gastric | Homo sapiens (Human) | CVCL_0139 |
| Experiment for Molecule Alteration |
Immunohistochemistry assay | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | S1P could lead to cytotoxic drug resistance in gastroesophegal cancer acting in an autocrine or paracrine manner via cell surface S1P receptors following transportation out of the cytosol. Alternatively S1P may mediate cytotoxic drug resistance acting intracellularly by counteracting apoptosis mediated by its pro-apoptotic precursor ceramide or interaction with known intracellular targets involved in cancer pathogenesis and cytotoxic drug resistance such as Histone deacetylase 1 (HDAC1) and Histone deacetylase 2 (HDAC 2) to which S1P directly binds and inhibits, and TNF Receptor-Associated Factor 2 (TRAF 2), or Protein Kinase C (PKC). S1P production controlled by SPHK1 and SGPL1 are key determinants of cytotoxic drug resistance and that decreasing S1P production in cancer cells could lead to increased cytotoxic sensitivity. | |||
| Key Molecule: F-box/WD repeat-containing protein 7 (FBXW7) | [43] | |||
| Resistant Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell proliferation | Activation | hsa05200 | |
| In Vitro Model | BGC-823 cells | Gastric | Homo sapiens (Human) | CVCL_3360 |
| MGC-803 cells | Gastric | Homo sapiens (Human) | CVCL_5334 | |
| SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 | |
| HGC27 cells | Gastric | Homo sapiens (Human) | CVCL_1279 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | miR-363 promotes gastric cancer cells proliferation by inhibiting FBW7 expression and was associated with chemo-resistance of gastric cancer cells. Silencing FBW7 largely phenocopied miR-363-induced resistance to chemotherapy agents and promoted proliferation in gastric cancer cells. In addition, an inverse correlation between miR-363 and FBW7 mRNA expression was observed in gastric cancer tissues. | |||
| Key Molecule: E3 SUMO-protein ligase EGR2 (EGR2) | [15] | |||
| Resistant Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell migration | Activation | hsa04670 | ||
| Cell proliferation | Activation | hsa05200 | ||
| EGR2 signaling pathway | Inhibition | hsa04625 | ||
| In Vitro Model | SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 |
| GES-1 cells | Gastric | Homo sapiens (Human) | CVCL_EQ22 | |
| MkN-45 cells | Gastric | Homo sapiens (Human) | CVCL_0434 | |
| NUGC3 cells | Gastric | Homo sapiens (Human) | CVCL_1612 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miR-20a promoted the growth, migration and invasion of GC cells, enhanced the chemoresistance of GC cells to cisplatin and docetaxel. Luciferase activity and Western blot confirmed that miR-20a negatively regulated EGR2 expression. Overexpression of EGR2 significantly attenuated the oncogenic effect of miR-20a. | |||
| Key Molecule: Hypoxia-inducible factor 2-alpha (EPAS1) | [45] | |||
| Resistant Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | BGC-823 cells | Gastric | Homo sapiens (Human) | CVCL_3360 |
| BEL-7402 cells | Liver | Homo sapiens (Human) | CVCL_5492 | |
| HepG2 cells | Liver | Homo sapiens (Human) | CVCL_0027 | |
| HEK293 cells | Kidney | Homo sapiens (Human) | CVCL_0045 | |
| Experiment for Molecule Alteration |
Western blotting assay | |||
| Experiment for Drug Resistance |
Flow cytometry assay | |||
| Mechanism Description | Over-expression of EPAS-1 increased the expression of PXR responsive genes, enhanced the proliferation of BGC-823 cells and boosted the resistance of BGC-823 cells against the cytotoxicity of chemotherapeutic drugs, e.g. Mitomycin C and Paclitaxel.EPAS-1 reduces BGC-823 cell apoptosis induced by Mitomycin C and Paclitaxel. | |||
| Key Molecule: Sphingosine-1-phosphate lyase 1 (SGPL1) | [9] | |||
| Resistant Disease | Gastric cardia adenocarcinoma [ICD-11: 2B72.2] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | AGS cells | Gastric | Homo sapiens (Human) | CVCL_0139 |
| Experiment for Molecule Alteration |
Immunohistochemistry assay | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | S1P could lead to cytotoxic drug resistance in gastroesophegal cancer acting in an autocrine or paracrine manner via cell surface S1P receptors following transportation out of the cytosol. Alternatively S1P may mediate cytotoxic drug resistance acting intracellularly by counteracting apoptosis mediated by its pro-apoptotic precursor ceramide or interaction with known intracellular targets involved in cancer pathogenesis and cytotoxic drug resistance such as Histone deacetylase 1 (HDAC1) and Histone deacetylase 2 (HDAC 2) to which S1P directly binds and inhibits, and TNF Receptor-Associated Factor 2 (TRAF 2), or Protein Kinase C (PKC). S1P production controlled by SPHK1 and SGPL1 are key determinants of cytotoxic drug resistance and that decreasing S1P production in cancer cells could lead to increased cytotoxic sensitivity. | |||
|
|
||||
| Key Molecule: hsa-mir-363 | [43] | |||
| Resistant Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell proliferation | Activation | hsa05200 | |
| In Vitro Model | BGC-823 cells | Gastric | Homo sapiens (Human) | CVCL_3360 |
| MGC-803 cells | Gastric | Homo sapiens (Human) | CVCL_5334 | |
| SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 | |
| HGC27 cells | Gastric | Homo sapiens (Human) | CVCL_1279 | |
| Experiment for Molecule Alteration |
RT-PCR | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | miR-363 promotes gastric cancer cells proliferation by inhibiting FBW7 expression and was associated with chemo-resistance of gastric cancer cells. Silencing FBW7 largely phenocopied miR-363-induced resistance to chemotherapy agents and promoted proliferation in gastric cancer cells. In addition, an inverse correlation between miR-363 and FBW7 mRNA expression was observed in gastric cancer tissues. | |||
| Key Molecule: hsa-mir-20a | [15] | |||
| Resistant Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell migration | Activation | hsa04670 | ||
| Cell proliferation | Activation | hsa05200 | ||
| EGR2 signaling pathway | Inhibition | hsa04625 | ||
| In Vitro Model | SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 |
| GES-1 cells | Gastric | Homo sapiens (Human) | CVCL_EQ22 | |
| MkN-45 cells | Gastric | Homo sapiens (Human) | CVCL_0434 | |
| NUGC3 cells | Gastric | Homo sapiens (Human) | CVCL_1612 | |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miR-20a promoted the growth, migration and invasion of GC cells, enhanced the chemoresistance of GC cells to cisplatin and docetaxel. Luciferase activity and Western blot confirmed that miR-20a negatively regulated EGR2 expression. Overexpression of EGR2 significantly attenuated the oncogenic effect of miR-20a. | |||
|
|
||||
| Key Molecule: Dihydroorotate dehydrogenase (DHODH) | [44] | |||
| Metabolic Type | Nucleic acid metabolism | |||
| Resistant Disease | Gastric adenocarcinoma [ICD-11: 2B72.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | AGS cells | Gastric | Homo sapiens (Human) | CVCL_0139 |
| HGC27 cells | Gastric | Homo sapiens (Human) | CVCL_1279 | |
| Experiment for Molecule Alteration |
qRT-PCR; Western blot analysis | |||
| Experiment for Drug Resistance |
IC50 assay | |||
| Mechanism Description | Mechanistically, pyrimidine biosynthesis augmented Notch signaling and transcriptionally increased c-Myc expression, leading to up-regulation of critical glycolytic enzymes. Further studies revealed that pyrimidine synthesis could stabilize gamma-secretase subunit Nicastrin at post-translational N-linked glycosylation level, thereby inducing the cleavage and activation of Notch. Besides, we found that up-regulation of the key enzymes for de novo pyrimidine synthesis CAD and DHODH conferred the chemotherapeutic resistance of gastric cancer via accelerating glycolysis, and pharmacologic inhibition of pyrimidine biosynthetic pathway sensitized cancer cells to chemotherapy in vitro and in vivo. | |||
| Key Molecule: Carbamoyl-phosphate synthetase 2, aspartate transcarbamylase, and dihydroorotase (CAD) | [44] | |||
| Metabolic Type | Nucleic acid metabolism | |||
| Resistant Disease | Gastric adenocarcinoma [ICD-11: 2B72.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | AGS cells | Gastric | Homo sapiens (Human) | CVCL_0139 |
| HGC27 cells | Gastric | Homo sapiens (Human) | CVCL_1279 | |
| Experiment for Molecule Alteration |
qRT-PCR; Western blot analysis | |||
| Experiment for Drug Resistance |
IC50 assay | |||
| Mechanism Description | Mechanistically, pyrimidine biosynthesis augmented Notch signaling and transcriptionally increased c-Myc expression, leading to up-regulation of critical glycolytic enzymes. Further studies revealed that pyrimidine synthesis could stabilize gamma-secretase subunit Nicastrin at post-translational N-linked glycosylation level, thereby inducing the cleavage and activation of Notch. Besides, we found that up-regulation of the key enzymes for de novo pyrimidine synthesis CAD and DHODH conferred the chemotherapeutic resistance of gastric cancer via accelerating glycolysis, and pharmacologic inhibition of pyrimidine biosynthetic pathway sensitized cancer cells to chemotherapy in vitro and in vivo. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: hsa-miR-33b-5p | [46] | |||
| Sensitive Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | MGC-803 cells | Gastric | Homo sapiens (Human) | CVCL_5334 |
| SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 | |
| GES-1 cells | Gastric | Homo sapiens (Human) | CVCL_EQ22 | |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
CCK8 assay; Colony formation assay; Flow cytometric apoptosis assay | |||
| Mechanism Description | miR33b-5p sensitizes gastric cancer cells to chemotherapy drugs via inhibiting HMGA2 expression. | |||
| Key Molecule: hsa-mir-34 | [47] | |||
| Sensitive Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | p53 signaling pathway | Inhibition | hsa04115 | |
| In Vitro Model | AGS cells | Gastric | Homo sapiens (Human) | CVCL_0139 |
| NCI-N87 cells | Gastric | Homo sapiens (Human) | CVCL_1603 | |
| MkN-45 cells | Gastric | Homo sapiens (Human) | CVCL_0434 | |
| KATO-3 cells | Gastric | Homo sapiens (Human) | CVCL_0371 | |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | Human gastric cancer kato III cells with miR-34 restoration reduced the expression of target genes Bcl-2, Notch, and HMGA2. MicroRNA miR-34 was recently found to be a direct target of p53, functioning downstream of the p53 pathway as a tumor suppressor, miR-34 impaired cell growth, accumulated the cells in G1 phase, increased caspase-3 activation, and, more significantly, inhibited tumorsphere formation and growth. | |||
|
|
||||
| Key Molecule: High mobility group protein HMGI-C (HMGA2) | [46] | |||
| Sensitive Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | MGC-803 cells | Gastric | Homo sapiens (Human) | CVCL_5334 |
| SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 | |
| GES-1 cells | Gastric | Homo sapiens (Human) | CVCL_EQ22 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay; Colony formation assay; Flow cytometric apoptosis assay | |||
| Mechanism Description | miR33b-5p sensitizes gastric cancer cells to chemotherapy drugs via inhibiting HMGA2 expression. | |||
| Key Molecule: Apoptosis regulator Bcl-2 (BCL2) | [47] | |||
| Sensitive Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | p53 signaling pathway | Inhibition | hsa04115 | |
| In Vitro Model | AGS cells | Gastric | Homo sapiens (Human) | CVCL_0139 |
| NCI-N87 cells | Gastric | Homo sapiens (Human) | CVCL_1603 | |
| MkN-45 cells | Gastric | Homo sapiens (Human) | CVCL_0434 | |
| KATO-3 cells | Gastric | Homo sapiens (Human) | CVCL_0371 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | Human gastric cancer kato III cells with miR-34 restoration reduced the expression of target genes Bcl-2, Notch, and HMGA2. MicroRNA miR-34 was recently found to be a direct target of p53, functioning downstream of the p53 pathway as a tumor suppressor, miR-34 impaired cell growth, accumulated the cells in G1 phase, increased caspase-3 activation, and, more significantly, inhibited tumorsphere formation and growth. | |||
| Key Molecule: High mobility group protein HMGI-C (HMGA2) | [47] | |||
| Sensitive Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | p53 signaling pathway | Inhibition | hsa04115 | |
| In Vitro Model | AGS cells | Gastric | Homo sapiens (Human) | CVCL_0139 |
| NCI-N87 cells | Gastric | Homo sapiens (Human) | CVCL_1603 | |
| MkN-45 cells | Gastric | Homo sapiens (Human) | CVCL_0434 | |
| KATO-3 cells | Gastric | Homo sapiens (Human) | CVCL_0371 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | Human gastric cancer kato III cells with miR-34 restoration reduced the expression of target genes Bcl-2, Notch, and HMGA2. MicroRNA miR-34 was recently found to be a direct target of p53, functioning downstream of the p53 pathway as a tumor suppressor, miR-34 impaired cell growth, accumulated the cells in G1 phase, increased caspase-3 activation, and, more significantly, inhibited tumorsphere formation and growth. | |||
| Key Molecule: Neurogenic locus notch homolog protein (NOTCH) | [47] | |||
| Sensitive Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | p53 signaling pathway | Inhibition | hsa04115 | |
| In Vitro Model | AGS cells | Gastric | Homo sapiens (Human) | CVCL_0139 |
| NCI-N87 cells | Gastric | Homo sapiens (Human) | CVCL_1603 | |
| MkN-45 cells | Gastric | Homo sapiens (Human) | CVCL_0434 | |
| KATO-3 cells | Gastric | Homo sapiens (Human) | CVCL_0371 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | Human gastric cancer kato III cells with miR-34 restoration reduced the expression of target genes Bcl-2, Notch, and HMGA2. MicroRNA miR-34 was recently found to be a direct target of p53, functioning downstream of the p53 pathway as a tumor suppressor, miR-34 impaired cell growth, accumulated the cells in G1 phase, increased caspase-3 activation, and, more significantly, inhibited tumorsphere formation and growth. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: hsa-mir-29c | [8] | |||
| Resistant Disease | Nasopharyngeal cancer [ICD-11: 2B6B.1] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | C666-1 cells | Throat | Homo sapiens (Human) | CVCL_7949 |
| SUNE-1 cells | Nasopharynx | Homo sapiens (Human) | CVCL_6946 | |
| In Vivo Model | Balb/c athymic nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miR-29c was downregulated and integrin beta-1 (ITGB1) was upregulated in Taxol-resistant NPC cells compared with parental NPC cells. Further investigations using a TUNEL assay and BAX/BCL-2 ratio, found that overexpression of miR-29c and knockdown of ITGB1 can resensitize drug-resistant NPC cells to Taxol and promote apoptosis. | |||
| Key Molecule: hsa-mir-29c | [8] | |||
| Resistant Disease | Nasopharyngeal cancer [ICD-11: 2B6B.1] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | C666-1 cells | Throat | Homo sapiens (Human) | CVCL_7949 |
| SUNE-1 cells | Nasopharynx | Homo sapiens (Human) | CVCL_6946 | |
| In Vivo Model | Balb/c athymic nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miR-29c was downregulated and integrin beta-1 (ITGB1) was upregulated in Taxol-resistant NPC cells compared with parental NPC cells. Further investigations using a TUNEL assay and BAX/BCL-2 ratio, found that overexpression of miR-29c and knockdown of ITGB1 can resensitize drug-resistant NPC cells to Taxol and promote apoptosis. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Multidrug resistance protein 1 (ABCB1) | [12] | |||
| Resistant Disease | Squamous cell carcinoma [ICD-11: 2B6E.3] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | KB-3-1 cells | Lung | Homo sapiens (Human) | CVCL_2088 |
| KB-8-5 cells | Mouth | Homo sapiens (Human) | CVCL_5994 | |
| KB-V1 cells | Mouth | Homo sapiens (Human) | CVCL_2089 | |
| In Vivo Model | Athymic nu/nu female mice xenograft model | Mus musculus | ||
| Experiment for Drug Resistance |
MTS assay | |||
| Mechanism Description | In a cell line expressing a high level of P-glycoprotein, the IC50 of TTI-237 increased 25-fold whereas those of paclitaxel and vincristine increased 806-fold and 925-fold. | |||
|
|
||||
| Key Molecule: Histone H3 | [10] | |||
| Metabolic Type | Glucose metabolism | |||
| Resistant Disease | Oral squamous cell carcinoma [ICD-11: 2B6E.0] | |||
| Molecule Alteration | Lactylation | . |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vivo Model | OSCC samples | Homo Sapiens | ||
| Mechanism Description | We found that histone Kla-induced BCAM was overexpressed in OSCC, and a high BCAM level was related to a lower immune cell score and inhibition of immune response. On the other hand, BCAM induced EMT and angiogenesis, leading to OSCC malignant progression via activating the Notch signaling pathway. However, the difference of the BCAM function in Pan-cancers might be attributed to tumor heterogeneity. Taken together, BCAM played a vital role in OSCC chemotherapy resistance and prognosis and contributed to inhibition of the immune process, suggesting that it might be a novel therapeutic target for OSCC. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: hsa-miR-455-3p | [4] | |||
| Resistant Disease | Esophageal squamous cell carcinoma [ICD-11: 2B70.3] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Wnt/beta-catenin/TGF-beta signaling pathway | Activation | hsa04310 | |
| In Vitro Model | ECA-109 cells | Esophagus | Homo sapiens (Human) | CVCL_6898 |
| AGS cells | Gastric | Homo sapiens (Human) | CVCL_0139 | |
| KYSE30 cells | Esophagus | Homo sapiens (Human) | CVCL_1351 | |
| H157 cells | Lung | Homo sapiens (Human) | CVCL_2458 | |
| In Vivo Model | Mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
RT-PCR | |||
| Experiment for Drug Resistance |
Tumor volume measurement; Luciferase assay | |||
| Mechanism Description | Antagonizing miR455-3p inhibits chemoresistance and aggressiveness in esophageal squamous cell carcinoma. Treatment with a miR455-3p antagomir dramatically chemosensitized ESCC cells and reduced the subpopulations of CD90+ and CD271+ T-ICs via deactivation of multiple stemness-associated pathways, including Wnt/beta-catenin and TGF-beta signaling. | |||
| Key Molecule: hsa-miR-193a-3p | [14] | |||
| Resistant Disease | Esophageal cancer [ICD-11: 2B70.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell proliferation | Activation | hsa05200 | ||
| In Vitro Model | KYSE150 cells | Esophagus | Homo sapiens (Human) | CVCL_1348 |
| KYSE510 cells | Esophagus | Homo sapiens (Human) | CVCL_1354 | |
| kYSE410 cells | Esophagus | Homo sapiens (Human) | CVCL_1352 | |
| kYSE450 cells | Esophagus | Homo sapiens (Human) | CVCL_1353 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Over-expression of miR-193a-3p increased the radioresistance and chemoresistance of oesophageal squamous cell carcinoma (ESCC) cells. In contrast, the down-regulation of miR-193a-3p decreased the radioresistance and chemoresistance of ESCC cells. In addition, miR-193a-3p inducing DNA damage has also been demonstrated through measuring the level of gamma-H2AX associated with miR-193a-3p. Moreover, a small interfering RNA(siRNA)-induced repression of the PSEN1 gene had an effect similar to that of miR-193a-3p up-regulation. The above processes also inhibited oesophageal cancer cells apoptosis. These findings suggest that miR-193a-3p contributes to the radiation and chemotherapy resistance of oesophageal carcinoma by down-regulating PSEN1. | |||
| Key Molecule: hsa-miR-193a-3p | [14] | |||
| Resistant Disease | Esophageal cancer [ICD-11: 2B70.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | KYSE150 cells | Esophagus | Homo sapiens (Human) | CVCL_1348 |
| KYSE510 cells | Esophagus | Homo sapiens (Human) | CVCL_1354 | |
| kYSE410 cells | Esophagus | Homo sapiens (Human) | CVCL_1352 | |
| kYSE450 cells | Esophagus | Homo sapiens (Human) | CVCL_1353 | |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Over-expression of miR-193a-3p increased the radioresistance and chemoresistance of oesophageal squamous cell carcinoma (ESCC) cells. The regulation role of miR-193a-3p on multi-chemoresistance and radioresistance were mediated by PSEN1. | |||
| Key Molecule: hsa-miR-193a-3p | [14] | |||
| Resistant Disease | Esophageal cancer [ICD-11: 2B70.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | KYSE150 cells | Esophagus | Homo sapiens (Human) | CVCL_1348 |
| KYSE510 cells | Esophagus | Homo sapiens (Human) | CVCL_1354 | |
| kYSE410 cells | Esophagus | Homo sapiens (Human) | CVCL_1352 | |
| kYSE450 cells | Esophagus | Homo sapiens (Human) | CVCL_1353 | |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Over-expression of miR-193a-3p increased the radioresistance and chemoresistance of oesophageal squamous cell carcinoma (ESCC) cells. The regulation role of miR-193a-3p on multi-chemoresistance and radioresistance were mediated by PSEN1. | |||
| Key Molecule: hsa-miR-193a-3p | [14] | |||
| Resistant Disease | Esophageal cancer [ICD-11: 2B70.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | KYSE150 cells | Esophagus | Homo sapiens (Human) | CVCL_1348 |
| KYSE510 cells | Esophagus | Homo sapiens (Human) | CVCL_1354 | |
| kYSE410 cells | Esophagus | Homo sapiens (Human) | CVCL_1352 | |
| kYSE450 cells | Esophagus | Homo sapiens (Human) | CVCL_1353 | |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Over-expression of miR-193a-3p increased the radioresistance and chemoresistance of oesophageal squamous cell carcinoma (ESCC) cells. The regulation role of miR-193a-3p on multi-chemoresistance and radioresistance were mediated by PSEN1. | |||
| Key Molecule: hsa-miR-193a-3p | [14] | |||
| Resistant Disease | Esophageal cancer [ICD-11: 2B70.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | KYSE150 cells | Esophagus | Homo sapiens (Human) | CVCL_1348 |
| KYSE510 cells | Esophagus | Homo sapiens (Human) | CVCL_1354 | |
| kYSE410 cells | Esophagus | Homo sapiens (Human) | CVCL_1352 | |
| kYSE450 cells | Esophagus | Homo sapiens (Human) | CVCL_1353 | |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Over-expression of miR-193a-3p increased the radioresistance and chemoresistance of oesophageal squamous cell carcinoma (ESCC) cells. The regulation role of miR-193a-3p on multi-chemoresistance and radioresistance were mediated by PSEN1. | |||
| Key Molecule: Histone H1.4 (H1-4) | [5] | |||
| Resistant Disease | Oesophagus adenocarcinoma [ICD-11: 2B70.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Discovered Using In-vivo Testing Model | |||
| In Vivo Model | Patient-derived esophageal cancer model | Homo sapiens | ||
| Experiment for Molecule Alteration |
Gene expression analysis | |||
| Experiment for Drug Resistance |
Drug sensitivity analysis | |||
| Mechanism Description | The results of drug sensitivity of risk genes showed that the high expression of HIST1H1E made tumor cells resistant to trametinib, selumetinib, RDEA119, Docetaxel and 17-AAG. The high expression of UBE2C makes tumor cells resistant to masitinib. The low expression of ERO1B makes the EC more sensitive to FK866 | |||
|
|
||||
| Key Molecule: Presenilin-1 (PSEN1) | [14] | |||
| Resistant Disease | Esophageal cancer [ICD-11: 2B70.1] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell proliferation | Activation | hsa05200 | ||
| In Vitro Model | KYSE150 cells | Esophagus | Homo sapiens (Human) | CVCL_1348 |
| KYSE510 cells | Esophagus | Homo sapiens (Human) | CVCL_1354 | |
| kYSE410 cells | Esophagus | Homo sapiens (Human) | CVCL_1352 | |
| kYSE450 cells | Esophagus | Homo sapiens (Human) | CVCL_1353 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Over-expression of miR-193a-3p increased the radioresistance and chemoresistance of oesophageal squamous cell carcinoma (ESCC) cells. In contrast, the down-regulation of miR-193a-3p decreased the radioresistance and chemoresistance of ESCC cells. In addition, miR-193a-3p inducing DNA damage has also been demonstrated through measuring the level of gamma-H2AX associated with miR-193a-3p. Moreover, a small interfering RNA(siRNA)-induced repression of the PSEN1 gene had an effect similar to that of miR-193a-3p up-regulation. The above processes also inhibited oesophageal cancer cells apoptosis. These findings suggest that miR-193a-3p contributes to the radiation and chemotherapy resistance of oesophageal carcinoma by down-regulating PSEN1. | |||
| Key Molecule: Sphingosine-1-phosphate lyase 1 (SGPL1) | [9] | |||
| Resistant Disease | Oesophagus adenocarcinoma [ICD-11: 2B70.0] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | OE33 cells | Esophagus | Homo sapiens (Human) | CVCL_0471 |
| Experiment for Molecule Alteration |
Immunohistochemistry assay | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | S1P could lead to cytotoxic drug resistance in gastroesophegal cancer acting in an autocrine or paracrine manner via cell surface S1P receptors following transportation out of the cytosol. Alternatively S1P may mediate cytotoxic drug resistance acting intracellularly by counteracting apoptosis mediated by its pro-apoptotic precursor ceramide or interaction with known intracellular targets involved in cancer pathogenesis and cytotoxic drug resistance such as Histone deacetylase 1 (HDAC1) and Histone deacetylase 2 (HDAC 2) to which S1P directly binds and inhibits, and TNF Receptor-Associated Factor 2 (TRAF 2), or Protein Kinase C (PKC). S1P production controlled by SPHK1 and SGPL1 are key determinants of cytotoxic drug resistance and that decreasing S1P production in cancer cells could lead to increased cytotoxic sensitivity. | |||
| Key Molecule: Sphingosine kinase 1 (SPHK1) | [9] | |||
| Resistant Disease | Oesophagus adenocarcinoma [ICD-11: 2B70.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | OE33 cells | Esophagus | Homo sapiens (Human) | CVCL_0471 |
| Experiment for Molecule Alteration |
Immunohistochemistry assay | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | S1P could lead to cytotoxic drug resistance in gastroesophegal cancer acting in an autocrine or paracrine manner via cell surface S1P receptors following transportation out of the cytosol. Alternatively S1P may mediate cytotoxic drug resistance acting intracellularly by counteracting apoptosis mediated by its pro-apoptotic precursor ceramide or interaction with known intracellular targets involved in cancer pathogenesis and cytotoxic drug resistance such as Histone deacetylase 1 (HDAC1) and Histone deacetylase 2 (HDAC 2) to which S1P directly binds and inhibits, and TNF Receptor-Associated Factor 2 (TRAF 2), or Protein Kinase C (PKC). S1P production controlled by SPHK1 and SGPL1 are key determinants of cytotoxic drug resistance and that decreasing S1P production in cancer cells could lead to increased cytotoxic sensitivity. | |||
| Key Molecule: Sphingosine-1-phosphate lyase 1 (SGPL1) | [9] | |||
| Resistant Disease | Esophageal squamous cell carcinoma [ICD-11: 2B70.3] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | OE21 cells | Esophagus | Homo sapiens (Human) | CVCL_2661 |
| Experiment for Molecule Alteration |
Immunohistochemistry assay | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | S1P could lead to cytotoxic drug resistance in gastroesophegal cancer acting in an autocrine or paracrine manner via cell surface S1P receptors following transportation out of the cytosol. Alternatively S1P may mediate cytotoxic drug resistance acting intracellularly by counteracting apoptosis mediated by its pro-apoptotic precursor ceramide or interaction with known intracellular targets involved in cancer pathogenesis and cytotoxic drug resistance such as Histone deacetylase 1 (HDAC1) and Histone deacetylase 2 (HDAC 2) to which S1P directly binds and inhibits, and TNF Receptor-Associated Factor 2 (TRAF 2), or Protein Kinase C (PKC). S1P production controlled by SPHK1 and SGPL1 are key determinants of cytotoxic drug resistance and that decreasing S1P production in cancer cells could lead to increased cytotoxic sensitivity. | |||
| Key Molecule: Sphingosine kinase 1 (SPHK1) | [9] | |||
| Resistant Disease | Esophageal squamous cell carcinoma [ICD-11: 2B70.3] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | OE21 cells | Esophagus | Homo sapiens (Human) | CVCL_2661 |
| Experiment for Molecule Alteration |
Immunohistochemistry assay | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | S1P could lead to cytotoxic drug resistance in gastroesophegal cancer acting in an autocrine or paracrine manner via cell surface S1P receptors following transportation out of the cytosol. Alternatively S1P may mediate cytotoxic drug resistance acting intracellularly by counteracting apoptosis mediated by its pro-apoptotic precursor ceramide or interaction with known intracellular targets involved in cancer pathogenesis and cytotoxic drug resistance such as Histone deacetylase 1 (HDAC1) and Histone deacetylase 2 (HDAC 2) to which S1P directly binds and inhibits, and TNF Receptor-Associated Factor 2 (TRAF 2), or Protein Kinase C (PKC). S1P production controlled by SPHK1 and SGPL1 are key determinants of cytotoxic drug resistance and that decreasing S1P production in cancer cells could lead to increased cytotoxic sensitivity. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: hsa-mir-200c | [42] | |||
| Sensitive Disease | Esophageal cancer [ICD-11: 2B70.1] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
Endoscopy; Computed tomography assay; Positron emission tomography assay | |||
| Mechanism Description | Serum miR-200c levels are useful for predicting the response to chemotherapy (cisplatin, 5-fluorouracil, and Adriamycin (ACF) or cisplatin, 5-fluorouracil, and docetaxel (DCF) ) in patients with esophageal cancer who underwent preoperative chemotherapy followed by surgery. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Sphingosine-1-phosphate lyase 1 (SGPL1) | [9] | |||
| Resistant Disease | Gastroesophageal cancer [ICD-11: 2B71.0] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Gastroesophageal cancer tissue | N.A. | ||
| Experiment for Molecule Alteration |
Immunohistochemistry assay | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | S1P could lead to cytotoxic drug resistance in gastroesophegal cancer acting in an autocrine or paracrine manner via cell surface S1P receptors following transportation out of the cytosol. Alternatively S1P may mediate cytotoxic drug resistance acting intracellularly by counteracting apoptosis mediated by its pro-apoptotic precursor ceramide or interaction with known intracellular targets involved in cancer pathogenesis and cytotoxic drug resistance such as Histone deacetylase 1 (HDAC1) and Histone deacetylase 2 (HDAC 2) to which S1P directly binds and inhibits, and TNF Receptor-Associated Factor 2 (TRAF 2), or Protein Kinase C (PKC). S1P production controlled by SPHK1 and SGPL1 are key determinants of cytotoxic drug resistance and that decreasing S1P production in cancer cells could lead to increased cytotoxic sensitivity. | |||
| Key Molecule: Sphingosine kinase 1 (SPHK1) | [9] | |||
| Resistant Disease | Gastroesophageal cancer [ICD-11: 2B71.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Gastroesophageal cancer tissue | N.A. | ||
| Experiment for Molecule Alteration |
Immunohistochemistry assay | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | S1P could lead to cytotoxic drug resistance in gastroesophegal cancer acting in an autocrine or paracrine manner via cell surface S1P receptors following transportation out of the cytosol. Alternatively S1P may mediate cytotoxic drug resistance acting intracellularly by counteracting apoptosis mediated by its pro-apoptotic precursor ceramide or interaction with known intracellular targets involved in cancer pathogenesis and cytotoxic drug resistance such as Histone deacetylase 1 (HDAC1) and Histone deacetylase 2 (HDAC 2) to which S1P directly binds and inhibits, and TNF Receptor-Associated Factor 2 (TRAF 2), or Protein Kinase C (PKC). S1P production controlled by SPHK1 and SGPL1 are key determinants of cytotoxic drug resistance and that decreasing S1P production in cancer cells could lead to increased cytotoxic sensitivity. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Multidrug resistance protein 1 (ABCB1) | [12] | |||
| Resistant Disease | Colorectal carcinoma [ICD-11: 2B91.3] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | LOVO cells | Colon | Homo sapiens (Human) | CVCL_0399 |
| In Vivo Model | Athymic nu/nu female mice xenograft model | Mus musculus | ||
| Experiment for Drug Resistance |
MTS assay | |||
| Mechanism Description | In a cell line expressing a high level of P-glycoprotein, the IC50 of TTI-237 increased 25-fold whereas those of paclitaxel and vincristine increased 806-fold and 925-fold. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: hsa-mir-223 | [48] | |||
| Sensitive Disease | Gallbladder cancer [ICD-11: 2C13.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | GBC-SD cells | Gallbladder | Homo sapiens (Human) | CVCL_6903 |
| NOZ cells | Gallbladder | Homo sapiens (Human) | CVCL_3079 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | miR223 increases gallbladder cancer cell sensitivity to docetaxel by downregulating STMN1. | |||
|
|
||||
| Key Molecule: Stathmin (STMN1) | [48] | |||
| Sensitive Disease | Gallbladder cancer [ICD-11: 2C13.0] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | GBC-SD cells | Gallbladder | Homo sapiens (Human) | CVCL_6903 |
| NOZ cells | Gallbladder | Homo sapiens (Human) | CVCL_3079 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | miR223 increases gallbladder cancer cell sensitivity to docetaxel by downregulating STMN1. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Polycomb complex protein BMI-1 (BMI1) | [3] | |||
| Resistant Disease | Endometrial cancer [ICD-11: 2C76.1] | |||
| Molecule Alteration | Mutation | . |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | PI3K/AKT signaling pathway | Activation | hsa04151 | |
| Experiment for Molecule Alteration |
Low throughput experiment assay | |||
| Experiment for Drug Resistance |
Disease-free survival analysis | |||
| Mechanism Description | Recently, Dong et al. demonstrated that loss of BMI1 in endometrial cancer cells reduces expression of drug resistance gene MRP1, suggesting that BMI1 is required for the drug resistance. Overexpression of BMI1 rescues tumor cells from the apoptosis induced by Okadaic acid and Epigallocatechin-3-gallate, well-known apoptotic agents. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Multidrug resistance protein 1 (ABCB1) | [12] | |||
| Resistant Disease | Cervical carcinoma [ICD-11: 2C77.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Hela cells | Cervix uteri | Homo sapiens (Human) | CVCL_0030 |
| In Vivo Model | Athymic nu/nu female mice xenograft model | Mus musculus | ||
| Experiment for Drug Resistance |
MTS assay | |||
| Mechanism Description | In a cell line expressing a high level of P-glycoprotein, the IC50 of TTI-237 increased 25-fold whereas those of paclitaxel and vincristine increased 806-fold and 925-fold. | |||
|
|
||||
| Key Molecule: Hypoxia-inducible factor 1-alpha (HIF1A) | [67] | |||
| Metabolic Type | Glucose metabolism | |||
| Resistant Disease | Cervical cancer [ICD-11: 2C77.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Hela cells | Cervix uteri | Homo sapiens (Human) | CVCL_0030 |
| Parental HeLa cells | Cervix | Homo sapiens (Human) | CVCL_0030 | |
| Experiment for Drug Resistance |
IC50 assay | |||
| Mechanism Description | Warburg effect activated HIF1-alpha-mediated signaling-induced autophagic pathway may have an important role in paclitaxel chemoresistance. | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
