Drug Information
Drug (ID: DG00227) and It's Reported Resistant Information
| Name |
Meropenem
|
||||
|---|---|---|---|---|---|
| Synonyms |
MEPM; MERONEM; Meropen; Merrem; Meropenem anhydrous; Mepem (TN); Meronem (TN); Meropen (TN); Meropenem (INN); Merrem (TN); Neopenem (TN); SM-7338; Meronem; Merrem I.V. (TN); (1R,5S,6S)-2-[(3S,5S)-5-(dimethylaminocarbonyl)pyrrolidin-3-ylthio]-6-[(R)-1-hydroxyethyl]-1-methylcarbapen-2-em-3-carboxylic acid trihydrate; (2S,3R,4R)-2-[(1S,2R)-1-carboxy-2-hydroxypropyl]-4-{[(3S,5R)-5-(dimethylcarbamoyl)pyrrolidin-3-yl]sulfanyl}-3-methyl-3,4-dihydro-2H-pyrrole-5-carboxylic acid; (4R,5S,6S)-3-[(3S,5S)-5-(dimethylcarbamoyl)pyrrolidin-3-yl]sulfanyl-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid; (4R,5S,6S)-3-{[(3S,5S)-5-(dimethylcarbamoyl)pyrrolidin-3-yl]thio}-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid; (5S,6S)-3-((3S,5S)-5-(dimethylcarbamoyl)pyrrolidin-3-ylthio)-6-((S)-1-hydroxyethyl)-4-methyl-7-oxo-1-aza-bicyclo[3.2.0]hept-2-ene-2-carboxylic acid; (6S)-2-{[(3S,5S)-5-(dimethylcarbamoyl)pyrrolidin-3-yl]sulfanyl}-6-[(1R)-1-hydroxyethyl]-1beta-methyl-2,3-didehydro-1-carbapenam-3-carboxylic acid
Click to Show/Hide
|
||||
| Indication |
In total 1 Indication(s)
|
||||
| Structure |
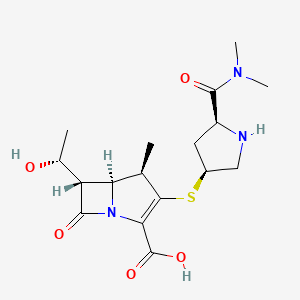
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Clinically Reported Resistance for This Drug
(7 diseases)
[2]
[2]
[1]
[3]
[4]
[4]
[5]
Disease(s) with Resistance Information Validated by in-vivo Model for This Drug
(1 diseases)
[6]
|
||||
| Target | Bacterial Penicillin binding protein (Bact PBP) | NOUNIPROTAC | [1] | ||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
C17H25N3O5S
|
||||
| IsoSMILES |
C[C@@H]1[C@@H]2[C@H](C(=O)N2C(=C1S[C@H]3C[C@H](NC3)C(=O)N(C)C)C(=O)O)[C@@H](C)O
|
||||
| InChI |
1S/C17H25N3O5S/c1-7-12-11(8(2)21)16(23)20(12)13(17(24)25)14(7)26-9-5-10(18-6-9)15(22)19(3)4/h7-12,18,21H,5-6H2,1-4H3,(H,24,25)/t7-,8-,9+,10+,11-,12-/m1/s1
|
||||
| InChIKey |
DMJNNHOOLUXYBV-PQTSNVLCSA-N
|
||||
| PubChem CID | |||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
| VARIDT ID | |||||
| INTEDE ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-01: Infectious/parasitic diseases
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Beta-lactamase-like protein (VARG) | [6] | |||
| Resistant Disease | Vibrio cholerae infection [ICD-11: 1A00.0] | |||
| Molecule Alteration | Expression | Inherence |
||
| Experimental Note | Discovered Using In-vivo Testing Model | |||
| In Vitro Model | Vibrio cholerae CVD101 | 1854 | ||
| Mechanism Description | A gel-shift analysis was used to determine if VarR bound to the promoter regions of the resistance genes. Consistent with the regulation of these resistance genes, VarR binds to three distinct intergenic regions, varRG, varGA and varBC located upstream and adjacent to varG, varA and varC, respectively. VarR can act as a repressor at the varRG promoter region; whilst this repression was relieved upon addition of Beta-lactams, these did not dissociate the VarR/varRG-DNA complex, indicating that the de-repression of varR by Beta-lactams is indirect. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: CATB10-Ib variant (CATB10) | [7] | |||
| Resistant Disease | Bacterial infection [ICD-11: 1A00-1C4Z] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Pseudomonas aeruginosa TS-103 | 287 | ||
| Pseudomonas aeruginosa TS-832035 | 287 | |||
| Experiment for Molecule Alteration |
Whole genome sequence assay; Allelic frequency measurement assay | |||
| Experiment for Drug Resistance |
Agar dilution method assay | |||
| Mechanism Description | P. aeruginosa TS-832035 produces a carbapenemase, coded by a blaVIM-1 determinant carried by the chromosomal class 1 integron In70.2 (containing also the aacA4, aphA15, and aadA1 genes in its cassette array),which induce the resistance to carbapenems. | |||
| Key Molecule: Metallo-beta-lactamase (VIM1) | [2] | |||
| Resistant Disease | Achromobacter xylosoxydans infection [ICD-11: 1A00-1C4Z] | |||
| Molecule Alteration | Expression | Inherence |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Escherichia coli | 668369 | ||
| Achromobacter xylosoxydans subsp. denitrificans AX-22 | 85698 | |||
| Escherichia coli MkD-135 | 562 | |||
| Pseudomonas aeruginosa 10145/3 | 287 | |||
| Experiment for Molecule Alteration |
DNA extraction and Sequencing assay | |||
| Experiment for Drug Resistance |
Macrodilution broth method assay | |||
| Mechanism Description | A. xylosoxydans AX22 exhibited broad-spectrum resistance to Beta-lactams and aminoglycosides. The Beta-lactam resistance pattern (including piperacillin, ceftazidime, and carbapenem resistance) was unusual for this species, and the high-level carbapenem resistance suggested the production of an acquired carbapenemase. In fact, carbapenemase activity was detected in a crude extract of AX22 (specific activity, 184 +/- 12 U/mg of protein), and this activity was reduced (>80%) after incubation of the crude extract with 2 mM EDTA, suggesting the presence of a metallo-Beta-lactamase determinant. | |||
|
|
||||
| Key Molecule: TolC family outer membrane protein (TOLC) | [8] | |||
| Resistant Disease | Bacterial infection [ICD-11: 1A00-1C4Z] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Acinetobacter baumannii AYE WT | 509173 | ||
| Acinetobacter baumannii AYE detaabuO | 509173 | |||
| Acinetobacter baumannii AYE detaabuO Omega abuO | 509173 | |||
| Experiment for Molecule Alteration |
Whole genome sequence assay | |||
| Experiment for Drug Resistance |
Disk diffusion test assay; E-strip test assay | |||
| Mechanism Description | AbuO, an OMP, confers broad-spectrum antimicrobial resistance via active efflux in A. baumannii. | |||
|
|
||||
| Key Molecule: Pyruvate decarboxylase 5 (PDC5) | [9], [10] | |||
| Resistant Disease | Bacterial infection [ICD-11: 1A00-1C4Z] | |||
| Molecule Alteration | Missense mutation | p.R79Q+p.T105A |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Escherichia coli TOP10 | 83333 | ||
| Pseudomonas aeruginosa isolates | 287 | |||
| Pseudomonas aeruginosa PAO1 | 208964 | |||
| Pseudomonas aeruginosa 12B | 287 | |||
| Pseudomonas aeruginosa kG2505 | 287 | |||
| Experiment for Molecule Alteration |
Whole genome sequence assay | |||
| Experiment for Drug Resistance |
Agar dilution method assay; Etest method assay | |||
| Mechanism Description | Reduced susceptibility to imipenem, ceftazidime, and cefepime was observed only with recombinant P. aeruginosa strains expressing an AmpC Beta-lactamase that had an alanine residue at position 105.Recently, several ESACs have been described from Escherichia coli contributing to reduced susceptibility to imipenem. | |||
| Key Molecule: Pyruvate decarboxylase 3 (PDC3) | [9], [10] | |||
| Resistant Disease | Bacterial infection [ICD-11: 1A00-1C4Z] | |||
| Molecule Alteration | Missense mutation | p.T97A |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Escherichia coli TOP10 | 83333 | ||
| Pseudomonas aeruginosa isolates | 287 | |||
| Pseudomonas aeruginosa PAO1 | 208964 | |||
| Pseudomonas aeruginosa 12B | 287 | |||
| Pseudomonas aeruginosa kG2505 | 287 | |||
| Experiment for Molecule Alteration |
Whole genome sequence assay | |||
| Experiment for Drug Resistance |
Agar dilution method assay; Etest method assay | |||
| Mechanism Description | Reduced susceptibility to imipenem, ceftazidime, and cefepime was observed only with recombinant P. aeruginosa strains expressing an AmpC Beta-lactamase that had an alanine residue at position 105.Recently, several ESACs have been described from Escherichia coli contributing to reduced susceptibility to imipenem. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Metallo-beta-lactamase (VIM1) | [2] | |||
| Resistant Disease | Escherichia coli infection [ICD-11: 1A03.0] | |||
| Molecule Alteration | Expression | Acquired |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Escherichia coli | 668369 | ||
| Achromobacter xylosoxydans subsp. denitrificans AX-22 | 85698 | |||
| Escherichia coli MkD-135 | 562 | |||
| Pseudomonas aeruginosa 10145/3 | 287 | |||
| Experiment for Molecule Alteration |
DNA extraction and Sequencing assay | |||
| Experiment for Drug Resistance |
Macrodilution broth method assay | |||
| Mechanism Description | Electroporation of Escherichia coli DH5alpha with the purified plasmid preparation yielded ampicillin-resistant transformants which contained a plasmid apparently identical to pAX22 (data not shown). DH5alpha(pAX22) produced carbapenemase activity (specific activity of crude extract, 202 +/- 14 U/mg of protein) and, compared to DH5alpha, exhibited a decreased susceptibility to several Beta-lactams. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: OXA-23 carbapenemase (BLA OXA-23) | [3] | |||
| Resistant Disease | Cutaneous bacterial infection [ICD-11: 1B21.4] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Acinetobacter baumannii isolates | 470 | ||
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
Broth microdilution method assay; Agar dilution method assay | |||
| Mechanism Description | The isolate was resistant to antibiotics other than ampicillin-sulbactam and colistin, suggesting drug resistance due to carbapenemase production by OXA-23.carbapenem resistance in the isolated carbapenem-resistant A. baumannii strain was at least partially conferred by bla OXA-23-like carbapenemase. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: CAM-1 carbapenemase (CAM1) | [1] | |||
| Resistant Disease | Pseudomonas infection [ICD-11: 1F45.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Escherichia coli TOP10 | 83333 | ||
| Pseudomonas aeruginosa N17-01167 | 287 | |||
| Pseudomonas aeruginosa N17-01173 | 287 | |||
| Pseudomonas aeruginosa N17-02436 | 287 | |||
| Pseudomonas aeruginosa N17-02437 | 287 | |||
| Experiment for Molecule Alteration |
Whole genome sequencing assay | |||
| Experiment for Drug Resistance |
Vitek 2 assay; Etest assay | |||
| Mechanism Description | A novel class B Beta-lactamase gene, blaCAM-1, exhibited resistance to imipenem, meropenem, doripenem, cefotaxime, ceftazidime, cefoxitin, piperacillin/tazobactam, ceftazidime/avibactam and ceftolozane/tazobactam. | |||
ICD-12: Respiratory system diseases
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: OmpK37 (OMPK37) | [11] | |||
| Sensitive Disease | Klebsiella pneumoniae infection [ICD-11: CA40.1] | |||
| Molecule Alteration | Expression | Inherence |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Escherichia coli | 668369 | ||
| Klebsiella pneumoniae strain CSUB10R | 573 | |||
| Klebsiella pneumoniae strain CSUB10S | 573 | |||
| Klebsiella pneumoniae strain LB4 | 573 | |||
| Klebsiella pneumoniae strain LB66 | 573 | |||
| Klebsiella pneumoniae strain SD8 | 573 | |||
| Experiment for Molecule Alteration |
Southern blotting assay | |||
| Experiment for Drug Resistance |
Microdilution method assay | |||
| Mechanism Description | Due to its porin deficiency, strain CSUB10R is more resistant to Beta-lactams than is parental strain CSUB10S. As expected, for k. pneumoniae CSUB10R expressing Ompk36 or Ompk35, the MICs reverted to values similar to those observed for strain CSUB10S. | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
