Molecule Information
General Information of the Molecule (ID: Mol00145)
| Name |
Phosphatase and tensin homolog (PTEN)
,Homo sapiens
|
||||
|---|---|---|---|---|---|
| Synonyms |
Mutated in multiple advanced cancers 1; Phosphatase and tensin homolog; MMAC1; TEP1
Click to Show/Hide
|
||||
| Molecule Type |
Protein
|
||||
| Gene Name |
PTEN
|
||||
| Gene ID | |||||
| Location |
chr10:87862638-87971930[+]
|
||||
| Sequence |
MTAIIKEIVSRNKRRYQEDGFDLDLTYIYPNIIAMGFPAERLEGVYRNNIDDVVRFLDSK
HKNHYKIYNLCAERHYDTAKFNCRVAQYPFEDHNPPQLELIKPFCEDLDQWLSEDDNHVA AIHCKAGKGRTGVMICAYLLHRGKFLKAQEALDFYGEVRTRDKKGVTIPSQRRYVYYYSY LLKNHLDYRPVALLFHKMMFETIPMFSGGTCNPQFVVCQLKVKIYSSNSGPTRREDKFMY FEFPQPLPVCGDIKVEFFHKQNKMLKKDKMFHFWVNTFFIPGPEETSEKVENGSLCDQEI DSICSIERADNDKEYLVLTLTKNDLDKANKDKANRYFSPNFKVKLYFTKTVEEPSNPEAS SSTSVTPDVSDNEPDHYRYSDTTDSDPENEPFDEDQHTQITKV Click to Show/Hide
|
||||
| Function |
Tumor suppressor. Acts as a dual-specificity protein phosphatase, dephosphorylating tyrosine-, serine- and threonine-phosphorylated proteins. Also acts as a lipid phosphatase, removing the phosphate in the D3 position of the inositol ring from phosphatidylinositol 3,4,5-trisphosphate, phosphatidylinositol 3,4-diphosphate, phosphatidylinositol 3-phosphate and inositol 1,3,4,5-tetrakisphosphate with order of substrate preference in vitro PtdIns(3,4,5)P3 > PtdIns(3,4)P2 > PtdIns3P > Ins(1,3,4,5)P4. The lipid phosphatase activity is critical for its tumor suppressor function. Antagonizes the PI3K-AKT/PKB signaling pathway by dephosphorylating phosphoinositides and thereby modulating cell cycle progression and cell survival. The unphosphorylated form cooperates with MAGI2 to suppress AKT1 activation. Dephosphorylates tyrosine-phosphorylated focal adhesion kinase and inhibits cell migration and integrin-mediated cell spreading and focal adhesion formation. Plays a role as a key modulator of the AKT-mTOR signaling pathway controlling the tempo of the process of newborn neurons integration during adult neurogenesis, including correct neuron positioning, dendritic development and synapse formation. May be a negative regulator of insulin signaling and glucose metabolism in adipose tissue. The nuclear monoubiquitinated form possesses greater apoptotic potential, whereas the cytoplasmic nonubiquitinated form induces less tumor suppressive ability. In motile cells, suppresses the formation of lateral pseudopods and thereby promotes cell polarization and directed movement.
Click to Show/Hide
|
||||
| Uniprot ID | |||||
| Ensembl ID | |||||
| HGNC ID | |||||
| Click to Show/Hide the Complete Species Lineage | |||||
Type(s) of Resistant Mechanism of This Molecule
Drug Resistance Data Categorized by Drug
Approved Drug(s)
28 drug(s) in total
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Ovarian cancer [ICD-11: 2C73.0] | [1] | |||
| Resistant Disease | Ovarian cancer [ICD-11: 2C73.0] | |||
| Resistant Drug | Cisplatin | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Ovarian cancer [ICD-11: 2C73] | |||
| The Specified Disease | Ovarian cancer | |||
| The Studied Tissue | Ovarian tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 8.10E-01 Fold-change: 7.35E-03 Z-score: 2.48E-01 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell proliferation | Inhibition | hsa05200 | |
| In Vitro Model | A2780 cells | Ovary | Homo sapiens (Human) | CVCL_0134 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CellTiter 96 aqueous one solution cell proliferation assay | |||
| Mechanism Description | miR-130a and miR-374a mimics decreased the sensitivity of A2780 cells to cisplatin, reversely, their inhibitors could resensitize A2780/DDP cells. Furthermore, overexpression of miR-130a could increase the MDR1 mRNA and P-gp levels in A2780 and A2780/DDP cells, whereas knockdown of miR-130a could inhibit MDR1 gene expression and upregulate the PTEN protein expression. In a conclusion, the deregulation of miR-374a and miR-130a may be involved in the development and regulation of cisplatin resistance in ovarian cancer cells. This role of miR-130a may be achieved by regulating the MDR1 and PTEN gene expression. | |||
| Disease Class: Melanoma [ICD-11: 2C30.0] | [7] | |||
| Resistant Disease | Melanoma [ICD-11: 2C30.0] | |||
| Resistant Drug | Cisplatin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Melanoma [ICD-11: 2C30] | |||
| The Specified Disease | Melanoma | |||
| The Studied Tissue | Skin | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 2.31E-01 Fold-change: -3.56E-02 Z-score: -1.22E+00 |
|||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | AKT/FAKT signaling pathway | Activation | hsa04151 | |
| In Vitro Model | A375 cells | Skin | Homo sapiens (Human) | CVCL_0132 |
| SkMEL1 cells | Skin | Homo sapiens (Human) | CVCL_0068 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay; Colony formation assay; Annexin V-fluorescein isothiocyanate (FITC) apoptosis analysis; Wound scratch healing or transwell invasion assay | |||
| Mechanism Description | PTEN can interact with AkT and FAk and inhibit their activity through their dephosphorylation, Akt and FAk signaling pathways are involved in miR301a/PTEN-promoting malignant phenotypes in MM cells, miR301a promotes MM progression via activation of Akt and FAk signaling pathways by down regulating PTEN. | |||
| Disease Class: Neuroblastoma [ICD-11: 2A00.11] | [10] | |||
| Resistant Disease | Neuroblastoma [ICD-11: 2A00.11] | |||
| Resistant Drug | Cisplatin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Brain cancer [ICD-11: 2A00] | |||
| The Specified Disease | Glioma | |||
| The Studied Tissue | Brainstem tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 3.69E-01 Fold-change: -4.98E-02 Z-score: -1.27E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | SH-SY5Y cells | Abdomen | Homo sapiens (Human) | CVCL_0019 |
| BE(2) -M17 cells | Brain | Homo sapiens (Human) | CVCL_0167 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Increased miR-21 expression might suppress the PTEN expression and eventually induce chemoresistance to cisplatin and increase cell proliferation. | |||
| Disease Class: Non-small cell lung cancer [ICD-11: 2C25.Y] | [17] | |||
| Resistant Disease | Non-small cell lung cancer [ICD-11: 2C25.Y] | |||
| Resistant Drug | Cisplatin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell proliferation | Activation | hsa05200 | |
| PTEN/AKT signaling pathway | Activation | hsa05235 | ||
| In Vitro Model | A549 cells | Lung | Homo sapiens (Human) | CVCL_0023 |
| H460 cells | Lung | Homo sapiens (Human) | CVCL_0459 | |
| Experiment for Molecule Alteration |
Western blot analysis; RT-qPCR | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Deregulation of LncRNA-AC078883.3 and microRNA-19a is involved in the development of chemoresistance to cisplatin via modulating signaling pathway of PTEN/AkT. | |||
| Disease Class: Ovarian cancer [ICD-11: 2C73.0] | [18] | |||
| Resistant Disease | Ovarian cancer [ICD-11: 2C73.0] | |||
| Resistant Drug | Cisplatin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | PTEN/PI3K/AKT signaling pathway | Regulation | N.A. | |
| In Vitro Model | SkOV3 cells | Ovary | Homo sapiens (Human) | CVCL_0532 |
| SkOV3/DDP cells | Ovary | Homo sapiens (Human) | CVCL_0532 | |
| Experiment for Molecule Alteration |
RT-qPCR; Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miRNA-21 enhances chemoresistance to cisplatin in epithelial ovarian cancer by negatively regulating PTEN. | |||
| Disease Class: Non-small cell lung cancer [ICD-11: 2C25.Y] | [19] | |||
| Resistant Disease | Non-small cell lung cancer [ICD-11: 2C25.Y] | |||
| Resistant Drug | Cisplatin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | AKT signaling pathway | Regulation | N.A. | |
| miR21/PTEN signaling pathway | Regulation | N.A. | ||
| In Vitro Model | A549 cells | Lung | Homo sapiens (Human) | CVCL_0023 |
| H460 cells | Lung | Homo sapiens (Human) | CVCL_0459 | |
| H1299 cells | Lung | Homo sapiens (Human) | CVCL_0060 | |
| Sk-MES-1 cells | Lung | Homo sapiens (Human) | CVCL_0630 | |
| NCI-H358 cells | Lung | Homo sapiens (Human) | CVCL_1559 | |
| 16HBE cells | Lung | Homo sapiens (Human) | CVCL_0112 | |
| H157 cells | Lung | Homo sapiens (Human) | CVCL_2458 | |
| Experiment for Molecule Alteration |
Western blot analysis; Luciferase reporter assay; RNA immunoprecipitation assay | |||
| Experiment for Drug Resistance |
MTT assay; Soft agar assay | |||
| Mechanism Description | miR21 acts as an oncogenic miRNA through targeting PTEN in many cancers. By negatively regulating the intracellular levels of PI3k, PTEN exerts a suppressive effect on tumor through AkT pathway. miR21 was involved in GAS5 regulation of NSCLC sensitivity to DDP through PTEN pathway. | |||
| Disease Class: Non-small cell lung cancer [ICD-11: 2C25.Y] | [19] | |||
| Resistant Disease | Non-small cell lung cancer [ICD-11: 2C25.Y] | |||
| Resistant Drug | Cisplatin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | AKT signaling pathway | Regulation | N.A. | |
| In Vitro Model | A549 cells | Lung | Homo sapiens (Human) | CVCL_0023 |
| H460 cells | Lung | Homo sapiens (Human) | CVCL_0459 | |
| H1299 cells | Lung | Homo sapiens (Human) | CVCL_0060 | |
| Sk-MES-1 cells | Lung | Homo sapiens (Human) | CVCL_0630 | |
| NCI-H358 cells | Lung | Homo sapiens (Human) | CVCL_1559 | |
| 16HBE cells | Lung | Homo sapiens (Human) | CVCL_0112 | |
| H157 cells | Lung | Homo sapiens (Human) | CVCL_2458 | |
| Experiment for Molecule Alteration |
Western blot analysis; Luciferase reporter assay; RNA immunoprecipitation assay | |||
| Experiment for Drug Resistance |
MTT assay; Soft agar assay | |||
| Mechanism Description | GAS5 could compete with PTEN for miR21 binding, GAS5 downregulation can induce trastuzumab resistance of breast cancer By negatively regulating the intracellular levels of PI3k, PTEN exerts a suppressive effect on tumor through AkT pathway. GAS5 regulated NSCLC chemo-sensitivity to DDP-based therapy through PTEN pathway. | |||
| Disease Class: Cervical cancer [ICD-11: 2C77.0] | [20] | |||
| Resistant Disease | Cervical cancer [ICD-11: 2C77.0] | |||
| Resistant Drug | Cisplatin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | PI3K/AKT signaling pathway | Activation | hsa04151 | |
| In Vitro Model | Hela cells | Cervix uteri | Homo sapiens (Human) | CVCL_0030 |
| Siha cells | Cervix uteri | Homo sapiens (Human) | CVCL_0032 | |
| Caski cells | Uterus | Homo sapiens (Human) | CVCL_1100 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | The low level of GAS5 can down-regulate PTEN by interacting with miR21 because PTEN is one of the genes in the PI3k/Akt/mTOR pathway that can be regulated by GAS5 negatively. The low expression of PTEN activates the PI3k/Akt pathway. | |||
| Disease Class: Lung cancer [ICD-11: 2C25.5] | [21], [22], [23] | |||
| Resistant Disease | Lung cancer [ICD-11: 2C25.5] | |||
| Resistant Drug | Cisplatin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell survival | Inhibition | hsa05200 | |
| PTEN/AKT signaling pathway | Regulation | N.A. | ||
| Wnt/Beta-catenin signaling pathway | Activation | hsa04310 | ||
| In Vitro Model | A549 cells | Lung | Homo sapiens (Human) | CVCL_0023 |
| H1299 cells | Lung | Homo sapiens (Human) | CVCL_0060 | |
| NCl-H226 cells | Lung | Homo sapiens (Human) | CVCL_1544 | |
| H446 cells | Lung | Homo sapiens (Human) | CVCL_1562 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis; RIP assay; Luciferase reporter assay | |||
| Experiment for Drug Resistance |
CCK8 assay; SA-beta-gal assay | |||
| Mechanism Description | Suppression of miR-221 could lead to increase of PTEN expression level and enhance the CDDP chemosensitivity. And miRNA 328 overexpression confers cisplatin resistance in non small cell lung cancer via targeting of PTEN. And microRNA-130b targets PTEN to induce resistance to cisplatin in lung cancer cells by activating Wnt/beta-catenin pathway. | |||
| Disease Class: Ovarian cancer [ICD-11: 2C73.0] | [24] | |||
| Resistant Disease | Ovarian cancer [ICD-11: 2C73.0] | |||
| Resistant Drug | Cisplatin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell colony | Activation | hsa05200 | |
| Cell proliferation | Activation | hsa05200 | ||
| In Vitro Model | SkOV3 cells | Ovary | Homo sapiens (Human) | CVCL_0532 |
| OVCA433 cells | Ovary | Homo sapiens (Human) | CVCL_0475 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miR-216a increases cisplatin resistance in ovarian cancer cells via downregulating PTEN. | |||
| Disease Class: Non-small cell lung cancer [ICD-11: 2C25.Y] | [25] | |||
| Resistant Disease | Non-small cell lung cancer [ICD-11: 2C25.Y] | |||
| Resistant Drug | Cisplatin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell viability | Activation | hsa05200 | ||
| PTEN/PI3K/AKT signaling pathway | Inhibition | hsa05235 | ||
| In Vitro Model | A549/DDP cells | Lung | Homo sapiens (Human) | CVCL_0023 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay; Flow cytometry assay | |||
| Mechanism Description | miR-181 downregulation promoted cell growth and metastasis and inhibited cell apoptosis and suppressed LC3 and ATG5 protein expression in A549/DDP cells through suppression of the PTEN/PI3k/AkT/mTOR pathway, whereas miR-181 overexpression recovered LC3 and ATG5 protein expression by promoting PTEN/PI3k/AkT/mTOR signaling. | |||
| Disease Class: Gastric cancer [ICD-11: 2B72.1] | [26], [27], [28] | |||
| Resistant Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Resistant Drug | Cisplatin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell viability | Activation | hsa05200 | ||
| PTEN/AKT signaling pathway | Activation | hsa05235 | ||
| In Vitro Model | SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 |
| SGC7901/VCR cells | Gastric | Homo sapiens (Human) | CVCL_VU58 | |
| SGC7901/ADR cells | Gastric | Homo sapiens (Human) | CVCL_VU57 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | miR-106a is up-regulated in the DDP-resistant SGC7901/DDP cells, Overexpression of miR-106a in the SGC7901 cells confers resistance to DDP, PTEN is a target gene of miR-106a, there was a consistent and strong inverse correlation between the miR-106a levels and PTEN, PTEN is a key signal molecule in miR-106a-regulated DDP resistance in SGC7901/DDP cells. | |||
| Disease Class: Non-small cell lung cancer [ICD-11: 2C25.Y] | [29], [30] | |||
| Resistant Disease | Non-small cell lung cancer [ICD-11: 2C25.Y] | |||
| Resistant Drug | Cisplatin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell migration | Activation | hsa04670 | ||
| Cell proliferation | Activation | hsa05200 | ||
| PTEN signaling pathway | Regulation | N.A. | ||
| Tumorigenesis | Activation | hsa05206 | ||
| In Vitro Model | A549 cells | Lung | Homo sapiens (Human) | CVCL_0023 |
| SPC-A1 cells | Lung | Homo sapiens (Human) | CVCL_6955 | |
| Sk-MES-1 cells | Lung | Homo sapiens (Human) | CVCL_0630 | |
| 16HBE cells | Lung | Homo sapiens (Human) | CVCL_0112 | |
| Experiment for Molecule Alteration |
Western blot analysis; Luciferase assay | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miR-205 promotes the growth of the NSCLC cell lines, miR-205 is inversely correlated with PTEN expression, miR-205 has the ability to promote growth, migration, invasion and chemoresistance of NSCLC cells by targeting PTEN. And miR-21 decreased the expression of PTEN and increased Bcl-2 in A549. Upregulation of miR-21 induces cholangiocarcinoma cell survival and gemcitabine resistance primarily through targeting the PTEN dependent PI3k/Akt pathway. Inhibition of miR-21 was shown to increase the sensitivity to topotecan in breast cancer cells partly by regulating BCL2 induced anti-apoptosis indirectly in MCF-7 cells. | |||
| Disease Class: Ovarian cancer [ICD-11: 2C73.0] | [31], [32], [33] | |||
| Resistant Disease | Ovarian cancer [ICD-11: 2C73.0] | |||
| Resistant Drug | Cisplatin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| PI3K/AKT/PTEN/mTOR signaling pathway | Activation | hsa04151 | ||
| In Vitro Model | SkOV3 cells | Ovary | Homo sapiens (Human) | CVCL_0532 |
| OVCAR3 cells | Ovary | Homo sapiens (Human) | CVCL_0465 | |
| SkOV3/CIS cells | Ovary | Homo sapiens (Human) | CVCL_UI88 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miR-93, a new family member of PTEN regulator, blocks PTEN translation leading to activation of the AkT pathway and played an important role in regulating cisplatin chemosensitivity pathway in ovarian cancer. | |||
| Disease Class: Osteosarcoma [ICD-11: 2B51.0] | [34] | |||
| Resistant Disease | Osteosarcoma [ICD-11: 2B51.0] | |||
| Resistant Drug | Cisplatin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| PI3K/AKT signaling pathway | Regulation | N.A. | ||
| In Vitro Model | SOSP-9607 cells | Bones | Homo sapiens (Human) | CVCL_4V80 |
| SOSP-9901 cells | Bones | Homo sapiens (Human) | N.A. | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miR-221 induce cell survival and cisplatin resistance in human osteosarcoma at least partly through targeting the PI3k/PTEN/Akt pathway. | |||
| Disease Class: Non-small cell lung cancer [ICD-11: 2C25.Y] | [35] | |||
| Resistant Disease | Non-small cell lung cancer [ICD-11: 2C25.Y] | |||
| Resistant Drug | Cisplatin | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell migration | Activation | hsa04670 | ||
| PTEN signaling pathway | Inhibition | hsa05235 | ||
| In Vitro Model | KB-3-1 cells | Lung | Homo sapiens (Human) | CVCL_2088 |
| KB-CP.5 cells | Lung | Homo sapiens (Human) | CVCL_IP04 | |
| KB-CP20 cells | Lung | Homo sapiens (Human) | CVCL_IP06 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | PTEN, a tumor suppressor gene, is an essential regulator of cell proliferation, differentiation, growth, and apoptosis. miR-21 can promote growth, migration, and invasion, chemo- or radioresistance of NSCLC cells by downregulation PTEN. | |||
|
|
||||
| Disease Class: Gastric cancer [ICD-11: 2B72.1] | [16] | |||
| Resistant Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Resistant Drug | Cisplatin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| PI3K/AKT signaling pathway | Activation | hsa04151 | ||
| In Vitro Model | MGC-803 cells | Gastric | Homo sapiens (Human) | CVCL_5334 |
| MFC cells | Gastric | Homo sapiens (Human) | CVCL_5J48 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay; FITC Annexin V Apoptosis Detection assay; Flow cytometric analysis | |||
| Mechanism Description | Exosomal transfer of tumor-associated macrophages derived miR21 confer DDP resistance in gastric cancer Exosomal miR21 can be directly transferred from macrophages to the gastric cancer cells, where it suppresses cell apoptosis and enhances activation of PI3k/AkT signaling pathway by down-regulation of PTEN. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Cervical cancer [ICD-11: 2C77.0] | [36] | |||
| Sensitive Disease | Cervical cancer [ICD-11: 2C77.0] | |||
| Sensitive Drug | Cisplatin | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Hela cells | Cervix uteri | Homo sapiens (Human) | CVCL_0030 |
| Siha cells | Cervix uteri | Homo sapiens (Human) | CVCL_0032 | |
| Caski cells | Uterus | Homo sapiens (Human) | CVCL_1100 | |
| ME-180 cells | Uterus | Homo sapiens (Human) | CVCL_1401 | |
| H8 cells | Uterus | Homo sapiens (Human) | CVCL_9389 | |
| HCE1 cells | Uterus | Homo sapiens (Human) | CVCL_A8SM | |
| Experiment for Molecule Alteration |
Western blot analysis; Luciferase reporter assay | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | CASC2 upregulated PTEN expression by direct inhibiting miR21 in the DDP-resistant cancer cells, leading to the down-regulation of p-AkT protein, CASC2 up-regulates PTEN as a ceRNA of miR21. Inhibiting miR21 increased the sensitivity of human glioblastoma cells U251 and LN229 to taxol. | |||
| Disease Class: Non-small cell lung cancer [ICD-11: 2C25.Y] | [37] | |||
| Sensitive Disease | Non-small cell lung cancer [ICD-11: 2C25.Y] | |||
| Sensitive Drug | Cisplatin | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell proliferation | Inhibition | hsa05200 | ||
| Cell viability | Inhibition | hsa05200 | ||
| miR18a/PTEN signaling pathway | Regulation | N.A. | ||
| In Vitro Model | A549 cells | Lung | Homo sapiens (Human) | CVCL_0023 |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay; Flow cytometry assay | |||
| Mechanism Description | TP53TG1 increased the sensitivity of NSCLC cells to cisplatin by modulating miR-18a/PTEN axis by promoting PTEN expression via inhibiting miR-18a. | |||
| Disease Class: Esophageal adenocarcinoma [ICD-11: 2B70.2] | [38] | |||
| Sensitive Disease | Esophageal adenocarcinoma [ICD-11: 2B70.2] | |||
| Sensitive Drug | Cisplatin | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell proliferation | Inhibition | hsa05200 | ||
| PI3K/AKT signaling pathway | Inhibition | hsa04151 | ||
| In Vitro Model | OE33 cellss | Esophagus | Homo sapiens (Human) | CVCL_0471 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | PTEN and TNF were demonstrated to be upregulated following miR-187 overexpression. TNF is a cytokine that regulates multiple cellular processes including proliferation and apoptosis. PTEN acts as a tumor suppressor and regulates the PI3k/AkT pathway, which has been identified as a radiation response pathway. The upregulation of PTEN enhances radiosensitivity via the downregulation of the PI3k/AkT pathway. | |||
| Disease Class: Non-small cell lung cancer [ICD-11: 2C25.Y] | [39] | |||
| Sensitive Disease | Non-small cell lung cancer [ICD-11: 2C25.Y] | |||
| Sensitive Drug | Cisplatin | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell proliferation | Inhibition | hsa05200 | ||
| In Vitro Model | A549 cells | Lung | Homo sapiens (Human) | CVCL_0023 |
| Experiment for Molecule Alteration |
Western blot analysis; Immunofluorescence analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Overexpression of miR-92b promotes, while knockdown of it inhabits A549 cell growth, miR-92b regulates the resistance of NSCLC A549 cells to CDDP, Anti-miR-92b sensitizes A549/CDDP cells to CDDP-induced apop-tosis, miR-92b down-regulates PTEN expression at mRNA and protein level in A549 cells, PTEN plays important roles in cell cycle detention and apoptosis, regulation of cell adherence, migration, differentiation and has the function of enhancing the sensitivity of cancer cells to certain anticancer agents. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Gastric cancer [ICD-11: 2B72.1] | [2] | |||
| Sensitive Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Sensitive Drug | Doxorubicin | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Gastric cancer [ICD-11: 2B72] | |||
| The Specified Disease | Gastric cancer | |||
| The Studied Tissue | Gastric tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 2.35E-01 Fold-change: 6.64E-02 Z-score: 1.67E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell viability | Inhibition | hsa05200 | ||
| In Vitro Model | SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay; Flow cytometry assay | |||
| Mechanism Description | Suppression of miR-21-5p expression sensitizes SGC7901/DOX cells to DOX via upregulating PTEN and TIMP3. | |||
| Disease Class: Breast cancer [ICD-11: 2C60.3] | [48] | |||
| Sensitive Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Sensitive Drug | Doxorubicin | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| PTEN/AKT/GSk3Beta signaling pathway | Activation | hsa05235 | ||
| In Vitro Model | MCF-7 cells | Breast | Homo sapiens (Human) | CVCL_0031 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTS assay; Flow cytometry assay | |||
| Mechanism Description | Down-regulation of miR-29a expression in MCF-7/ADR cells increased PTEN expression levels, resulting in decreased phospho-Akt (p-Akt) and phospho-GSk3beta (p-GSk3beta) expression. Conversely, upregulation of miR-29a expression in MCF-7/S cells is associated with decreasing PTEN expression and increasing p-Akt and p-GSk3beta expression. PTEN and GSk3beta are targeted by miR-29a, and miR-29a may contribute to ADR resistance through inhibition of the PTEN/AkT/GSk3beta pathway in breast cancer cells. | |||
| Disease Class: Diffuse large B-cell lymphoma [ICD-11: 2A81.0] | [40] | |||
| Sensitive Disease | Diffuse large B-cell lymphoma [ICD-11: 2A81.0] | |||
| Sensitive Drug | Doxorubicin | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | PI3K/AKT signaling pathway | Inhibition | hsa04151 | |
| In Vitro Model | CRL2631 cells | Bone marrow | Homo sapiens (Human) | CVCL_3611 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miR-21 impacts the PI3k/AkT signaling pathway through the regulation of PTEN, thereby affecting cellular sensitivity to the CHOP chemotherapeutic regimen. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Breast cancer [ICD-11: 2C60.3] | [11] | |||
| Resistant Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Resistant Drug | Doxorubicin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Breast cancer [ICD-11: 2C60] | |||
| The Specified Disease | Breast cancer | |||
| The Studied Tissue | Breast tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 2.58E-08 Fold-change: -5.23E-02 Z-score: -5.71E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| In Vitro Model | MCF-7 cells | Breast | Homo sapiens (Human) | CVCL_0031 |
| MCF-7/ADR cells | Breast | Homo sapiens (Human) | CVCL_1452 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miR-21 regulates ADR resistance of breast cancer cells, at least in part, by targeting the tumor suppressor gene PTEN. | |||
| Disease Class: Bladder cancer [ICD-11: 2C94.0] | [12] | |||
| Resistant Disease | Bladder cancer [ICD-11: 2C94.0] | |||
| Resistant Drug | Doxorubicin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Bladder cancer [ICD-11: 2C94] | |||
| The Specified Disease | Bladder cancer | |||
| The Studied Tissue | Bladder tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 6.11E-08 Fold-change: -1.87E-01 Z-score: -1.22E+01 |
|||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell proliferation | Activation | hsa05200 | ||
| PI3K/AKT signaling pathway | Activation | hsa04151 | ||
| In Vitro Model | T24 cells | Bladder | Homo sapiens (Human) | CVCL_0554 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | A negative correlation between expression of miR-21 and pten was established in vivo. cell proliferation and chemoresistance to doxorubicin were promoted by overexpression of miR-21 in t24 cells. Bcl-2 up-regulation could be achieved by miR-21 overexpression, which prevented t24 cells from apoptosis induced by doxorubicin. | |||
| Disease Class: Hepatocellular carcinoma [ICD-11: 2C12.2] | [44] | |||
| Resistant Disease | Hepatocellular carcinoma [ICD-11: 2C12.2] | |||
| Resistant Drug | Doxorubicin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | PI3K/AKT signaling pathway | Activation | hsa04151 | |
| In Vitro Model | HepG2 cells | Liver | Homo sapiens (Human) | CVCL_0027 |
| SMMC7721 cells | Uterus | Homo sapiens (Human) | CVCL_0534 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
TUNEL assays | |||
| Mechanism Description | lncARSR physically associates with PTEN mRNA, promotes PTEN mRNA degradation, decreases PTEN expression, and activates PI3k/Akt pathway. Upregulated lncARSR promotes doxorubicin resistance in HCC via modulating PTEN-PI3k/Akt pathway. | |||
| Disease Class: Breast cancer [ICD-11: 2C60.3] | [45] | |||
| Resistant Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Resistant Drug | Doxorubicin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| PI3K/AKT signaling pathway | Activation | hsa04151 | ||
| In Vitro Model | MCF-7 cells | Breast | Homo sapiens (Human) | CVCL_0031 |
| MCF-7/ADR cells | Breast | Homo sapiens (Human) | CVCL_1452 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay; Colony Formation assay; FITC Annexin V Apoptosis assay | |||
| Mechanism Description | microRNA-130b targets PTEN to mediate drug resistance and proliferation of breast cancer cells via the PI3k/Akt signaling pathway. PTEN acted as a tumor inhibitor gene by specifically reversely regulating the PI3k/Akt pathway, miR130b may activate PI3k/Akt signaling by silencing PTEN. | |||
| Disease Class: Melanoma [ICD-11: 2C30.0] | [7] | |||
| Resistant Disease | Melanoma [ICD-11: 2C30.0] | |||
| Resistant Drug | Doxorubicin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | AKT/FAKT signaling pathway | Activation | hsa04151 | |
| In Vitro Model | A375 cells | Skin | Homo sapiens (Human) | CVCL_0132 |
| SkMEL1 cells | Skin | Homo sapiens (Human) | CVCL_0068 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay; Colony formation assay; Annexin V-fluorescein isothiocyanate (FITC) apoptosis analysis; Wound scratch healing or transwell invasion assay | |||
| Mechanism Description | PTEN can interact with AkT and FAk and inhibit their activity through their dephosphorylation, Akt and FAk signaling pathways are involved in miR301a/PTEN-promoting malignant phenotypes in MM cells, miR301a promotes MM progression via activation of Akt and FAk signaling pathways by down regulating PTEN. | |||
| Disease Class: Breast cancer [ICD-11: 2C60.3] | [46] | |||
| Resistant Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Resistant Drug | Doxorubicin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell viability | Activation | hsa05200 | ||
| PTEN/AKT/NF-kappaB signaling pathway | Regulation | N.A. | ||
| In Vitro Model | MCF-7 cells | Breast | Homo sapiens (Human) | CVCL_0031 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay; Flow cytometry assay | |||
| Mechanism Description | AkT/NF-kB pathway contributes to the miR-132/-212-mediated drug resistance phenotype in breast cancer cells, which is likely regulated by suppressing PTEN expression at the molecular level. | |||
| Disease Class: Breast cancer [ICD-11: 2C60.3] | [42] | |||
| Resistant Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Resistant Drug | Doxorubicin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell proliferation | Activation | hsa05200 | ||
| In Vitro Model | MCF-7 cells | Breast | Homo sapiens (Human) | CVCL_0031 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | PTEN plays major roles in suppressing cancer and embryonic development, cell migration and apoptosis, miR-222 and -29a could regulate the expression of PTEN, maybe through which the two miRNAs conferred Adr and Doc resistance in MCF-7 cells. | |||
| Disease Class: Gastric cancer [ICD-11: 2B72.1] | [27] | |||
| Resistant Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Resistant Drug | Doxorubicin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| PTEN/AKT signaling pathway | Inhibition | hsa05235 | ||
| In Vitro Model | SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 |
| SGC7901/VCR cells | Gastric | Homo sapiens (Human) | CVCL_VU58 | |
| SGC7901/ADR cells | Gastric | Homo sapiens (Human) | CVCL_VU57 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miR-19a/b are upregulated in multidrug-resistant gastric cancer cell line, miR-19a/b suppress the sensitivity of gastric cancer cells to anticancer drugs, miR-19a/b accelerate the efflux of ADR through P-gp upregulation. | |||
| Disease Class: Chronic myeloid leukemia [ICD-11: 2A20.0] | [47] | |||
| Resistant Disease | Chronic myeloid leukemia [ICD-11: 2A20.0] | |||
| Resistant Drug | Doxorubicin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| PI3K/AKT/mTOR signaling pathway | Activation | hsa04151 | ||
| In Vitro Model | K562 cells | Blood | Homo sapiens (Human) | CVCL_0004 |
| K562/A02 cells | Blood | Homo sapiens (Human) | CVCL_0368 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miR-21 is associated with inactivation of PTEN, a know tumor suppressor gene, resulting in activation of PI3k/Akt/mTOR signaling pathway, Akt promotes cell survival by inhibiting apoptosis through its ability to phosphorylate/inactivate downstream targets of apoptotic machinery. ADR sensitivity is associated with up-regulation of PTEN resulting from the inhibition of miR-21 expression. | |||
|
|
||||
| Disease Class: Breast cancer [ICD-11: 2C60.3] | [43] | |||
| Resistant Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Resistant Drug | Doxorubicin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell proliferation | Activation | hsa05200 | |
| Epithelial mesenchymal transition signaling pathway | Activation | hsa01521 | ||
| In Vitro Model | MCF-7 cells | Breast | Homo sapiens (Human) | CVCL_0031 |
| MCF-7/ADR cells | Breast | Homo sapiens (Human) | CVCL_1452 | |
| Experiment for Molecule Alteration |
Dual-luciferase reporter assay; qRT-PCR | |||
| Experiment for Drug Resistance |
CCK8 assay; Flow cytometric analysis | |||
| Mechanism Description | miR93 contributes to inducing EMT and drug resistance of breast cancer cells by targeting PTEN. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Malignant glioma [ICD-11: 2A00.2] | [3] | |||
| Sensitive Disease | Malignant glioma [ICD-11: 2A00.2] | |||
| Sensitive Drug | Temozolomide | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Brain cancer [ICD-11: 2A00] | |||
| The Specified Disease | Brain cancer | |||
| The Studied Tissue | Nervous tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 3.13E-109 Fold-change: 1.46E-01 Z-score: 2.48E+01 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell growth | Inhibition | hsa05200 | |
| In Vitro Model | U251 cells | Brain | Homo sapiens (Human) | CVCL_0021 |
| LN229 cells | Brain | Homo sapiens (Human) | CVCL_0393 | |
| SNB19 cells | Brain | Homo sapiens (Human) | CVCL_0535 | |
| U373 cells | Brain | Homo sapiens (Human) | CVCL_2219 | |
| U118 cells | Brain | Homo sapiens (Human) | CVCL_0633 | |
| NHA cells | Brain | Homo sapiens (Human) | N.A. | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay; BrdU incorporation assay | |||
| Mechanism Description | CASC2 up-regulated PTEN protein and down-regulated p-AkT protein through regulating miR181a, and the effect of CASC2 on PTEN and p-AkT could be partially restored by miR181a. | |||
| Disease Class: Glioma [ICD-11: 2A00.1] | [3] | |||
| Sensitive Disease | Glioma [ICD-11: 2A00.1] | |||
| Sensitive Drug | Temozolomide | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | PTEN signaling pathway | Activation | hsa05235 | |
| In Vitro Model | U251 cells | Brain | Homo sapiens (Human) | CVCL_0021 |
| LN229 cells | Brain | Homo sapiens (Human) | CVCL_0393 | |
| SNB19 cells | Brain | Homo sapiens (Human) | CVCL_0535 | |
| U373 cells | Brain | Homo sapiens (Human) | CVCL_2219 | |
| U118 cells | Brain | Homo sapiens (Human) | CVCL_0633 | |
| NHA cells | Brain | Homo sapiens (Human) | N.A. | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay; BrdU incorporation assay | |||
| Mechanism Description | CASC2 up-regulated PTEN protein and down-regulated p-AkT protein through regulating miR181a, and the effect of CASC2 on PTEN and p-AkT could be partially restored by miR181a. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Prostate cancer [ICD-11: 2C82.0] | [4] | |||
| Sensitive Disease | Prostate cancer [ICD-11: 2C82.0] | |||
| Sensitive Drug | Cabazitaxel | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Prostate cancer [ICD-11: 2C82] | |||
| The Specified Disease | Prostate cancer | |||
| The Studied Tissue | Prostate | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 2.27E-02 Fold-change: 1.09E-01 Z-score: 2.44E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | CAL27 cells | Oral | Homo sapiens (Human) | CVCL_1107 |
| LOVO cells | Colon | Homo sapiens (Human) | CVCL_0399 | |
| BxPC-3 cells | Pancreas | Homo sapiens (Human) | CVCL_0186 | |
| C4-2 cells | Prostate | Homo sapiens (Human) | CVCL_4782 | |
| HuTu80 cells | Small intestine | Homo sapiens (Human) | CVCL_1301 | |
| DU145-DR cells | Brain | Homo sapiens (Human) | CVCL_4Y36 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | TUBB3 expression was upregulated in DTX-resistant and CBZ-resistant cells. TUBB3 knockdown re-sensitized DTX-resistant cells to DTX and CBZ-resistant cells to CBZ. Additionally, TUBB3 knockdown re-sensitized DTX-resistant cell lines to CBZ, indicating that TUBB3 mediates cross-resistance between DTX and CBZ. Knockdown of TUBB3 enhanced PTEN expression, and PTEN knockout enhanced TUBB3 expression. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Prostate cancer [ICD-11: 2C82.0] | [4] | |||
| Resistant Disease | Prostate cancer [ICD-11: 2C82.0] | |||
| Resistant Drug | Cabazitaxel | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | CAL27 cells | Oral | Homo sapiens (Human) | CVCL_1107 |
| LOVO cells | Colon | Homo sapiens (Human) | CVCL_0399 | |
| BxPC-3 cells | Pancreas | Homo sapiens (Human) | CVCL_0186 | |
| C4-2 cells | Prostate | Homo sapiens (Human) | CVCL_4782 | |
| HuTu80 cells | Small intestine | Homo sapiens (Human) | CVCL_1301 | |
| DU145-DR cells | Brain | Homo sapiens (Human) | CVCL_4Y36 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | TUBB3 expression was upregulated in DTX-resistant and CBZ-resistant cells. TUBB3 knockdown re-sensitized DTX-resistant cells to DTX and CBZ-resistant cells to CBZ. Additionally, TUBB3 knockdown re-sensitized DTX-resistant cell lines to CBZ, indicating that TUBB3 mediates cross-resistance between DTX and CBZ. Knockdown of TUBB3 enhanced PTEN expression, and PTEN knockout enhanced TUBB3 expression. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Renal carcinoma [ICD-11: 2C90.2] | [5] | |||
| Sensitive Disease | Renal carcinoma [ICD-11: 2C90.2] | |||
| Sensitive Drug | Paclitaxel | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Kidney cancer [ICD-11: 2C90] | |||
| The Specified Disease | Renal cancer | |||
| The Studied Tissue | Kidney | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 3.24E-05 Fold-change: 1.07E-01 Z-score: 6.36E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | PI3K/AKT signaling pathway | Inhibition | hsa04151 | |
| In Vitro Model | 786-O cells | Kidney | Homo sapiens (Human) | CVCL_1051 |
| ACHN cells | Pleural effusion | Homo sapiens (Human) | CVCL_1067 | |
| HK-2 cells | Kidney | Homo sapiens (Human) | CVCL_0302 | |
| RCC10 cells | Kidney | Homo sapiens (Human) | CVCL_6265 | |
| RCC4 cells | Kidney | Homo sapiens (Human) | CVCL_0498 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Celltiter96 Aqueous Non Radioactive Cell Proliferation Assay | |||
| Mechanism Description | Tumor suppressor genes like PTEN, PDCD4 and TIMP3, are target genes of miR21. PTEN is a potent inhibitor of PI3k/Akt pathway, as well as a direct target of miR21. | |||
| Disease Class: Cervical cancer [ICD-11: 2C77.0] | [67] | |||
| Sensitive Disease | Cervical cancer [ICD-11: 2C77.0] | |||
| Sensitive Drug | Paclitaxel | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell proliferation | Activation | hsa05200 | ||
| PTEN/AKT signaling pathway | Regulation | N.A. | ||
| In Vitro Model | Hela cells | Cervix uteri | Homo sapiens (Human) | CVCL_0030 |
| Siha cells | Cervix uteri | Homo sapiens (Human) | CVCL_0032 | |
| Caski cells | Uterus | Homo sapiens (Human) | CVCL_1100 | |
| ME-180 cells | Uterus | Homo sapiens (Human) | CVCL_1401 | |
| C33A cells | Uterus | Homo sapiens (Human) | CVCL_1094 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Annexin V-FITC/PI staining for cell apoptosis assay; Hoechst 33258 staining for cell apoptosis assay; MTT assay | |||
| Mechanism Description | miR21 inhibitor suppresses cell proliferation and colony formation through regulating the PTEN/AkT pathway and improves paclitaxel sensitivity in cervical cancer cells. | |||
| Disease Class: Colon cancer [ICD-11: 2B90.1] | [68] | |||
| Sensitive Disease | Colon cancer [ICD-11: 2B90.1] | |||
| Sensitive Drug | Paclitaxel | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell proliferation | Inhibition | hsa05200 | ||
| In Vitro Model | HT-29 cells | Colon | Homo sapiens (Human) | CVCL_0320 |
| HCT15 cells | Colon | Homo sapiens (Human) | CVCL_0292 | |
| Experiment for Molecule Alteration |
Northern blotting analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Overexpression of miR-22 enhanced the anticancer effect of paclitaxel in the p53-mutated cells through increasing cell apoptosis and reducing cell proliferation and survival. The anticancer role of miR-22 was mediated by activation of PTEN signaling, subsequent inhibition of Akt Ser473 phosphorylation and MTDH expression, as well as upregulation of Bax and active caspase-3 levels. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Ovarian cancer [ICD-11: 2C73.0] | [65] | |||
| Resistant Disease | Ovarian cancer [ICD-11: 2C73.0] | |||
| Resistant Drug | Paclitaxel | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell invasion | Activation | hsa05200 | ||
| Cell migration | Activation | hsa04670 | ||
| Cell proliferation | Activation | hsa05200 | ||
| PTEN/AKT signaling pathway | Regulation | N.A. | ||
| In Vitro Model | SkOV3 cells | Ovary | Homo sapiens (Human) | CVCL_0532 |
| OVCAR3 cells | Ovary | Homo sapiens (Human) | CVCL_0465 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay; Flow cytometry assay | |||
| Mechanism Description | microRNA-17-5p induces drug resistance and invasion of ovarian carcinoma cells by targeting PTEN signaling. | |||
| Disease Class: Lung adenocarcinoma [ICD-11: 2C25.0] | [66] | |||
| Resistant Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | |||
| Resistant Drug | Paclitaxel | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell invasion | Activation | hsa05200 | ||
| Cell migration | Activation | hsa04670 | ||
| Cell proliferation | Activation | hsa05200 | ||
| PI3K/AKT signaling pathway | Regulation | N.A. | ||
| In Vitro Model | A549 cells | Lung | Homo sapiens (Human) | CVCL_0023 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay; Transwell assay | |||
| Mechanism Description | Paclitaxel- and cisplatin-resistant A549 cells acquired metastatic properties and EMT phenotype and had reduced PTEN expression as compared to sensitive cells. miR 181a was identified as a differentially expressed miRNA in drug-resistant A549 cells, and miR-181a mimic and inhibitor were shown to affect migration, invasion, morphology and expression of EMT-associated genes. PTEN was identified as a direct target of miR-181a. Our findings demonstrate that miR-181a expression in lung adenocarcinoma is associated with EMT progression, potentially through targeting of PTEN. Regulation of miR-181a may provide a novel strategy for overcoming resistance to paclitaxel and cisplatin in lung adenocarcinoma. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Non-small cell lung cancer [ICD-11: 2C25.Y] | [6] | |||
| Resistant Disease | Non-small cell lung cancer [ICD-11: 2C25.Y] | |||
| Resistant Drug | Gefitinib | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Lung cancer [ICD-11: 2C25] | |||
| The Specified Disease | Lung cancer | |||
| The Studied Tissue | Lung tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.01E-01 Fold-change: -9.25E-03 Z-score: -1.64E+00 |
|||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | NSCLC cells | Lung | Homo sapiens (Human) | N.A. |
| In Vivo Model | A retrospective survey in conducting clinical studies | Homo sapiens | ||
| Experiment for Molecule Alteration |
Next-generation sequencing assay | |||
| Experiment for Drug Resistance |
Liquid biopsy; ATP-binding pocket affinity comparison assay | |||
| Mechanism Description | Known mechanisms are secondary resistance mutations occurring in the ATP-binding domain (such as T790M and C797S), mutation or amplification of bypass signallings (such as AXL, Hh, ERBb2, CRIPTO, etc), activating mutations in the downstream pathways (PI3k, AkT, MEk, RAF), low levels of mRNA or polymorphisms of the pro-apoptotic protein BIM, induction of a transcription programme for EMT and phenotypical changes, or induction of elevated tumour PD-L1 levels. | |||
| Disease Class: Non-small cell lung cancer [ICD-11: 2C25.Y] | [59] | |||
| Resistant Disease | Non-small cell lung cancer [ICD-11: 2C25.Y] | |||
| Resistant Drug | Gefitinib | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | AKT/ERK signaling pathway | Activation | hsa04010 | |
| Cell apoptosis | Inhibition | hsa04210 | ||
| Cell invasion | Activation | hsa05200 | ||
| In Vitro Model | PC9 cells | Lung | Homo sapiens (Human) | CVCL_B260 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay; Flow cytometry assay | |||
| Mechanism Description | miR-21 was up-regulated concomitantly to down-regulation of Pten in pc-9/GR cells in comparison with pc-9 cells. Moreover, over-expression of miR-21 significantly decreased gefitinib sensitivity by down-regulating Pten expression and activating Akt and ERk pathways in pc-9 cells, while miR-21 knockdown dramatically restored gefitinib sensitivity of pc-9/GR cells by up-regulation of Pten expression and inactivation of AkT and ERk pathways, in vivo and in vitro. | |||
| Disease Class: Non-small cell lung cancer [ICD-11: 2C25.Y] | [60] | |||
| Resistant Disease | Non-small cell lung cancer [ICD-11: 2C25.Y] | |||
| Resistant Drug | Gefitinib | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell proliferation | Activation | hsa05200 | ||
| PI3K/AKT signaling pathway | Activation | hsa04151 | ||
| In Vitro Model | PC9 cells | Lung | Homo sapiens (Human) | CVCL_B260 |
| PC9R cells | Lung | Homo sapiens (Human) | CVCL_D778 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miR-21 overexpression is associated with the acquired resistance of EGFR-TkI in NSCLC, which might be caused by miR-21's function of activating PI3k/AkT pathway through inhibiting PTEN and PDCD4. | |||
| Disease Class: Non-small cell lung cancer [ICD-11: 2C25.Y] | [6] | |||
| Resistant Disease | Non-small cell lung cancer [ICD-11: 2C25.Y] | |||
| Resistant Drug | Gefitinib | |||
| Molecule Alteration | Structural variation | Copy number loss |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | NSCLC cells | Lung | Homo sapiens (Human) | N.A. |
| In Vivo Model | A retrospective survey in conducting clinical studies | Homo sapiens | ||
| Experiment for Molecule Alteration |
Next-generation sequencing assay | |||
| Experiment for Drug Resistance |
Liquid biopsy; ATP-binding pocket affinity comparison assay | |||
| Mechanism Description | Known mechanisms are secondary resistance mutations occurring in the ATP-binding domain (such as T790M and C797S), mutation or amplification of bypass signallings (such as AXL, Hh, ERBb2, CRIPTO, etc), activating mutations in the downstream pathways (PI3k, AkT, MEk, RAF), low levels of mRNA or polymorphisms of the pro-apoptotic protein BIM, induction of a transcription programme for EMT and phenotypical changes, or induction of elevated tumour PD-L1 levels. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Non-small cell lung cancer [ICD-11: 2C25.Y] | [61] | |||
| Sensitive Disease | Non-small cell lung cancer [ICD-11: 2C25.Y] | |||
| Sensitive Drug | Gefitinib | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | PTEN/AKT signaling pathway | Activation | hsa05235 | |
| In Vitro Model | 293T cells | Breast | Homo sapiens (Human) | CVCL_0063 |
| NCI-HCC827 cells | Lung | Homo sapiens (Human) | CVCL_2063 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTS assay | |||
| Mechanism Description | The knockdown of miR-214 resulted in not only PTEN un-regulation, but also the inactivation of p-AkT. This evidence indicated that miR-214 could regulate PTEN/AkT signaling pathway in EGFR mutant NSCLC cells. Furthermore, the knockdown of miR-214 re-sensitized HCC827/GR to gefitinib. Taken together, these evidences suggested that miR-214 may regulate the acquired resistance to gefinib in EGFR mutant cell lines by targeting PTEN/AkT signaling pathway. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Pancreatic cancer [ICD-11: 2C10.3] | [8] | |||
| Resistant Disease | Pancreatic cancer [ICD-11: 2C10.3] | |||
| Resistant Drug | Gemcitabine | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Pancreatic cancer [ICD-11: 2C10] | |||
| The Specified Disease | Pancreatic cancer | |||
| The Studied Tissue | Pancreas | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.32E-02 Fold-change: -3.94E-02 Z-score: -2.68E+00 |
|||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | AKT signaling pathway | Activation | hsa04151 | |
| Cell apoptosis | Inhibition | hsa04210 | ||
| Cell proliferation | Activation | hsa05200 | ||
| In Vitro Model | LPc006 cells | Pancreas | Homo sapiens (Human) | N.A. |
| LPc028 cells | Pancreas | Homo sapiens (Human) | N.A. | |
| LPc033 cells | Pancreas | Homo sapiens (Human) | N.A. | |
| LPc067 cells | Pancreas | Homo sapiens (Human) | N.A. | |
| LPc111 cells | Pancreas | Homo sapiens (Human) | N.A. | |
| LPc167 cells | Pancreas | Homo sapiens (Human) | N.A. | |
| PP437 cells | Pancreas | Homo sapiens (Human) | N.A. | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Fluorescence microscopy | |||
| Mechanism Description | miR-21 regulates expression of PTEN and phosphorylation of its downstream kinase Akt and (b) the reduction of phospho-Akt (pAkt) correlated with the enhancement of gemcitabine-induced apoptosis and antitumor activity in vitro and in vivo, suggesting that Akt pathway plays a significant role in mediating drug resistance in PDAC cells. | |||
| Disease Class: Pancreatic ductal adenocarcinoma [ICD-11: 2C10.0] | [62] | |||
| Resistant Disease | Pancreatic ductal adenocarcinoma [ICD-11: 2C10.0] | |||
| Resistant Drug | Gemcitabine | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell migration | Activation | hsa04670 | |
| In Vitro Model | PANC-1 cells | Pancreas | Homo sapiens (Human) | CVCL_0480 |
| HPAC cells | Pancreas | Homo sapiens (Human) | CVCL_3517 | |
| BxPc3 cells | Pancreas | Homo sapiens (Human) | CVCL_0186 | |
| Capan cells | Pancreas | Homo sapiens (Human) | CVCL_0237 | |
| HPAF cells | Pancreas | Homo sapiens (Human) | CVCL_B284 | |
| PL-45 cells | Pancreas | Homo sapiens (Human) | CVCL_3567 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Histone acetylation levels at miR-21 promoter were increased in PDAC cells after treatment with gemcitabine. Enhanced invasion and metastasis, increased miR-21 expression, decreased PTEN, elevated pAkT level were demonstrated in gemcitabine-resistant HPAC and PANC-1 cells. Pre-miR-21 transfection or TSA treatment further increased invasion and metastasis ability, decreased PTEN, and elevated pAkT levels in these two lines. In contrast, anti-miR-21 transfection could reverse invasion and metastasis, and PTEN and pAkT expressions induced by gemcitabine. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Cholangiocarcinoma [ICD-11: 2C12.0] | [63] | |||
| Sensitive Disease | Cholangiocarcinoma [ICD-11: 2C12.0] | |||
| Sensitive Drug | Gemcitabine | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell proliferation | Activation | hsa05200 | |
| PI3K signaling pathway | Activation | hsa04151 | ||
| In Vitro Model | H69 cells | Lung | Homo sapiens (Human) | CVCL_8121 |
| KMCH-1 cells | Gallbladder | Homo sapiens (Human) | CVCL_7970 | |
| Mz-ChA-1 cells | Gallbladder | Homo sapiens (Human) | CVCL_6932 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Celltiter 96 aqueous one solution cell proliferation assay | |||
| Mechanism Description | miR-21, miR-141, and miR-200b werehighly over-expressed in malignant cholangiocytes. Inhibi-tion of miR-21 and miR-200b increased sensitivity to gem-citabine, whereas inhibition of miR-141 decreased cellgrowth. miR-21 modulates gemcitabine-induced apo-ptosis by phosphatase and tensin homolog deleted onchromosome 10 (PTEN) -dependent activation of PI 3-ki-nase signaling. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Hepatocellular carcinoma [ICD-11: 2C12.2] | [9] | |||
| Resistant Disease | Hepatocellular carcinoma [ICD-11: 2C12.2] | |||
| Resistant Drug | Fluorouracil | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Liver cancer [ICD-11: 2C12] | |||
| The Specified Disease | Liver cancer | |||
| The Studied Tissue | Liver tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.86E-04 Fold-change: -4.60E-02 Z-score: -3.95E+00 |
|||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell viability | Activation | hsa05200 | |
| In Vitro Model | Huh-7 cells | Liver | Homo sapiens (Human) | CVCL_0336 |
| HepG2 cells | Liver | Homo sapiens (Human) | CVCL_0027 | |
| PLC/PRF/5 cells | Liver | Homo sapiens (Human) | CVCL_0485 | |
| HLE cells | Liver | Homo sapiens (Human) | CVCL_1281 | |
| HLF cells | Liver | Homo sapiens (Human) | CVCL_2947 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Hepatocellular carcinoma cells transfected with pre-miR-21 were significantly resistant to IFN-alpha/5-FU. Transfection of anti-miR-21 rendered HCC cells sensitive to IFN-alpha/5-FU, and such sensitivity was weakened by transfection of siRNAs of target molecules, PETN and PDCD4, miR-21 induces chemoresistance to IFN-alpha and 5-FU, mediated through PETN and PDCD4. | |||
| Disease Class: Esophageal cancer [ICD-11: 2B70.1] | [52] | |||
| Resistant Disease | Esophageal cancer [ICD-11: 2B70.1] | |||
| Resistant Drug | Fluorouracil | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | TE-1 cells | Esophagus | Homo sapiens (Human) | CVCL_1759 |
| EC9706 cells | Esophagus | Homo sapiens (Human) | CVCL_E307 | |
| KYSE150 cells | Esophagus | Homo sapiens (Human) | CVCL_1348 | |
| EC109 cells | Esophagus | Homo sapiens (Human) | CVCL_6898 | |
| EC9706-R cells | Esophagus | Homo sapiens (Human) | CVCL_E307 | |
| Het-1A cells | Esophagus | Homo sapiens (Human) | CVCL_3702 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay; Annexin V-FITC Apoptosis Detection assay | |||
| Mechanism Description | Involvement of microRNA-141-3p in 5-fluorouracil and oxaliplatin chemo-resistance in esophageal cancer cells via down-regulation of PTEN. | |||
| Disease Class: Pancreatic cancer [ICD-11: 2C10.3] | [53] | |||
| Resistant Disease | Pancreatic cancer [ICD-11: 2C10.3] | |||
| Resistant Drug | Fluorouracil | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell invasion | Activation | hsa05200 | |
| Cell migration | Activation | hsa04670 | ||
| Cell proliferation | Activation | hsa05200 | ||
| PI3K/AKT/mTOR signaling pathway | Regulation | N.A. | ||
| In Vitro Model | PANC-1 cells | Pancreas | Homo sapiens (Human) | CVCL_0480 |
| PATU8988 cells | Pancreas | Homo sapiens (Human) | CVCL_1846 | |
| 293TN cells | Pancreas | Homo sapiens (Human) | CVCL_UL49 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | miR-21 regulates 5-FU drug resistance in pancreatic cancer by reducing the expression of its targets, PTEN and PDCD4. And PTEN and PDCD4, as tumor suppressors, not only can inhibit tumor growth and invasion, but also can downregulate the 5-FU resistance induced by miR-21 in pancreatic cancer cells. | |||
| Disease Class: Colorectal cancer [ICD-11: 2B91.1] | [54] | |||
| Resistant Disease | Colorectal cancer [ICD-11: 2B91.1] | |||
| Resistant Drug | Fluorouracil | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell invasion | Activation | hsa05200 | ||
| Cell migration | Activation | hsa04670 | ||
| Cell proliferation | Activation | hsa05200 | ||
| PTEN/AKT/PI3K signaling pathway | Activation | hsa05235 | ||
| In Vitro Model | SW480 cells | Colon | Homo sapiens (Human) | CVCL_0546 |
| COLO205 cells | Colon | Homo sapiens (Human) | CVCL_F402 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay; Flow cytometry assay | |||
| Mechanism Description | The expression level of miRNA-17-5p was found increased in chemoresistant patients. Significantly higher expression levels of miR-17-5p were found in CRC patients with distant metastases and higher clinical stages. kaplan-Meier analysis showed that CRC patients with higher levels of miR-17-5p had reduced survival, especially in patients who had previously received chemotherapy. Overexpression of miR-17-5p promoted COLO205 cell invasiveness. PTEN was a target of miR-17-5p in the colon cancer cells, and their context-specific interactions were responsible for multiple drug-resistance. Chemotherapy was found to increase the expression levels of miR-17-5p, which further repressed PTEN levels, contributing to the development of chemo-resistance. | |||
| Disease Class: Gastric cancer [ICD-11: 2B72.1] | [27] | |||
| Resistant Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Resistant Drug | Fluorouracil | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| PTEN/AKT signaling pathway | Inhibition | hsa05235 | ||
| In Vitro Model | SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 |
| SGC7901/VCR cells | Gastric | Homo sapiens (Human) | CVCL_VU58 | |
| SGC7901/ADR cells | Gastric | Homo sapiens (Human) | CVCL_VU57 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miR-19a/b are upregulated in multidrug-resistant gastric cancer cell line, miR-19a/b suppress the sensitivity of gastric cancer cells to anticancer drugs, miR-19a/b accelerate the efflux of ADR through P-gp upregulation. | |||
|
|
||||
| Disease Class: Colorectal cancer [ICD-11: 2B91.1] | [50] | |||
| Resistant Disease | Colorectal cancer [ICD-11: 2B91.1] | |||
| Resistant Drug | Fluorouracil | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | PTEN/AKT signaling pathway | Regulation | N.A. | |
| In Vitro Model | SW480 cells | Colon | Homo sapiens (Human) | CVCL_0546 |
| HCT116 cells | Colon | Homo sapiens (Human) | CVCL_0291 | |
| HCT-8 cells | Colon | Homo sapiens (Human) | CVCL_2478 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | miR-1246, miR-21-5p, miR-96-5p and miR-1229-5p from serum exosomes involved in chemotherapy resistance may be new therapeutic targets, downregulating these miRNAs may promote CRC cell sensitivity to chemotherapeutic drugs. | |||
| Disease Class: Hepatocellular carcinoma [ICD-11: 2C12.2] | [51] | |||
| Resistant Disease | Hepatocellular carcinoma [ICD-11: 2C12.2] | |||
| Resistant Drug | Fluorouracil | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell proliferation | Activation | hsa05200 | |
| Epithelial mesenchymal transition signaling pathway | Activation | hsa01521 | ||
| PI3K/AKT signaling pathway | Activation | hsa04151 | ||
| In Vitro Model | BEL-7402 cells | Liver | Homo sapiens (Human) | CVCL_5492 |
| HepG2 cells | Liver | Homo sapiens (Human) | CVCL_0027 | |
| Hep3B cells | Liver | Homo sapiens (Human) | CVCL_0326 | |
| HEK293T cells | Kidney | Homo sapiens (Human) | CVCL_0063 | |
| SMMC7721 cells | Uterus | Homo sapiens (Human) | CVCL_0534 | |
| MHCC97-H cells | Liver | Homo sapiens (Human) | CVCL_4972 | |
| Bel/5-FU cells | Liver | Homo sapiens (Human) | CVCL_5493 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay; Wound healing assay; Transwell assay | |||
| Mechanism Description | Over-expression of miR-32-5p activated the PI3k/Akt pathway by suppressing PTEN and induced multidrug resistance via exosomes through promoting angiogenesis and epithelial-mesenchymal transition. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Gastric cancer [ICD-11: 2B72.1] | [55] | |||
| Sensitive Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Sensitive Drug | Fluorouracil | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell proliferation | Inhibition | hsa05200 | |
| PI3K/AKT signaling pathway | Inhibition | hsa04151 | ||
| In Vitro Model | BGC-823 cells | Gastric | Homo sapiens (Human) | CVCL_3360 |
| MGC-803 cells | Gastric | Homo sapiens (Human) | CVCL_5334 | |
| SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 | |
| AGS cells | Gastric | Homo sapiens (Human) | CVCL_0139 | |
| MkN-45 cells | Gastric | Homo sapiens (Human) | CVCL_0434 | |
| Experiment for Molecule Alteration |
Luciferase reporter assay; Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay; Flow cytometric analysis | |||
| Mechanism Description | miR147 suppressed the proliferation and enhanced the chemosensitivity of gastric cancer cells to 5-FU by promoting cell apoptosis through directly targeting PTEN and regulating the PI3k/AkT signaling pathway. knockdown of pten reverses the effects of miR147 downregulation on gastric cancer cells. | |||
| Disease Class: Renal carcinoma [ICD-11: 2C90.2] | [5] | |||
| Sensitive Disease | Renal carcinoma [ICD-11: 2C90.2] | |||
| Sensitive Drug | Fluorouracil | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | PI3K/AKT signaling pathway | Inhibition | hsa04151 | |
| In Vitro Model | 786-O cells | Kidney | Homo sapiens (Human) | CVCL_1051 |
| ACHN cells | Pleural effusion | Homo sapiens (Human) | CVCL_1067 | |
| HK-2 cells | Kidney | Homo sapiens (Human) | CVCL_0302 | |
| RCC10 cells | Kidney | Homo sapiens (Human) | CVCL_6265 | |
| RCC4 cells | Kidney | Homo sapiens (Human) | CVCL_0498 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Celltiter96 Aqueous Non Radioactive Cell Proliferation Assay | |||
| Mechanism Description | Tumor suppressor genes like PTEN, PDCD4 and TIMP3, are target genes of miR21. PTEN is a potent inhibitor of PI3k/Akt pathway, as well as a direct target of miR21. | |||
| Disease Class: Colorectal cancer [ICD-11: 2B91.1] | [56] | |||
| Sensitive Disease | Colorectal cancer [ICD-11: 2B91.1] | |||
| Sensitive Drug | Fluorouracil | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | AKT signaling pathway | Inhibition | hsa04151 | |
| In Vitro Model | HCT116 cells | Colon | Homo sapiens (Human) | CVCL_0291 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay; Annexin V/ PI staining; Caspase-3 activity assay | |||
| Mechanism Description | Levels of PTEN and E-cadherin were reduced by knockdown of miR200c in HCT-116 cells, PTEN inactivate the AkT signaling pathway, and E-cadherin is one of the major downstream regulators of miRNA-200c contributing to EMT, which is also important to inhibit tumor invasion and proliferation as well as to induce cell apoptosis. | |||
| Disease Class: Colorectal cancer [ICD-11: 2B91.1] | [57] | |||
| Sensitive Disease | Colorectal cancer [ICD-11: 2B91.1] | |||
| Sensitive Drug | Fluorouracil | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell viability | Inhibition | hsa05200 | ||
| PTEN/PI3K/AKT signaling pathway | Activation | hsa05235 | ||
| In Vitro Model | HCT8 cells | Colon | Homo sapiens (Human) | CVCL_2478 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay; Transwell assay; Flow cytometry assay | |||
| Mechanism Description | miR-543 enhanced drug resistance by down-regulating the expression of phosphatase and tensin homolog (PTEN), which negatively regulates protein kinase B (AkT) activation while an elevated expression of PTEN reversed the chemoresistance of miR-543-overexpressing HCT8 cells to 5-FU. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Breast cancer [ICD-11: 2C60.3] | [13], [14] | |||
| Resistant Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Resistant Drug | Alpelisib | |||
| Molecule Alteration | Structural variation | Copy number loss |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | PI3K signaling pathway | Activation | hsa04151 | |
| Experiment for Molecule Alteration |
Whole genome sequencing assay; Whole exome sequencing assay | |||
| Experiment for Drug Resistance |
Tetrazolium-based MTT assay | |||
| Mechanism Description | We conclude that parallel genetic evolution of separate metastatic sites with different PTEN genomic alterations leads to a convergent PTEN-null phenotype resistant to PI(3)kalpha inhibition. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Glioblastoma [ICD-11: 2A00.02] | [15] | |||
| Resistant Disease | Glioblastoma [ICD-11: 2A00.02] | |||
| Resistant Drug | Carmustine | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell proliferation | Activation | hsa05200 | ||
| PI3K/PTEN/AKT signaling axis | Activation | hsa04151 | ||
| In Vitro Model | U251 cells | Brain | Homo sapiens (Human) | CVCL_0021 |
| U87 cells | Brain | Homo sapiens (Human) | CVCL_0022 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | miR-221 regulated cell proliferation and BCNU resistance in glioma cells. Overexpression of miR-221 led to cell survival and BCNU resistance and reduced cell apoptosis induced by BCNU, whereas knockdown of miR-221 inhibited cell proliferation and prompted BCNU sensitivity and cell apoptosis. Further investigation revealed that miR-221 down-regulated PTEN and activated Akt, which resulted in cell survival and BCNU resistance. Overexpression of PTEN lacking 3'UTR or PI3-k/Akt specific inhibitor wortmannin attenuated miR-221-mediated BCNU resistance and prompted cell apoptosis. We propose that miR-221 regulated cell proliferation and BCNU resistance in glioma cells by targeting PI3-k/PTEN/Akt signaling axis. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Diffuse large B-cell lymphoma [ICD-11: 2A81.0] | [40] | |||
| Sensitive Disease | Diffuse large B-cell lymphoma [ICD-11: 2A81.0] | |||
| Sensitive Drug | Cyclophosphamide | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | PI3K/AKT signaling pathway | Inhibition | hsa04151 | |
| In Vitro Model | CRL2631 cells | Bone marrow | Homo sapiens (Human) | CVCL_3611 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miR-21 impacts the PI3k/AkT signaling pathway through the regulation of PTEN, thereby affecting cellular sensitivity to the CHOP chemotherapeutic regimen. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Leukemia [ICD-11: 2B33.6] | [41] | |||
| Resistant Disease | Leukemia [ICD-11: 2B33.6] | |||
| Resistant Drug | Daunorubicin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | PI3K/AKT signaling pathway | Activation | hsa04151 | |
| In Vitro Model | K562 cells | Blood | Homo sapiens (Human) | CVCL_0004 |
| K562/DNR cells | Blood | Homo sapiens (Human) | CVCL_4T87 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | DNR-induced drug resistance is associated with upregulation of miR-21 in the leukaemia cell line k562. miR-21 may regulate the survival of leukaemia cell lines by targeting PTEN expression and causing subsequent changes in the PI3k/Akt pathway. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Breast cancer [ICD-11: 2C60.3] | [42] | |||
| Resistant Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Resistant Drug | Docetaxel | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell proliferation | Activation | hsa05200 | ||
| In Vitro Model | MCF-7 cells | Breast | Homo sapiens (Human) | CVCL_0031 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | PTEN plays major roles in suppressing cancer and embryonic development, cell migration and apoptosis, miR-222 and -29a could regulate the expression of PTEN, maybe through which the two miRNAs conferred Adr and Doc resistance in MCF-7 cells. | |||
| Disease Class: Non-small cell lung cancer [ICD-11: 2C25.Y] | [35] | |||
| Resistant Disease | Non-small cell lung cancer [ICD-11: 2C25.Y] | |||
| Resistant Drug | Docetaxel | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell migration | Activation | hsa04670 | ||
| PTEN signaling pathway | Inhibition | hsa05235 | ||
| In Vitro Model | KB-3-1 cells | Lung | Homo sapiens (Human) | CVCL_2088 |
| KB-CP.5 cells | Lung | Homo sapiens (Human) | CVCL_IP04 | |
| KB-CP20 cells | Lung | Homo sapiens (Human) | CVCL_IP06 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | PTEN, a tumor suppressor gene, is an essential regulator of cell proliferation, differentiation, growth, and apoptosis. miR-21 can promote growth, migration, and invasion, chemo- or radioresistance of NSCLC cells by downregulation PTEN. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Renal carcinoma [ICD-11: 2C90.2] | [5] | |||
| Sensitive Disease | Renal carcinoma [ICD-11: 2C90.2] | |||
| Sensitive Drug | Dovitinib lactate | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | PI3K/AKT signaling pathway | Inhibition | hsa04151 | |
| In Vitro Model | 786-O cells | Kidney | Homo sapiens (Human) | CVCL_1051 |
| ACHN cells | Pleural effusion | Homo sapiens (Human) | CVCL_1067 | |
| HK-2 cells | Kidney | Homo sapiens (Human) | CVCL_0302 | |
| RCC10 cells | Kidney | Homo sapiens (Human) | CVCL_6265 | |
| RCC4 cells | Kidney | Homo sapiens (Human) | CVCL_0498 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Celltiter96 Aqueous Non Radioactive Cell Proliferation Assay | |||
| Mechanism Description | Tumor suppressor genes like PTEN, PDCD4 and TIMP3, are target genes of miR21. PTEN is a potent inhibitor of PI3k/Akt pathway, as well as a direct target of miR21. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Lung cancer [ICD-11: 2C25.5] | [49] | |||
| Sensitive Disease | Lung cancer [ICD-11: 2C25.5] | |||
| Sensitive Drug | Erlotinib | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell proliferation | Inhibition | hsa05200 | ||
| PTEN/PI3K/AKT signaling pathway | Inhibition | hsa05235 | ||
| In Vitro Model | PC9 cells | Lung | Homo sapiens (Human) | CVCL_B260 |
| Experiment for Molecule Alteration |
Luciferase reporter assay; Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay; Flow cytometric analysis | |||
| Mechanism Description | Inhibition of miR23a increases the sensitivity of lung cancer stem cells to erlotinib through PTEN/PI3k/Akt pathway. Transfection with miR23a inhibitors promoted the erlotinib-dependent inhibition of PI3k/AkT pathway, thus, suppressing the proliferation and inducing apoptosis in PC9 CSCs. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Breast cancer [ICD-11: 2C60.3] | [58] | |||
| Sensitive Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Sensitive Drug | Fulvestrant | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell invasion | Inhibition | hsa05200 | ||
| Cell proliferation | Inhibition | hsa05200 | ||
| PI3K/AKT/mTOR signaling pathway | Regulation | N.A. | ||
| In Vitro Model | MCF-7 cells | Breast | Homo sapiens (Human) | CVCL_0031 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miR-21 is a miRNA that is overexpressed in most tumor types, and acts as an oncogene by targeting many suppressor genes related to proliferation, apoptosis, and invasion. miR-21 facilitates tumor growth and invasion by targeting programmed cell death 4 (PDCD4), PTEN, and Bcl-2. silencing of miR-21 sensitized ER+ breast cancer cells to TAM and FUL induced cell apoptosis. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Hepatocellular carcinoma [ICD-11: 2C12.2] | [9] | |||
| Resistant Disease | Hepatocellular carcinoma [ICD-11: 2C12.2] | |||
| Resistant Drug | IFN-alpha | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell viability | Activation | hsa05200 | |
| In Vitro Model | Huh-7 cells | Liver | Homo sapiens (Human) | CVCL_0336 |
| HepG2 cells | Liver | Homo sapiens (Human) | CVCL_0027 | |
| PLC/PRF/5 cells | Liver | Homo sapiens (Human) | CVCL_0485 | |
| HLE cells | Liver | Homo sapiens (Human) | CVCL_1281 | |
| HLF cells | Liver | Homo sapiens (Human) | CVCL_2947 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Hepatocellular carcinoma cells transfected with pre-miR-21 were significantly resistant to IFN-alpha/5-FU. Transfection of anti-miR-21 rendered HCC cells sensitive to IFN-alpha/5-FU, and such sensitivity was weakened by transfection of siRNAs of target molecules, PETN and PDCD4, miR-21 induces chemoresistance to IFN-alpha and 5-FU, mediated through PETN and PDCD4. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Colorectal cancer [ICD-11: 2B91.1] | [54] | |||
| Resistant Disease | Colorectal cancer [ICD-11: 2B91.1] | |||
| Resistant Drug | Irinotecan | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell invasion | Activation | hsa05200 | ||
| Cell migration | Activation | hsa04670 | ||
| Cell proliferation | Activation | hsa05200 | ||
| PTEN/AKT/PI3K signaling pathway | Activation | hsa05235 | ||
| In Vitro Model | SW480 cells | Colon | Homo sapiens (Human) | CVCL_0546 |
| COLO205 cells | Colon | Homo sapiens (Human) | CVCL_F402 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay; Flow cytometry assay | |||
| Mechanism Description | The expression level of miRNA-17-5p was found increased in chemoresistant patients. Significantly higher expression levels of miR-17-5p were found in CRC patients with distant metastases and higher clinical stages. kaplan-Meier analysis showed that CRC patients with higher levels of miR-17-5p had reduced survival, especially in patients who had previously received chemotherapy. Overexpression of miR-17-5p promoted COLO205 cell invasiveness. PTEN was a target of miR-17-5p in the colon cancer cells, and their context-specific interactions were responsible for multiple drug-resistance. Chemotherapy was found to increase the expression levels of miR-17-5p, which further repressed PTEN levels, contributing to the development of chemo-resistance. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Breast cancer [ICD-11: 2C60.3] | [64] | |||
| Resistant Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Resistant Drug | Lapatinib | |||
| Molecule Alteration | Structural variation | Copy number loss |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | HER2-amplified breast cancer cells | Breast | Homo sapiens (Human) | N.A. |
| Experiment for Molecule Alteration |
Multi-region sequencing assay; Single-cell sequencing assay | |||
| Mechanism Description | Similarly, PTEN loss or PIk3CA mutation was found to lower the clinical benefit of lapatinib in HER2-amplified metastatic breast cancer and to be responsible for lapatinib resistance in breast cancer cell lines. Tumor-promoting mutations seem to be involved in three major biological processes: cell survival, sensitive to mutations in EGFR, HER2, PIk3CA, BRAF, PTEN, MYC and others; cell fate, influenced by mutations in APC, NOTCH, AR, GATA2, kLF4 and genomic stability, altered by mutations in TP53, ATM, BRCA1, BRCA2 and others. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Colorectal cancer [ICD-11: 2B91.1] | [50] | |||
| Resistant Disease | Colorectal cancer [ICD-11: 2B91.1] | |||
| Resistant Drug | Oxaliplatin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | PTEN/AKT signaling pathway | Regulation | N.A. | |
| In Vitro Model | SW480 cells | Colon | Homo sapiens (Human) | CVCL_0546 |
| HCT116 cells | Colon | Homo sapiens (Human) | CVCL_0291 | |
| HCT-8 cells | Colon | Homo sapiens (Human) | CVCL_2478 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | miR-1246, miR-21-5p, miR-96-5p and miR-1229-5p from serum exosomes involved in chemotherapy resistance may be new therapeutic targets, downregulating these miRNAs may promote CRC cell sensitivity to chemotherapeutic drugs. | |||
|
|
||||
| Disease Class: Esophageal cancer [ICD-11: 2B70.1] | [52] | |||
| Resistant Disease | Esophageal cancer [ICD-11: 2B70.1] | |||
| Resistant Drug | Oxaliplatin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | TE-1 cells | Esophagus | Homo sapiens (Human) | CVCL_1759 |
| EC9706 cells | Esophagus | Homo sapiens (Human) | CVCL_E307 | |
| KYSE150 cells | Esophagus | Homo sapiens (Human) | CVCL_1348 | |
| EC109 cells | Esophagus | Homo sapiens (Human) | CVCL_6898 | |
| EC9706-R cells | Esophagus | Homo sapiens (Human) | CVCL_E307 | |
| Het-1A cells | Esophagus | Homo sapiens (Human) | CVCL_3702 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay; Annexin V-FITC Apoptosis Detection assay | |||
| Mechanism Description | Involvement of microRNA-141-3p in 5-fluorouracil and oxaliplatin chemo-resistance in esophageal cancer cells via down-regulation of PTEN. | |||
| Disease Class: Colorectal cancer [ICD-11: 2B91.1] | [54] | |||
| Resistant Disease | Colorectal cancer [ICD-11: 2B91.1] | |||
| Resistant Drug | Oxaliplatin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell invasion | Activation | hsa05200 | ||
| Cell migration | Activation | hsa04670 | ||
| Cell proliferation | Activation | hsa05200 | ||
| PTEN/AKT/PI3K signaling pathway | Activation | hsa05235 | ||
| In Vitro Model | SW480 cells | Colon | Homo sapiens (Human) | CVCL_0546 |
| COLO205 cells | Colon | Homo sapiens (Human) | CVCL_F402 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay; Flow cytometry assay | |||
| Mechanism Description | The expression level of miRNA-17-5p was found increased in chemoresistant patients. Significantly higher expression levels of miR-17-5p were found in CRC patients with distant metastases and higher clinical stages. kaplan-Meier analysis showed that CRC patients with higher levels of miR-17-5p had reduced survival, especially in patients who had previously received chemotherapy. Overexpression of miR-17-5p promoted COLO205 cell invasiveness. PTEN was a target of miR-17-5p in the colon cancer cells, and their context-specific interactions were responsible for multiple drug-resistance. Chemotherapy was found to increase the expression levels of miR-17-5p, which further repressed PTEN levels, contributing to the development of chemo-resistance. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Renal carcinoma [ICD-11: 2C90.2] | [5] | |||
| Sensitive Disease | Renal carcinoma [ICD-11: 2C90.2] | |||
| Sensitive Drug | Oxaliplatin | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | PI3K/AKT signaling pathway | Inhibition | hsa04151 | |
| In Vitro Model | 786-O cells | Kidney | Homo sapiens (Human) | CVCL_1051 |
| ACHN cells | Pleural effusion | Homo sapiens (Human) | CVCL_1067 | |
| HK-2 cells | Kidney | Homo sapiens (Human) | CVCL_0302 | |
| RCC10 cells | Kidney | Homo sapiens (Human) | CVCL_6265 | |
| RCC4 cells | Kidney | Homo sapiens (Human) | CVCL_0498 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Celltiter96 Aqueous Non Radioactive Cell Proliferation Assay | |||
| Mechanism Description | Tumor suppressor genes like PTEN, PDCD4 and TIMP3, are target genes of miR21. PTEN is a potent inhibitor of PI3k/Akt pathway, as well as a direct target of miR21. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Diffuse large B-cell lymphoma [ICD-11: 2A81.0] | [40] | |||
| Sensitive Disease | Diffuse large B-cell lymphoma [ICD-11: 2A81.0] | |||
| Sensitive Drug | Prednisone | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | PI3K/AKT signaling pathway | Inhibition | hsa04151 | |
| In Vitro Model | CRL2631 cells | Bone marrow | Homo sapiens (Human) | CVCL_3611 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miR-21 impacts the PI3k/AkT signaling pathway through the regulation of PTEN, thereby affecting cellular sensitivity to the CHOP chemotherapeutic regimen. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Hepatocellular carcinoma [ICD-11: 2C12.2] | [69] | |||
| Resistant Disease | Hepatocellular carcinoma [ICD-11: 2C12.2] | |||
| Resistant Drug | Sorafenib | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | mTOR signaling pathway | Activation | hsa04150 | |
| In Vitro Model | Huh-7 cells | Liver | Homo sapiens (Human) | CVCL_0336 |
| HepG2 cells | Liver | Homo sapiens (Human) | CVCL_0027 | |
| Hep3B cells | Liver | Homo sapiens (Human) | CVCL_0326 | |
| PLC/PRF/5 cells | Liver | Homo sapiens (Human) | CVCL_0485 | |
| SNU182 cells | Liver | Homo sapiens (Human) | CVCL_0090 | |
| SNU398 cells | Liver | Homo sapiens (Human) | CVCL_0077 | |
| SNU449 cells | Liver | Homo sapiens (Human) | CVCL_0454 | |
| SNU475 cells | Liver | Homo sapiens (Human) | CVCL_0497 | |
| Experiment for Molecule Alteration |
Western blot analysis; Luciferase activity assay | |||
| Experiment for Drug Resistance |
Cell viability assay; Caspase-3/7 activity assay; WB analysis | |||
| Mechanism Description | miR494 overexpression increased sorafenib resistance via mTOR pathway activation in HCC cell lines, by targeting p27, pten, and puma. | |||
| Disease Class: Hepatocellular carcinoma [ICD-11: 2C12.2] | [70] | |||
| Resistant Disease | Hepatocellular carcinoma [ICD-11: 2C12.2] | |||
| Resistant Drug | Sorafenib | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell colony | Activation | hsa05200 | |
| Cell invasion | Activation | hsa05200 | ||
| Cell migration | Activation | hsa04670 | ||
| Cell proliferation | Activation | hsa05200 | ||
| PTEN/AKT signaling pathway | Inhibition | hsa05235 | ||
| In Vitro Model | BEL-7402 cells | Liver | Homo sapiens (Human) | CVCL_5492 |
| HepG2 cells | Liver | Homo sapiens (Human) | CVCL_0027 | |
| Hep3B cells | Liver | Homo sapiens (Human) | CVCL_0326 | |
| PLC/PRF/5 cells | Liver | Homo sapiens (Human) | CVCL_0485 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | miR-19a-3p induces sorafenib resistance through downregulation of PTEN expression. | |||
| Disease Class: Liver cancer [ICD-11: 2C12.6] | [71] | |||
| Resistant Disease | Liver cancer [ICD-11: 2C12.6] | |||
| Resistant Drug | Sorafenib | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell migration | Activation | hsa04670 | |
| Cell proliferation | Activation | hsa05200 | ||
| PI3K/AKT signaling pathway | Activation | hsa04151 | ||
| TGF-beta signaling pathway | Activation | hsa04350 | ||
| In Vitro Model | Huh-7 cells | Liver | Homo sapiens (Human) | CVCL_0336 |
| HepG2 cells | Liver | Homo sapiens (Human) | CVCL_0027 | |
| HCCLM3 cells | Liver | Homo sapiens (Human) | CVCL_6832 | |
| Hep3B cells | Liver | Homo sapiens (Human) | CVCL_0326 | |
| BEL-7404 cells | Liver | Homo sapiens (Human) | CVCL_6568 | |
| PLC/PRF/5 cells | Liver | Homo sapiens (Human) | CVCL_0485 | |
| SNU449 cells | Liver | Homo sapiens (Human) | CVCL_0454 | |
| Skhep1 cells | Liver | Homo sapiens (Human) | CVCL_0525 | |
| HLE cells | Liver | Homo sapiens (Human) | CVCL_1281 | |
| In Vivo Model | BALB/c nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis; Immunofluorescence analysis | |||
| Experiment for Drug Resistance |
Flow cytometry assay | |||
| Mechanism Description | Overexpression of miR-216a/217 activates the PI3k/Akt and TGF-beta pathways by targeting PTEN and SMAD7, contributing to hepatocarcinogenesis, sorafenib resistance and tumor recurrence in HCC. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Renal cell carcinoma [ICD-11: 2C90.0] | [72] | |||
| Resistant Disease | Renal cell carcinoma [ICD-11: 2C90.0] | |||
| Resistant Drug | Sunitinib | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell proliferation | Activation | hsa05200 | |
| In Vitro Model | Caki-1 cells | Kidney | Homo sapiens (Human) | CVCL_0234 |
| Experiment for Molecule Alteration |
Western blot analysis; RT-qPCR | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miR-130b promoted cell growth and was associated with sunitinib resistance through regulating PTEN expression. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Breast cancer [ICD-11: 2C60.3] | [73] | |||
| Resistant Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Resistant Drug | Tamoxifen | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell proliferation | Activation | hsa05200 | ||
| In Vitro Model | MCF-7 cells | Breast | Homo sapiens (Human) | CVCL_0031 |
| BT474 cells | Breast | Homo sapiens (Human) | CVCL_0179 | |
| MCF7/TAMR cells | Breast | Homo sapiens (Human) | CVCL_EG55 | |
| CAMA-1 cells | Breast | Homo sapiens (Human) | CVCL_1115 | |
| HEK293 FT cells | Kidney | Homo sapiens (Human) | CVCL_6911 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Promega assay | |||
| Mechanism Description | Tamoxifen-resistant cells express miRNA-519a at high levels, which directly represses the expression of PTEN, RB1, and CDkN1A, central nodes of a dense network, allowing the cells to proliferate, even in the presence of tamoxifen. miRNA-519a increases viability and S-phase population of the cell cycle, but does not affect EMT or invasion. miRNA-519a-expressing cells evade tamoxifen-induced apoptosis. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Breast cancer [ICD-11: 2C60.3] | [74] | |||
| Sensitive Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Sensitive Drug | Tamoxifen | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell proliferation | Activation | hsa05200 | ||
| In Vitro Model | MCF-7 cells | Breast | Homo sapiens (Human) | CVCL_0031 |
| Experiment for Molecule Alteration |
Western blot analysis; RT-qPCR | |||
| Experiment for Drug Resistance |
CCK8 assay; Flow cytometry assay | |||
| Mechanism Description | Low expression of ADAMTS9-AS2 inhibits PTEN expression and enhances tamoxifen resistance through targeting microRNA-130a-5p. | |||
| Disease Class: Breast cancer [ICD-11: 2C60.3] | [58] | |||
| Sensitive Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Sensitive Drug | Tamoxifen | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell invasion | Inhibition | hsa05200 | ||
| Cell proliferation | Inhibition | hsa05200 | ||
| PI3K/AKT/mTOR signaling pathway | Regulation | N.A. | ||
| In Vitro Model | MCF-7 cells | Breast | Homo sapiens (Human) | CVCL_0031 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miR-21 is a miRNA that is overexpressed in most tumor types, and acts as an oncogene by targeting many suppressor genes related to proliferation, apoptosis, and invasion. miR-21 facilitates tumor growth and invasion by targeting programmed cell death 4 (PDCD4), PTEN, and Bcl-2. silencing of miR-21 sensitized ER+ breast cancer cells to TAM and FUL induced cell apoptosis. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Ovarian cancer [ICD-11: 2C73.0] | [75] | |||
| Resistant Disease | Ovarian cancer [ICD-11: 2C73.0] | |||
| Resistant Drug | Tirbanibulin | |||
| Molecule Alteration | Missense mutation | p.G129R (c.385G>A) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | RMUG-S | Ovary | Homo sapiens (Human) | CVCL_3158 |
| RMUG-L | Endometrium | Homo sapiens (Human) | CVCL_3157 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay; EdU assay; Annexin V and 7-aminoactinomycin D assay; Flow cytometry analysis | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: HER2 positive breast cancer [ICD-11: 2C60.8] | [76] | |||
| Resistant Disease | HER2 positive breast cancer [ICD-11: 2C60.8] | |||
| Resistant Drug | Trastuzumab | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell proliferation | Activation | hsa05200 | |
| mTOR signaling pathway | Activation | hsa04150 | ||
| In Vitro Model | SkBR3 cells | Breast | Homo sapiens (Human) | CVCL_0033 |
| Experiment for Molecule Alteration |
Western blot analysis; Flow cytometric assay | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Down-regulation of LncRNA GAS5 causes trastuzumab resistance in breast cancer.Expression of the LncRNA GAS5 was decreased in trastuzumab-resistant SkBR-3/Tr cells and in breast cancer tissue from trastuzumab-treated patients. GAS5 suppresses cancer proliferation by acting as a molecular sponge for miR-21, leading to the de-repression of phosphatase and tensin homologs (PTEN), the endogenous target of miR-21. Moreover, mTOR activation associated with reduced GAS5 expression was required to suppress PTEN. This work identifies GAS5 as a novel prognostic marker and candidate drug target for HER2-positive breast cancer. | |||
| Disease Class: Breast cancer [ICD-11: 2C60.3] | [77] | |||
| Resistant Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Resistant Drug | Trastuzumab | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| PI3K signaling pathway | Activation | hsa04151 | ||
| In Vitro Model | SkBR3 cells | Breast | Homo sapiens (Human) | CVCL_0033 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | A target prediction analysis coupled with in vitro and in vivo validations revealed that miR-21 levels inversely correlated with the expression of PTEN and PDCD4, which differentially influenced the drug sensitivity of HER2-positive breast cancer cells.miR-21 was able to affect the response to both trastuzumab and chemotherapy, triggering an IL-6/STAT3/NF-kB-mediated signaling loop and activating the PI3k pathway. These findings support the ability of miR-21 signaling to sustain EMT and shape the tumor immune microenvironment in HER2-positive breast cancer. | |||
| Disease Class: Breast cancer [ICD-11: 2C60.3] | [78], [79] | |||
| Resistant Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Resistant Drug | Trastuzumab | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | AKT signaling pathway | Activation | hsa04151 | |
| Cell apoptosis | Inhibition | hsa04210 | ||
| Cell migration | Activation | hsa04670 | ||
| Cell proliferation | Activation | hsa05200 | ||
| In Vitro Model | SkBR3 cells | Breast | Homo sapiens (Human) | CVCL_0033 |
| BT474 cells | Breast | Homo sapiens (Human) | CVCL_0179 | |
| MDA-MB-453 cells | Breast | Homo sapiens (Human) | CVCL_0418 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay; Flow cytometry assay | |||
| Mechanism Description | PTEN is a tumor suppressing dual phosphatase that antagonizes the function of phosphatidylinositol 3-kinase (PI3k) and negatively regulates AkT activities, and PTEN phosphorylation is a crucial mechanism mediating the anti-tumor effect of trastuzumab by reducing and inhibiting the ErbB2 receptor-bound SRC. Ectopic expression of miR-21 in the previously sensitive cells confers trastuzumab resistance via PTEN inhibition. And miR-221 promotes the invasiveness and trastuzumab resistance of HER2-positive breast cancers by targeting the tumor suppressor gene PTEN. | |||
| Disease Class: Gastric cancer [ICD-11: 2B72.1] | [80] | |||
| Resistant Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Resistant Drug | Trastuzumab | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| miR21/PTEN signaling pathway | Activation | hsa05206 | ||
| In Vitro Model | NCI-N87 cells | Gastric | Homo sapiens (Human) | CVCL_1603 |
| MkN-45 cells | Gastric | Homo sapiens (Human) | CVCL_0434 | |
| NUGC4 cells | Gastric | Homo sapiens (Human) | CVCL_3082 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | The miR-21/PTEN pathway regulated the sensitivity of HER2-positive GC cell lines to trastuzumab through modulation apoptosis. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Melanoma [ICD-11: 2C30.0] | [81] | |||
| Resistant Disease | Melanoma [ICD-11: 2C30.0] | |||
| Resistant Drug | Vemurafenib | |||
| Molecule Alteration | Missense mutation | p.R159S |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | PI3K signaling pathway | Activation | hsa04151 | |
| Experiment for Molecule Alteration |
Whole-exome sequencing assay | |||
| Experiment for Drug Resistance |
Progression-free survival assay | |||
| Mechanism Description | Recent whole-exome and RNA sequencing studies have identified a wide array of acquired mutations that confer resistance, including those that reactivate the MAPk pathway (NRAS, kRAS, and MEk1/2 mutations, NF1 loss, BRAF amplification, and BRAF splice variants) and those that activate the PI3k pathway (PIk3CA, PIk3R1, and AkT1/2 mutations and PTEN loss). Of the 6 samples with putative resistance-conferring alterations, 15C harbored an acquired missense PTENR159S mutation in the phosphatase domain, 25C harbored a known acquired MEkQ60L mutation. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Gastric cancer [ICD-11: 2B72.1] | [27] | |||
| Resistant Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Resistant Drug | Vincristine | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| PTEN/AKT signaling pathway | Inhibition | hsa05235 | ||
| In Vitro Model | SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 |
| SGC7901/VCR cells | Gastric | Homo sapiens (Human) | CVCL_VU58 | |
| SGC7901/ADR cells | Gastric | Homo sapiens (Human) | CVCL_VU57 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miR-19a/b are upregulated in multidrug-resistant gastric cancer cell line, miR-19a/b suppress the sensitivity of gastric cancer cells to anticancer drugs, miR-19a/b accelerate the efflux of ADR through P-gp upregulation. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Diffuse large B-cell lymphoma [ICD-11: 2A81.0] | [40] | |||
| Sensitive Disease | Diffuse large B-cell lymphoma [ICD-11: 2A81.0] | |||
| Sensitive Drug | Vincristine | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | PI3K/AKT signaling pathway | Inhibition | hsa04151 | |
| In Vitro Model | CRL2631 cells | Bone marrow | Homo sapiens (Human) | CVCL_3611 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miR-21 impacts the PI3k/AkT signaling pathway through the regulation of PTEN, thereby affecting cellular sensitivity to the CHOP chemotherapeutic regimen. | |||
Clinical Trial Drug(s)
4 drug(s) in total
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Bladder cancer [ICD-11: 2C94.0] | [82] | |||
| Resistant Disease | Bladder cancer [ICD-11: 2C94.0] | |||
| Resistant Drug | Pictilisib | |||
| Molecule Alteration | Missense mutation | p.N48I (c.143A>T) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | MEK/ERK signaling pathway | Activation | hsa04011 | |
| In Vitro Model | 5637 cells | Bladder | Homo sapiens (Human) | CVCL_0126 |
| J82 cells | Bladder | Homo sapiens (Human) | CVCL_0359 | |
| RT4 cells | Bladder | Homo sapiens (Human) | CVCL_0036 | |
| T24 cells | Bladder | Homo sapiens (Human) | CVCL_0554 | |
| TCCSuP cells | Bladder | Homo sapiens (Human) | CVCL_1738 | |
| In Vivo Model | NSG mouse PDX model | Mus musculus | ||
| Experiment for Drug Resistance |
MTS assay; FACS assay | |||
| Mechanism Description | Pictilisib activated the compensatory MEK/ERK pathways that likely contributed to pictilisib resistance, which was reversed by co-treatment with the RAF inhibitor sorafenib. RNA-sequencing of tumors resistant to treatment suggested that LSP1 down-regulation correlated with drug resistance. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Breast adenocarcinoma [ICD-11: 2C60.1] | [83] | |||
| Sensitive Disease | Breast adenocarcinoma [ICD-11: 2C60.1] | |||
| Sensitive Drug | MTOR inhibitors | |||
| Molecule Alteration | Nonsense | p.R233* (c.697C>T) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Mechanism Description | The nonsense p.R233* (c.697C>T) in gene PTEN cause the sensitivity of MTOR inhibitors by unusual activation of pro-survival pathway. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Breast adenocarcinoma [ICD-11: 2C60.1] | [84] | |||
| Sensitive Disease | Breast adenocarcinoma [ICD-11: 2C60.1] | |||
| Sensitive Drug | BAY1125976 | |||
| Molecule Alteration | Missense mutation | p.L108R (c.323T>G) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | LNCaP cells | Prostate | Homo sapiens (Human) | CVCL_0395 |
| MDA-MB-231 cells | Breast | Homo sapiens (Human) | CVCL_0062 | |
| Hela cells | Cervix uteri | Homo sapiens (Human) | CVCL_0030 | |
| NCI-H460 cells | Lung | Homo sapiens (Human) | CVCL_0459 | |
| MDA-MB-453 cells | Breast | Homo sapiens (Human) | CVCL_0418 | |
| MDA-MB-468 cells | Breast | Homo sapiens (Human) | CVCL_0419 | |
| HCC70 cells | Breast | Homo sapiens (Human) | CVCL_1270 | |
| A2058 cells | Skin | Homo sapiens (Human) | CVCL_1059 | |
| Caco2 cells | Colon | Homo sapiens (Human) | CVCL_0025 | |
| MCF-7 cells | Breast | Homo sapiens (Human) | CVCL_0031 | |
| ZR75-1 cells | Breast | Homo sapiens (Human) | CVCL_0588 | |
| DU-145 cells | Prostate | Homo sapiens (Human) | CVCL_0105 | |
| NCI-60 cells | N.A. | Homo sapiens (Human) | N.A. | |
| EVSA-T cells | Ascites | Homo sapiens (Human) | CVCL_1207 | |
| KU-19 cells | Blood | Bos taurus (Bovine) | CVCL_VN09 | |
| T47D cells | Pleural effusion | Homo sapiens (Human) | CVCL_0553 | |
| CAL-120 cells | Pleural effusion | Homo sapiens (Human) | CVCL_1104 | |
| BT-549 cells | Breast | Homo sapiens (Human) | CVCL_1092 | |
| BT-474 cells | Breast | Homo sapiens (Human) | CVCL_0179 | |
| BT-20 cells | Mammary gland | Homo sapiens (Human) | CVCL_0178 | |
| B16F10 cells | Skin | Mus musculus (Mouse) | CVCL_0159 | |
| In Vivo Model | Female NMRI (nu/nu) mouse xenograft model | Mus musculus | ||
| Mechanism Description | The missense mutation p.L108R (c.323T>G) in gene PTEN cause the sensitivity of BAY1125976 by unusual activation of pro-survival pathway | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Thyroid gland cancer [ICD-11: 2D10.0] | [85] | |||
| Sensitive Disease | Thyroid gland cancer [ICD-11: 2D10.0] | |||
| Sensitive Drug | Perifosine | |||
| Molecule Alteration | Nonsense | p.R130* (c.388C>T) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | SW1736 cells | Thyroid | Homo sapiens (Human) | CVCL_3883 |
| C643 cells | Thyroid gland | Homo sapiens (Human) | CVCL_5969 | |
| HTH7 cells | Thyroid gland | Homo sapiens (Human) | CVCL_6289 | |
| SW1736 cells | Thyroid | Homo sapiens (Human) | CVCL_3883 | |
| HTH7 cells | Thyroid gland | Homo sapiens (Human) | CVCL_6289 | |
| Hth74 cells | Thyroid gland | Homo sapiens (Human) | CVCL_6288 | |
| Hth74 cells | Thyroid gland | Homo sapiens (Human) | CVCL_6288 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | The nonsense p.R130* (c.388C>T) in gene PTEN cause the sensitivity of Perifosine by unusual activation of pro-survival pathway. | |||
Discontinued Drug(s)
1 drug(s) in total
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Prostate cancer [ICD-11: 2C82.0] | [86] | |||
| Sensitive Disease | Prostate cancer [ICD-11: 2C82.0] | |||
| Sensitive Drug | AZD-6482 | |||
| Molecule Alteration | Missense mutation | p.A126G (c.377C>G) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | PC3 cells | Prostate | Homo sapiens (Human) | CVCL_0035 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Presto blue assay; Clonogenic cell survival assay | |||
Preclinical Drug(s)
3 drug(s) in total
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: ER negative breast cancer [ICD-11: 2C60.7] | [87] | |||
| Sensitive Disease | ER negative breast cancer [ICD-11: 2C60.7] | |||
| Sensitive Drug | DHM25 | |||
| Molecule Alteration | Missense mutation | p.V275* (c.823_824delGTinsTA) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | MCF-7 cells | Breast | Homo sapiens (Human) | CVCL_0031 |
| MDA-MB-231 cells | Breast | Homo sapiens (Human) | CVCL_0062 | |
| BT474 cells | Breast | Homo sapiens (Human) | CVCL_0179 | |
| ZR75-1 cells | Breast | Homo sapiens (Human) | CVCL_0588 | |
| MDA-MB-453 cells | Breast | Homo sapiens (Human) | CVCL_0418 | |
| MDA-MB-468 cells | Breast | Homo sapiens (Human) | CVCL_0419 | |
| BT549 cells | Breast | Homo sapiens (Human) | CVCL_1092 | |
| T47-D cells | Pleural effusion | Homo sapiens (Human) | CVCL_0553 | |
| In Vivo Model | NOD/SCID/gamac null mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Disease Class: Breast adenocarcinoma [ICD-11: 2C60.1] | [87] | |||
| Sensitive Disease | Breast adenocarcinoma [ICD-11: 2C60.1] | |||
| Sensitive Drug | DHM25 | |||
| Molecule Alteration | Missense mutation | p.L108R (c.323T>G) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | MCF-7 cells | Breast | Homo sapiens (Human) | CVCL_0031 |
| MDA-MB-231 cells | Breast | Homo sapiens (Human) | CVCL_0062 | |
| BT474 cells | Breast | Homo sapiens (Human) | CVCL_0179 | |
| ZR75-1 cells | Breast | Homo sapiens (Human) | CVCL_0588 | |
| MDA-MB-453 cells | Breast | Homo sapiens (Human) | CVCL_0418 | |
| MDA-MB-468 cells | Breast | Homo sapiens (Human) | CVCL_0419 | |
| BT549 cells | Breast | Homo sapiens (Human) | CVCL_1092 | |
| T47-D cells | Pleural effusion | Homo sapiens (Human) | CVCL_0553 | |
| Hs578T cells | Breast | Homo sapiens (Human) | CVCL_0332 | |
| In Vivo Model | NOD/SCID/gamac null mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Melanoma [ICD-11: 2C30.0] | [88] | |||
| Sensitive Disease | Melanoma [ICD-11: 2C30.0] | |||
| Sensitive Drug | MK-2206/PD184352 | |||
| Molecule Alteration | FS-deletion | p.V317fs (c.950_953) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | A375 cells | Skin | Homo sapiens (Human) | CVCL_0132 |
| Experiment for Molecule Alteration |
Sanger sequencing assay; SNP array; qPCR | |||
| Experiment for Drug Resistance |
CellTiter-Glo assay | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Thyroid gland cancer [ICD-11: 2D10.0] | [89] | |||
| Sensitive Disease | Thyroid gland cancer [ICD-11: 2D10.0] | |||
| Sensitive Drug | MK2206 | |||
| Molecule Alteration | Nonsense | p.R130* (c.388C>T) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | SW1736 cells | Thyroid | Homo sapiens (Human) | CVCL_3883 |
| C643 cells | Thyroid gland | Homo sapiens (Human) | CVCL_5969 | |
| HTH7 cells | Thyroid gland | Homo sapiens (Human) | CVCL_6289 | |
| Hth74 cells | Thyroid gland | Homo sapiens (Human) | CVCL_6288 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | The nonsense p.R130* (c.388C>T) in gene PTEN cause the sensitivity of MK2206 by unusual activation of pro-survival pathway. | |||
Investigative Drug(s)
1 drug(s) in total
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Epithelial ovarian cancer [ICD-11: 2B5D.0] | [90] | |||
| Resistant Disease | Epithelial ovarian cancer [ICD-11: 2B5D.0] | |||
| Resistant Drug | Platinum | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell viability | Inhibition | hsa05200 | ||
| PI3K/AKT signaling pathway | Inhibition | hsa04151 | ||
| In Vitro Model | A2780 cells | Ovary | Homo sapiens (Human) | CVCL_0134 |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay; Flow cytometry assay | |||
| Mechanism Description | Hypoxic TAMs derived exosomes containing miR-223 were internalized by EOC cells and promoted drug resistance of EOC cells and exosmic miR-223 inactivate PI3k/AkT pathway through PTEN targeting. | |||
|
|
||||
| Disease Class: Epithelial ovarian cancer [ICD-11: 2B5D.0] | [91] | |||
| Resistant Disease | Epithelial ovarian cancer [ICD-11: 2B5D.0] | |||
| Resistant Drug | Platinum | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| PI3K/AKT signaling pathway | Activation | hsa04151 | ||
| In Vitro Model | Epithelial ovarian cancer patients | Ovary | Homo sapiens (Human) | N.A. |
| Experiment for Molecule Alteration |
ELISA assay | |||
| Experiment for Drug Resistance |
CellTiter 96 aqueous one solution cell proliferation assay | |||
| Mechanism Description | miR-130a may mediate the generation of platinum resistance in epithelial ovarian cancer through inhibiting PTEN to activate PI3k/AkT signaling pathway and increasing BCL-2 to inhibit tumor cell apoptosis. | |||
Disease- and Tissue-specific Abundances of This Molecule
ICD Disease Classification 02

| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Nervous tissue | |
| The Specified Disease | Brain cancer | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 3.13E-109; Fold-change: 7.80E-01; Z-score: 1.98E+00 | |
|
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |
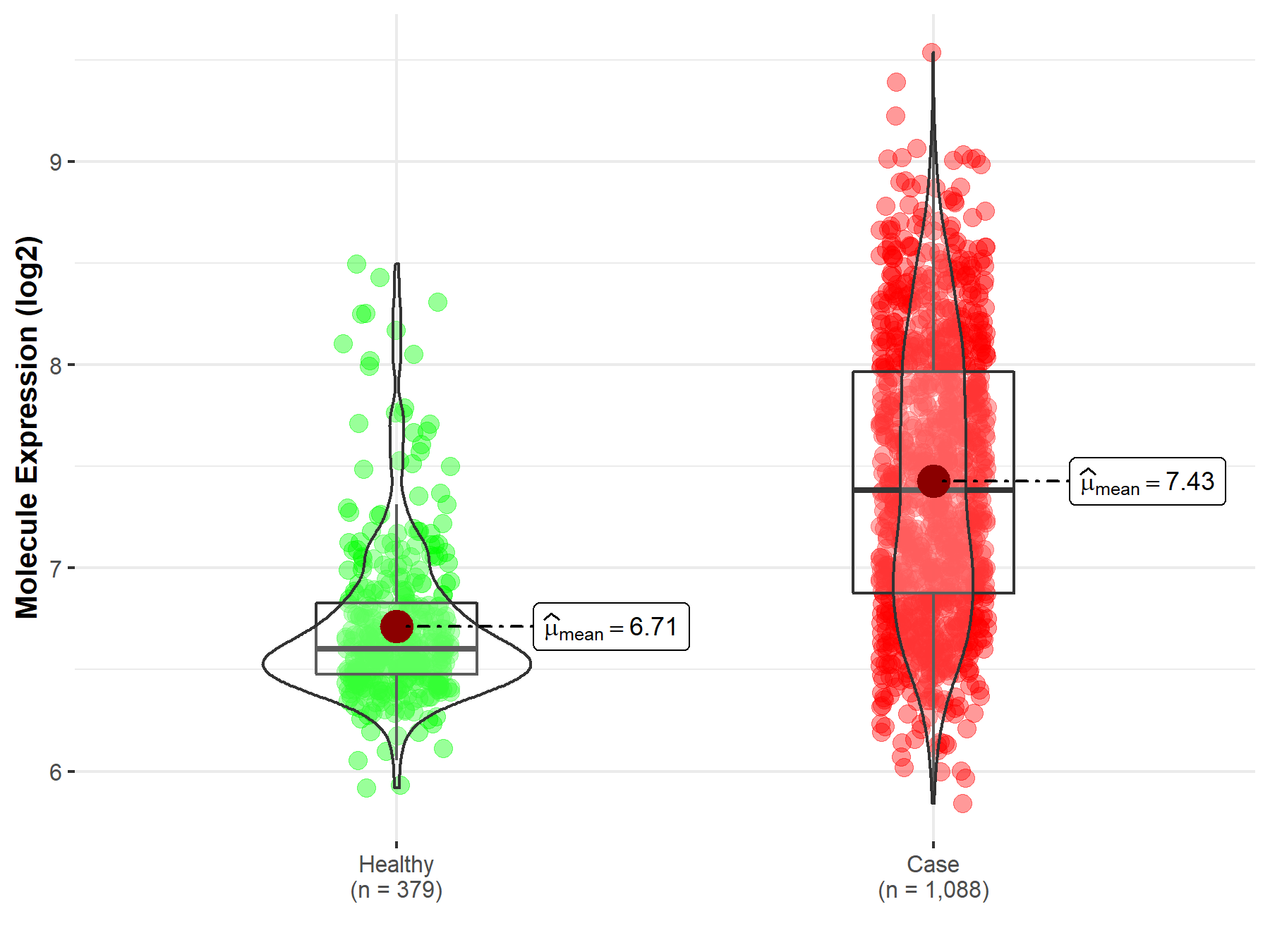
|
Click to View the Clearer Original Diagram |
| The Studied Tissue | Brainstem tissue | |
| The Specified Disease | Glioma | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 3.69E-01; Fold-change: -2.16E-01; Z-score: -8.82E-01 | |
|
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |

|
Click to View the Clearer Original Diagram |
| The Studied Tissue | White matter | |
| The Specified Disease | Glioma | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 2.64E-01; Fold-change: -8.57E-02; Z-score: -1.05E-01 | |
|
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |
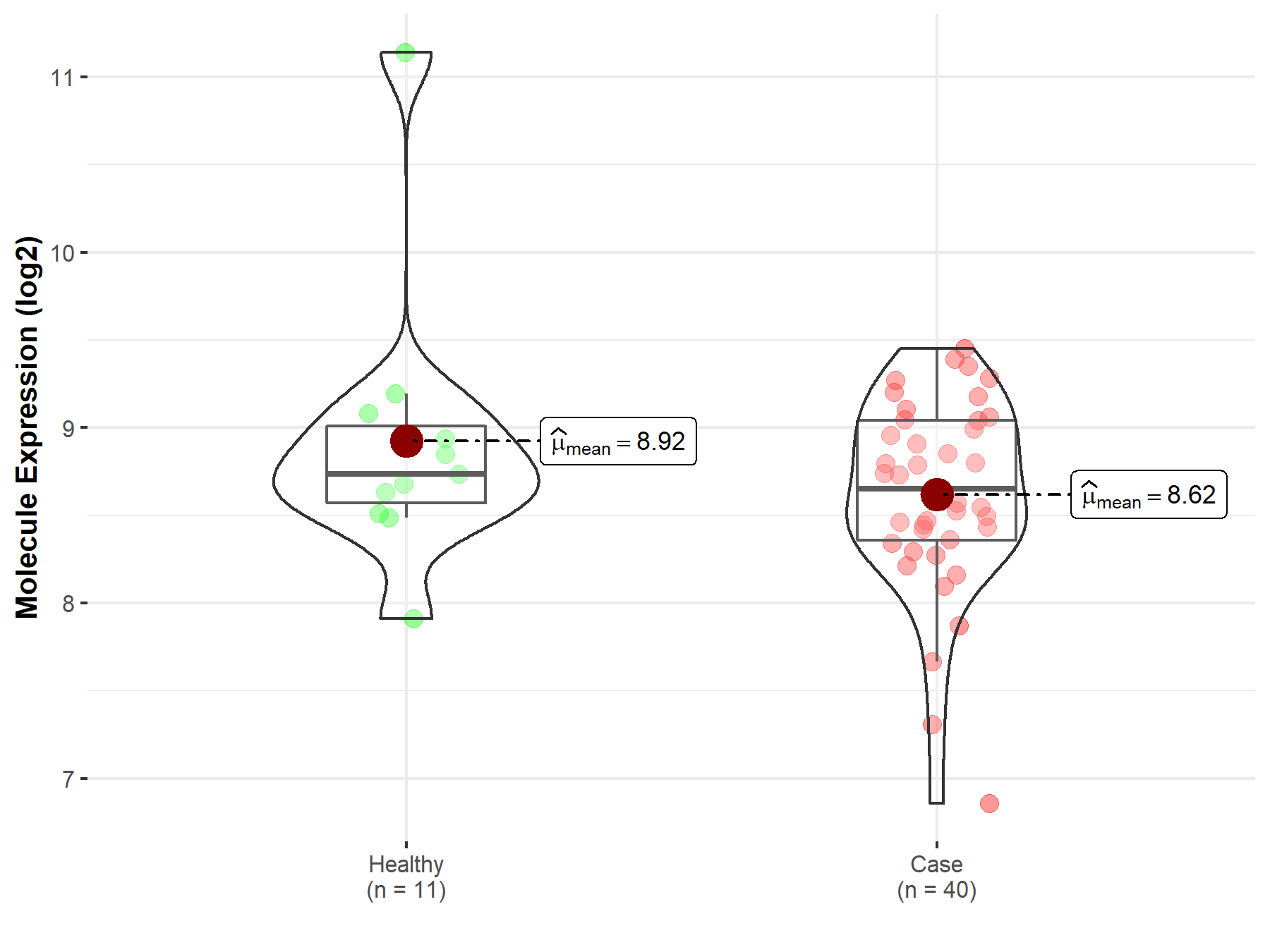
|
Click to View the Clearer Original Diagram |
| The Studied Tissue | Brainstem tissue | |
| The Specified Disease | Neuroectodermal tumor | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 6.54E-19; Fold-change: 1.46E+00; Z-score: 8.50E+00 | |
|
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |
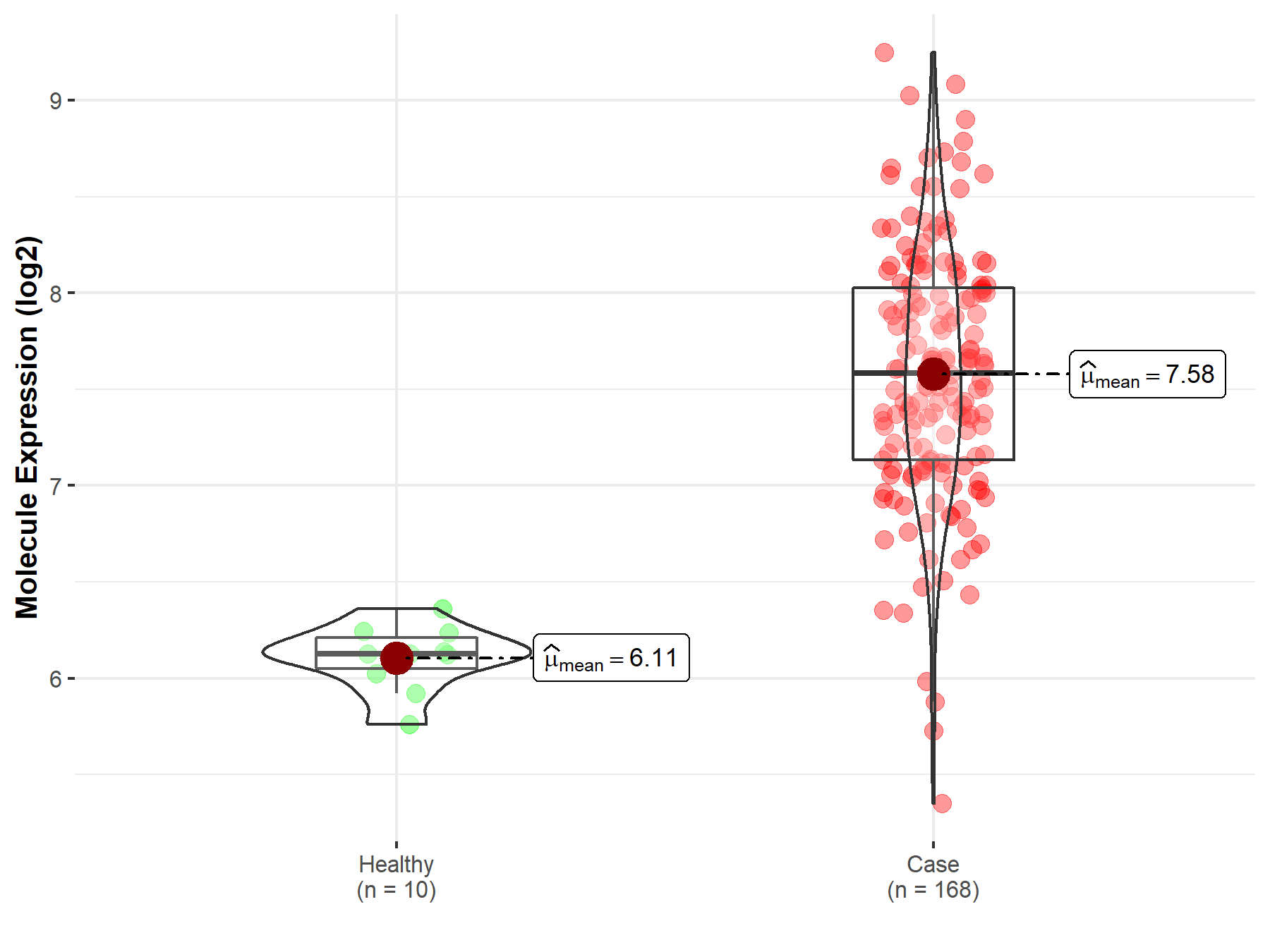
|
Click to View the Clearer Original Diagram |
| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Whole blood | |
| The Specified Disease | Myelofibrosis | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.68E-01; Fold-change: -8.17E-02; Z-score: -4.67E-01 | |
|
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |

|
Click to View the Clearer Original Diagram |
| The Studied Tissue | Whole blood | |
| The Specified Disease | Polycythemia vera | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 7.47E-05; Fold-change: 1.40E-01; Z-score: 7.67E-01 | |
|
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |
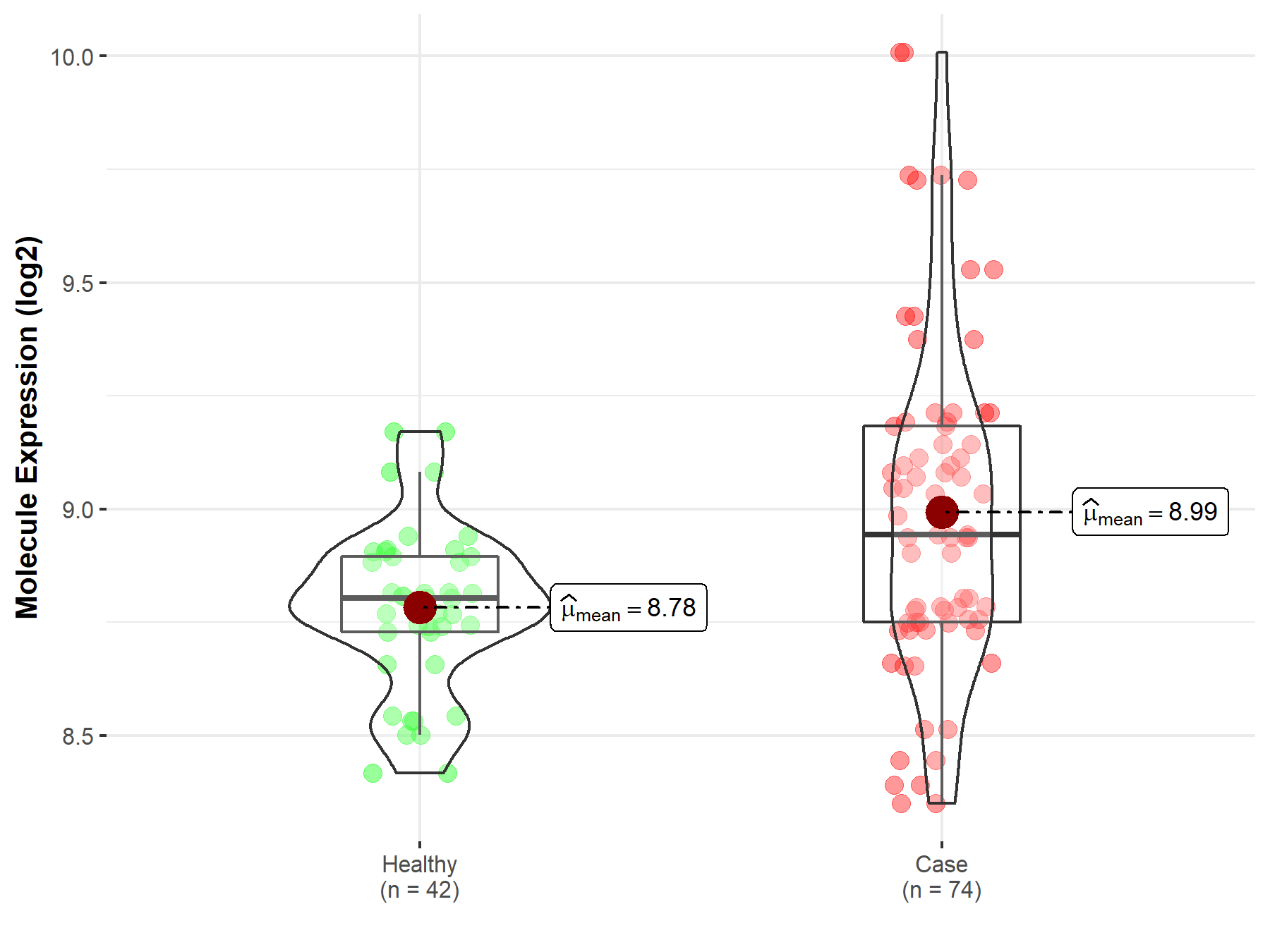
|
Click to View the Clearer Original Diagram |
| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Esophagus | |
| The Specified Disease | Esophageal cancer | |
| The Expression Level of Disease Section Compare with the Adjacent Tissue | p-value: 3.52E-01; Fold-change: -1.36E-01; Z-score: -4.85E-01 | |
|
Molecule expression in the normal tissue adjacent to the diseased tissue of patients
Molecule expression in the diseased tissue of patients
|
||
| Disease-specific Molecule Abundances |
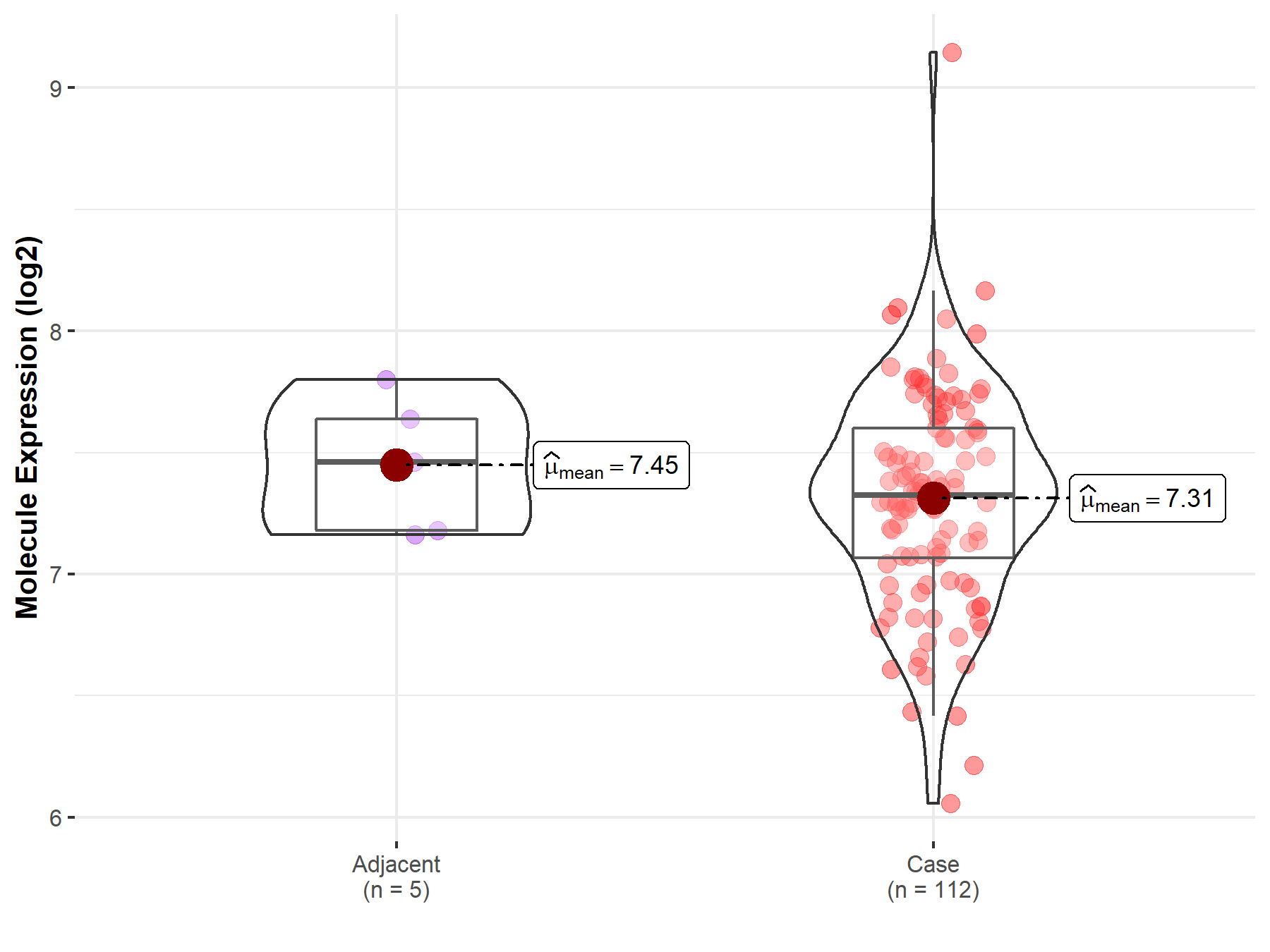
|
Click to View the Clearer Original Diagram |
| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Gastric tissue | |
| The Specified Disease | Gastric cancer | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 2.35E-01; Fold-change: 1.82E-01; Z-score: 5.32E-01 | |
| The Expression Level of Disease Section Compare with the Adjacent Tissue | p-value: 7.04E-03; Fold-change: -4.82E-01; Z-score: -1.31E+00 | |
|
Molecule expression in the normal tissue adjacent to the diseased tissue of patients
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |
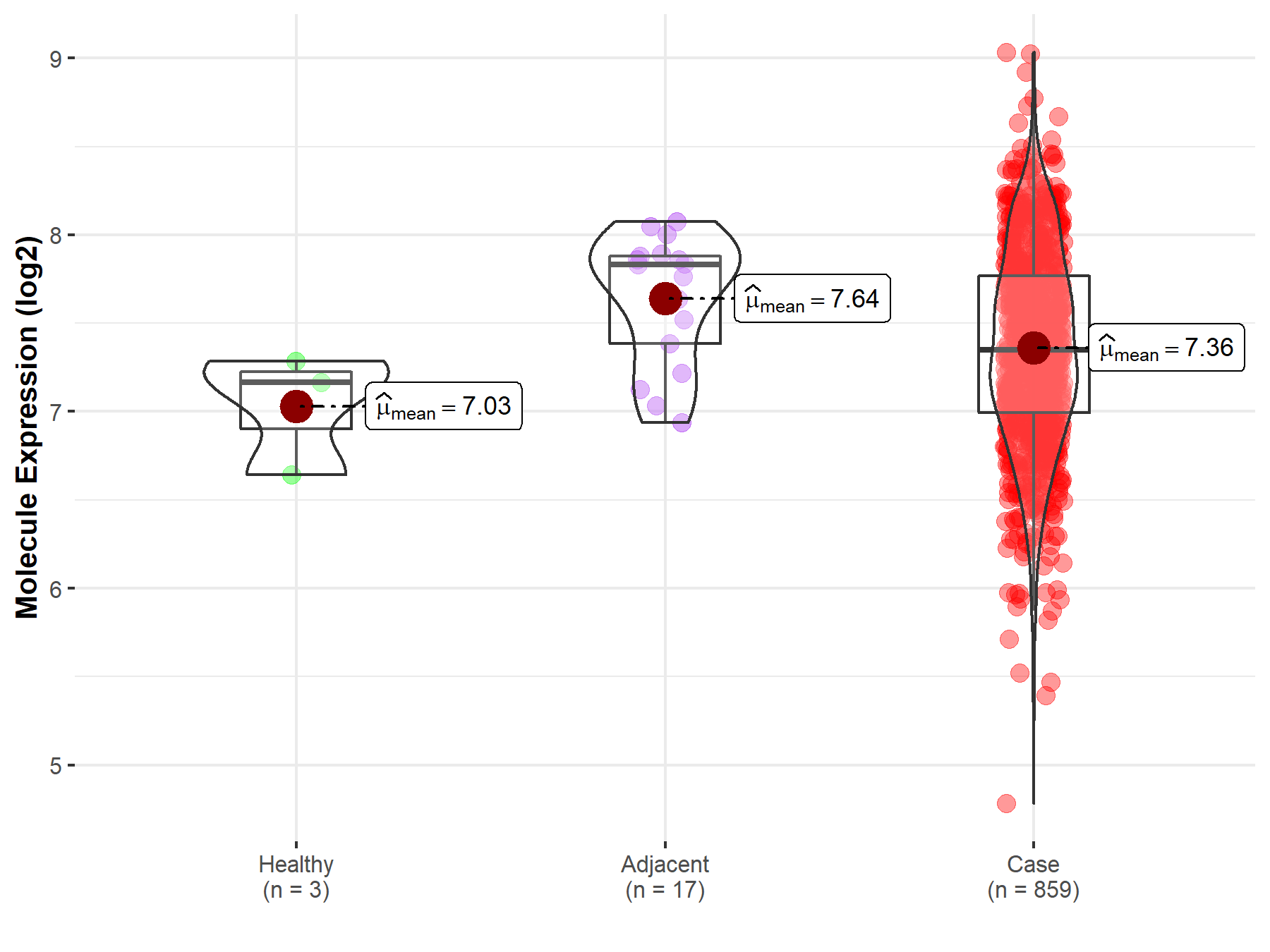
|
Click to View the Clearer Original Diagram |
| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Colon | |
| The Specified Disease | Colon cancer | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 2.96E-40; Fold-change: -4.80E-01; Z-score: -1.62E+00 | |
| The Expression Level of Disease Section Compare with the Adjacent Tissue | p-value: 1.67E-31; Fold-change: -5.03E-01; Z-score: -1.22E+00 | |
|
Molecule expression in the normal tissue adjacent to the diseased tissue of patients
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |
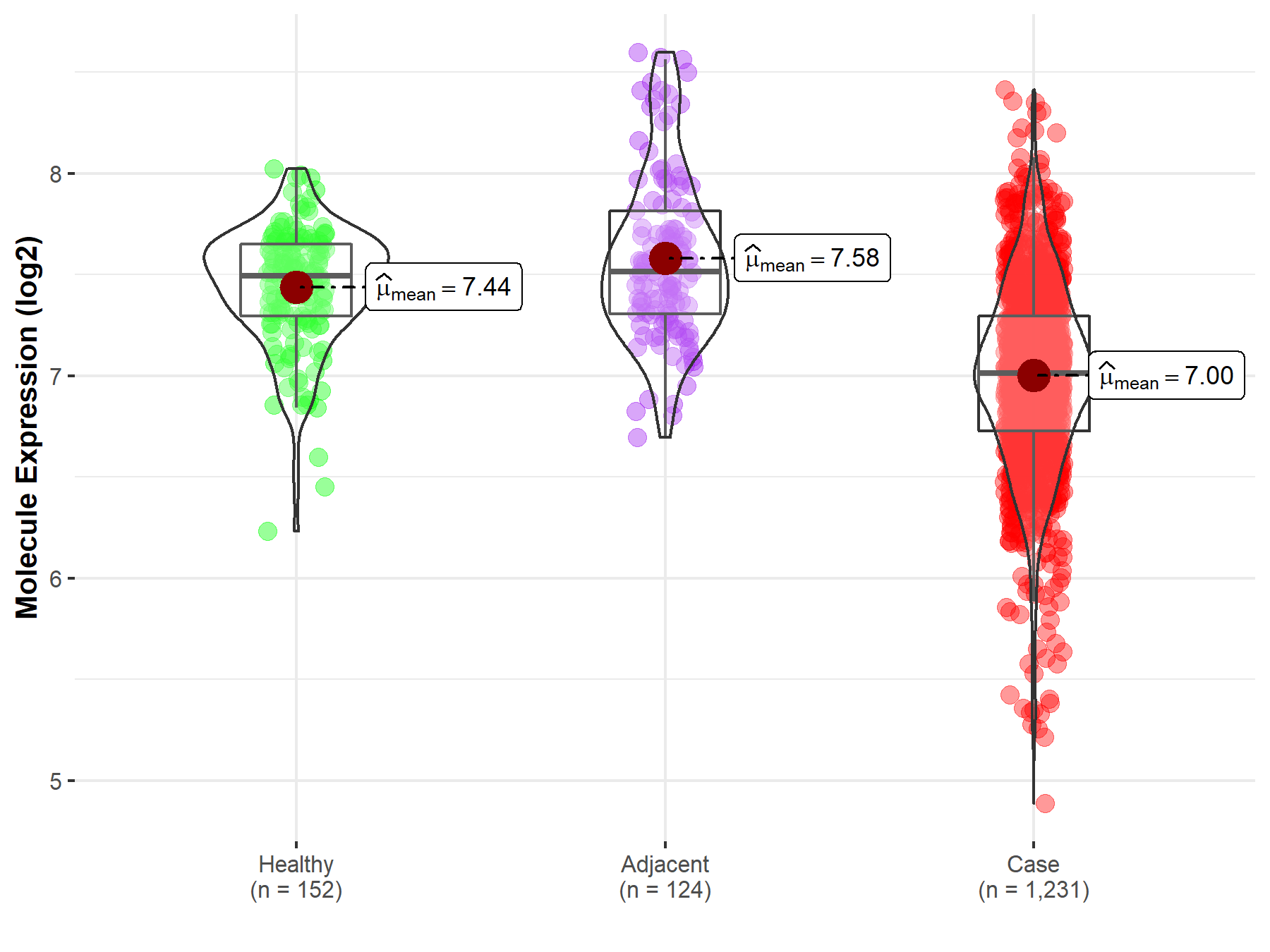
|
Click to View the Clearer Original Diagram |
| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Pancreas | |
| The Specified Disease | Pancreatic cancer | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.32E-02; Fold-change: -1.60E-01; Z-score: -5.75E-01 | |
| The Expression Level of Disease Section Compare with the Adjacent Tissue | p-value: 2.92E-03; Fold-change: -9.14E-02; Z-score: -2.63E-01 | |
|
Molecule expression in the normal tissue adjacent to the diseased tissue of patients
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |
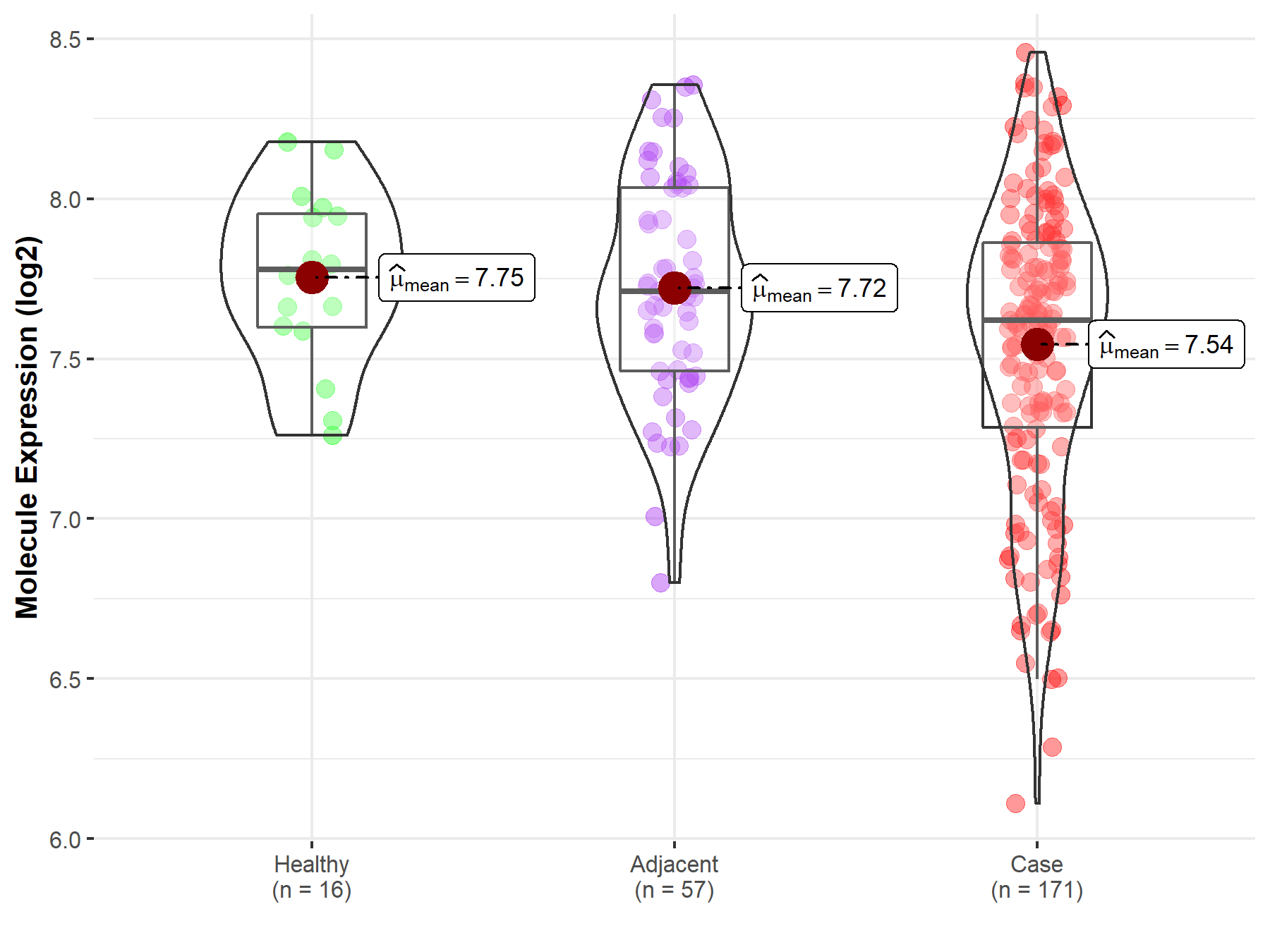
|
Click to View the Clearer Original Diagram |
| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Liver | |
| The Specified Disease | Liver cancer | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.86E-04; Fold-change: -1.58E-01; Z-score: -4.82E-01 | |
| The Expression Level of Disease Section Compare with the Adjacent Tissue | p-value: 1.23E-08; Fold-change: -1.87E-01; Z-score: -5.74E-01 | |
| The Expression Level of Disease Section Compare with the Other Disease Section | p-value: 1.41E-01; Fold-change: -5.49E-01; Z-score: -1.20E+00 | |
|
Molecule expression in the normal tissue adjacent to the diseased tissue of patients
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
Molecule expression in tissue other than the diseased tissue of patients
|
||
| Disease-specific Molecule Abundances |
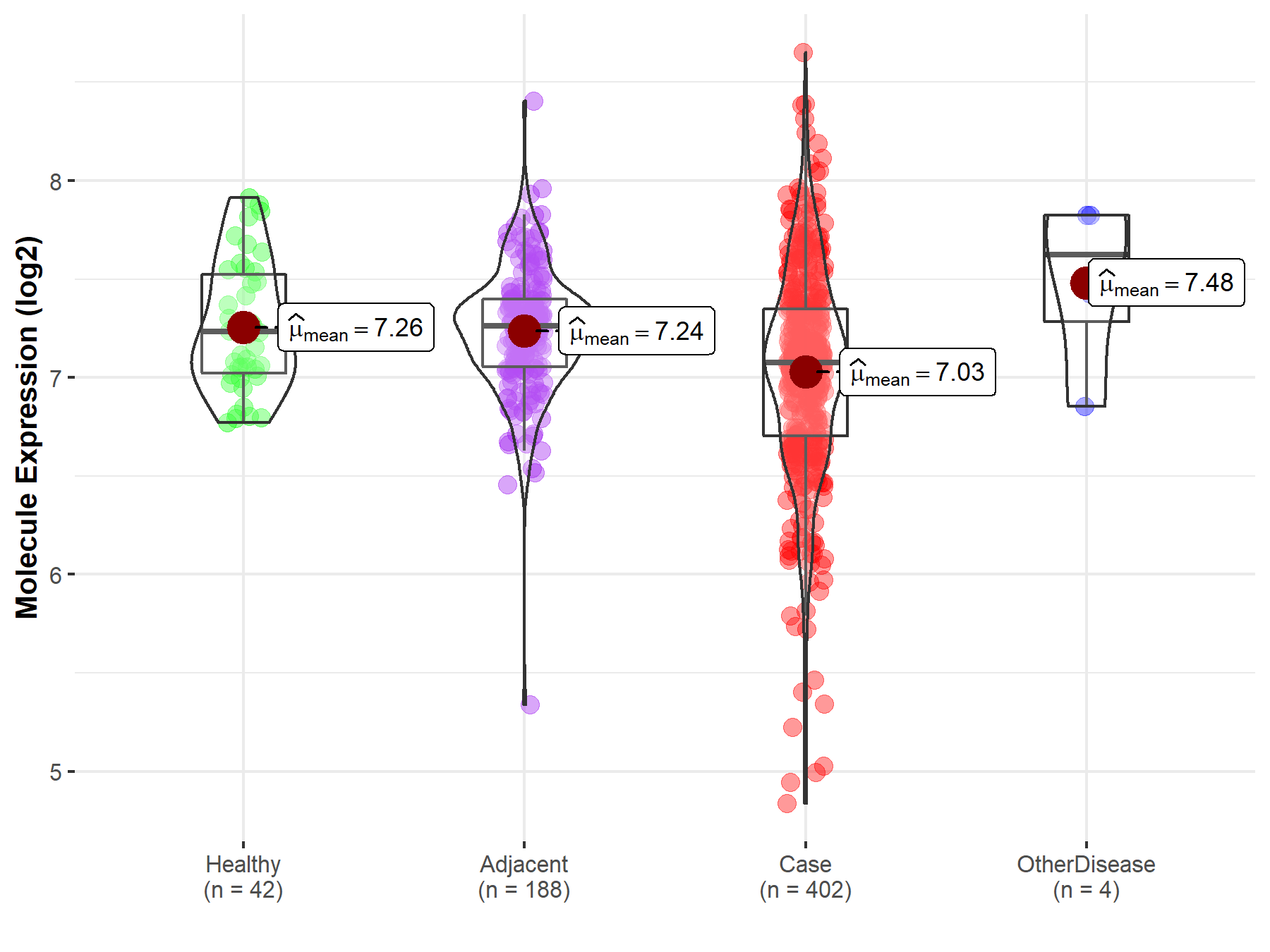
|
Click to View the Clearer Original Diagram |
| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Lung | |
| The Specified Disease | Lung cancer | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.01E-01; Fold-change: 7.96E-03; Z-score: 2.51E-02 | |
| The Expression Level of Disease Section Compare with the Adjacent Tissue | p-value: 3.79E-07; Fold-change: -9.00E-02; Z-score: -2.62E-01 | |
|
Molecule expression in the normal tissue adjacent to the diseased tissue of patients
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |
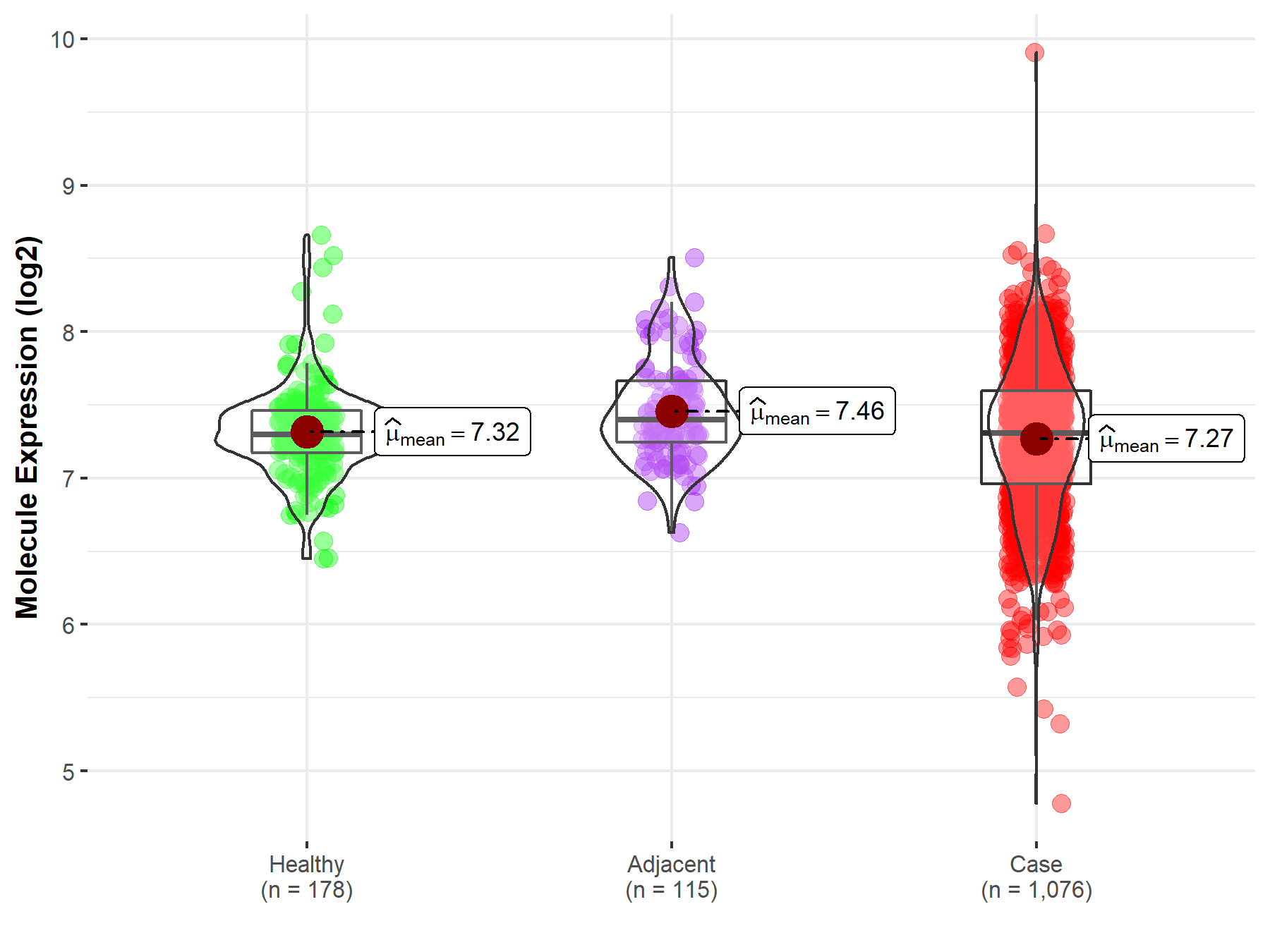
|
Click to View the Clearer Original Diagram |
| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Skin | |
| The Specified Disease | Melanoma | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 2.31E-01; Fold-change: -2.61E-01; Z-score: -3.41E-01 | |
|
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |

|
Click to View the Clearer Original Diagram |
| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Breast tissue | |
| The Specified Disease | Breast cancer | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 2.58E-08; Fold-change: -2.77E-01; Z-score: -3.67E-01 | |
| The Expression Level of Disease Section Compare with the Adjacent Tissue | p-value: 8.13E-05; Fold-change: -2.24E-01; Z-score: -3.91E-01 | |
|
Molecule expression in the normal tissue adjacent to the diseased tissue of patients
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |
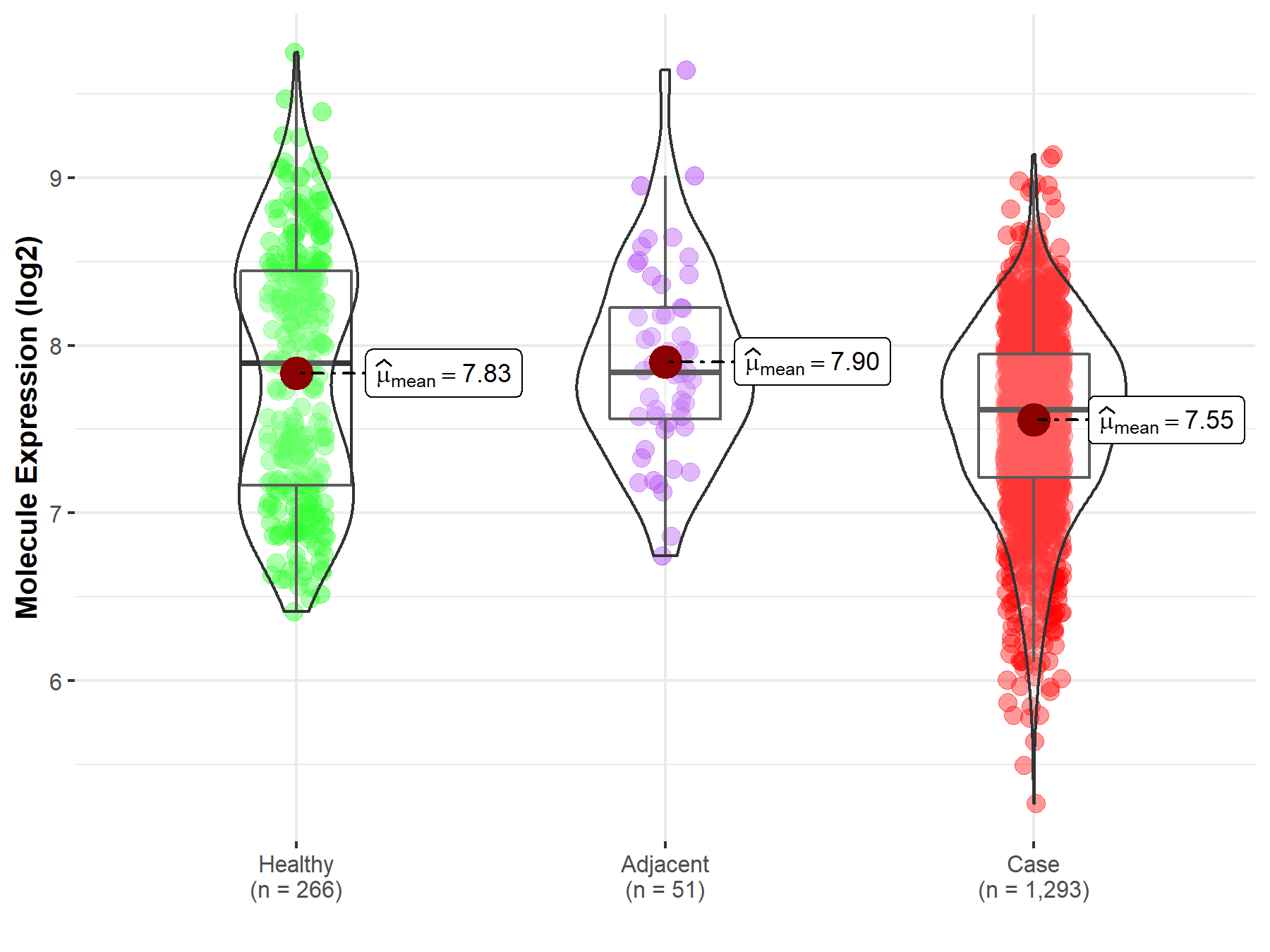
|
Click to View the Clearer Original Diagram |
| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Ovary | |
| The Specified Disease | Ovarian cancer | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 8.10E-01; Fold-change: -1.33E-01; Z-score: -2.86E-01 | |
| The Expression Level of Disease Section Compare with the Adjacent Tissue | p-value: 3.26E-02; Fold-change: 2.59E-01; Z-score: 7.76E-01 | |
|
Molecule expression in the normal tissue adjacent to the diseased tissue of patients
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |
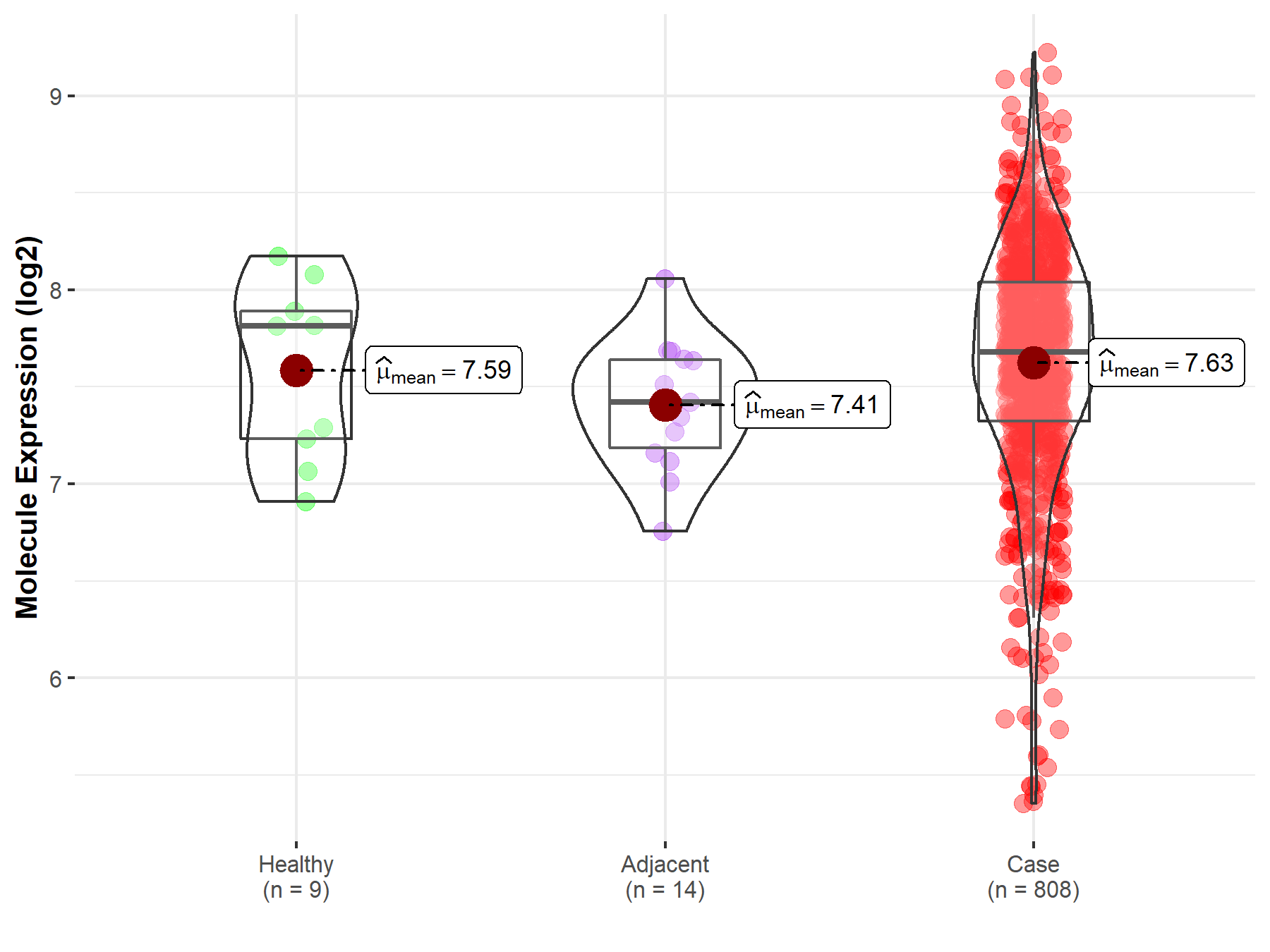
|
Click to View the Clearer Original Diagram |
| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Cervix uteri | |
| The Specified Disease | Cervical cancer | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.07E-02; Fold-change: -3.53E-01; Z-score: -8.17E-01 | |
|
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |
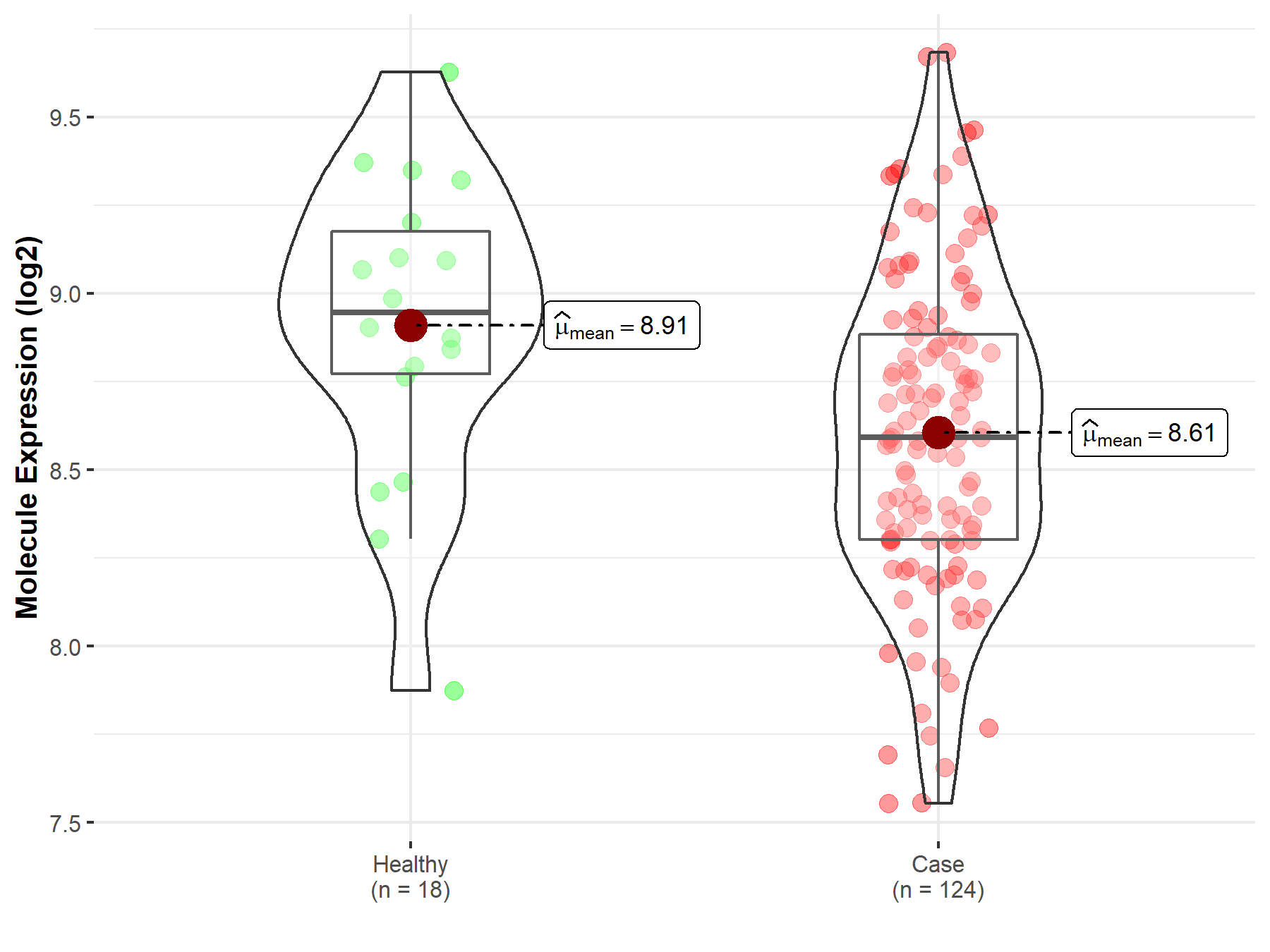
|
Click to View the Clearer Original Diagram |
| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Prostate | |
| The Specified Disease | Prostate cancer | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 2.27E-02; Fold-change: 1.46E+00; Z-score: 1.25E+00 | |
|
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |
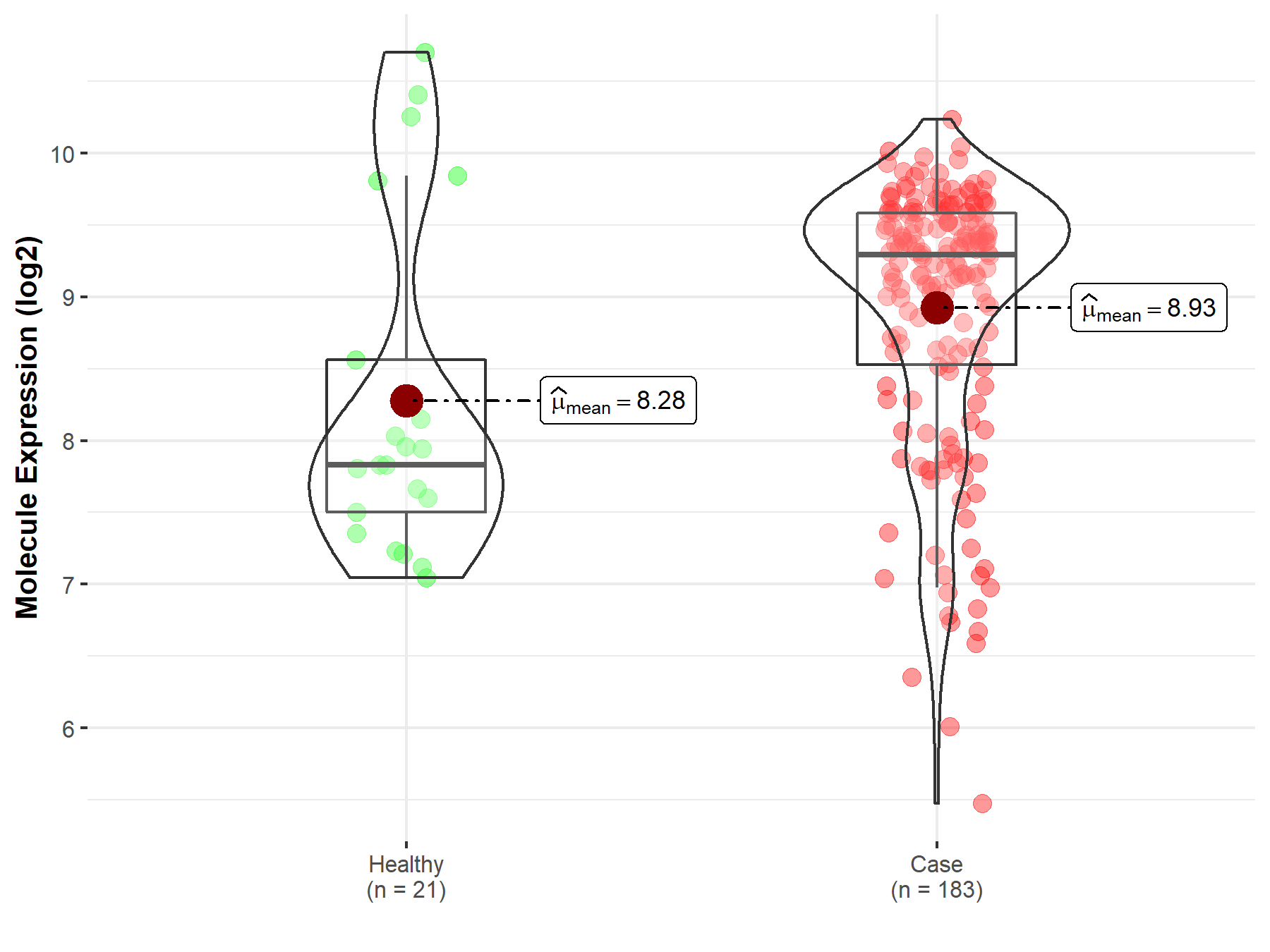
|
Click to View the Clearer Original Diagram |
| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Kidney | |
| The Specified Disease | Kidney cancer | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 3.24E-05; Fold-change: 6.62E-01; Z-score: 2.52E+00 | |
| The Expression Level of Disease Section Compare with the Adjacent Tissue | p-value: 1.84E-20; Fold-change: 8.80E-01; Z-score: 1.94E+00 | |
|
Molecule expression in the normal tissue adjacent to the diseased tissue of patients
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |

|
Click to View the Clearer Original Diagram |
| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Bladder tissue | |
| The Specified Disease | Bladder cancer | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 6.11E-08; Fold-change: -1.11E+00; Z-score: -5.59E+00 | |
|
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |
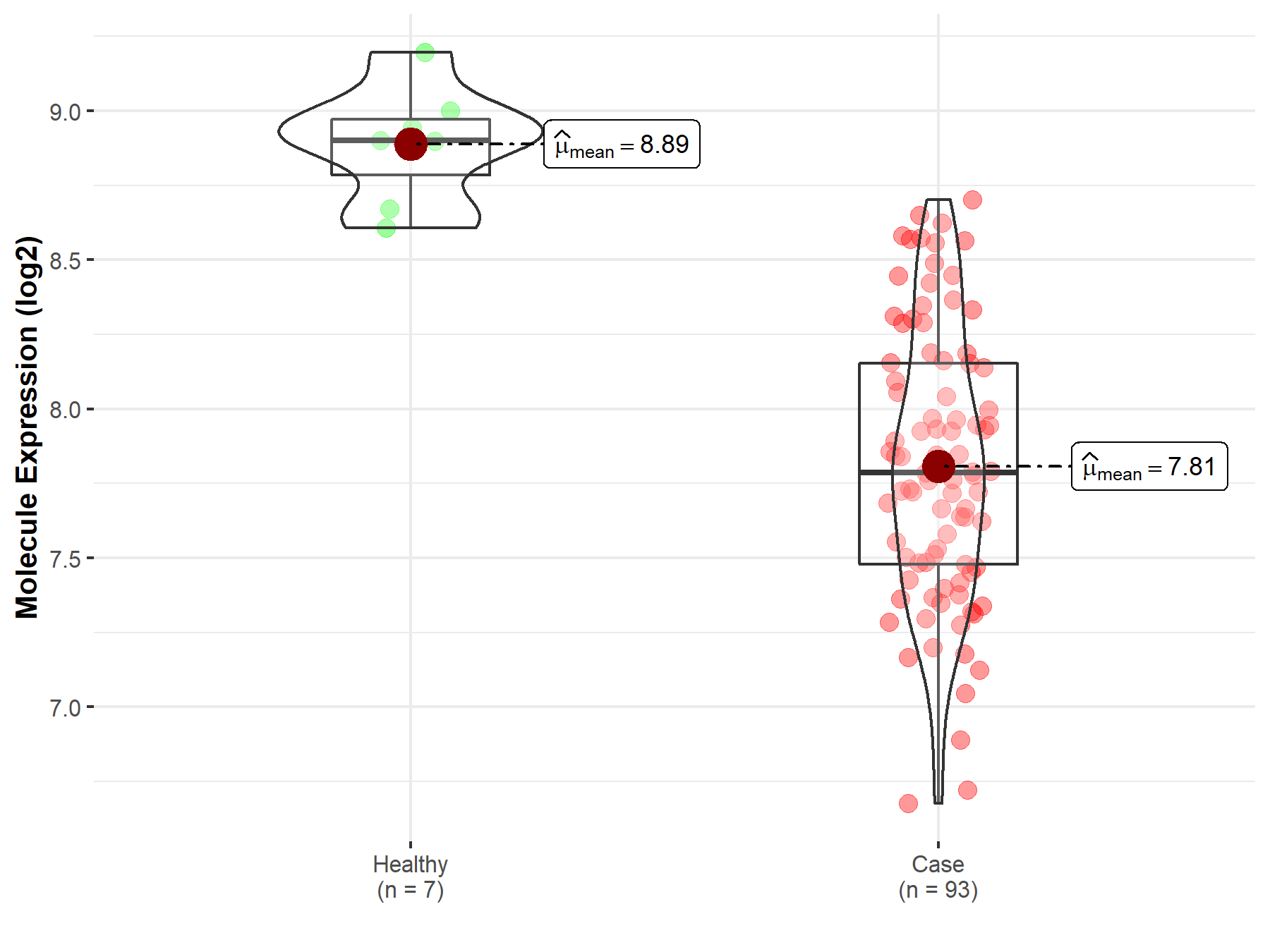
|
Click to View the Clearer Original Diagram |
| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Thyroid | |
| The Specified Disease | Thyroid cancer | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 3.41E-05; Fold-change: -1.65E-01; Z-score: -6.59E-01 | |
| The Expression Level of Disease Section Compare with the Adjacent Tissue | p-value: 4.48E-01; Fold-change: -9.49E-02; Z-score: -3.54E-01 | |
|
Molecule expression in the normal tissue adjacent to the diseased tissue of patients
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |

|
Click to View the Clearer Original Diagram |
Tissue-specific Molecule Abundances in Healthy Individuals

|
||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
