Drug Information
Drug (ID: DG01537) and It's Reported Resistant Information
| Name |
Tirbanibulin
|
||||
|---|---|---|---|---|---|
| Synonyms |
KX2-391; 897016-82-9; Tirbanibulin; KX-01; N-benzyl-2-(5-(4-(2-morpholinoethoxy)phenyl)pyridin-2-yl)acetamide; UNII-4V9848RS5G; KX-2-391; KX-2391; CHEMBL571546; 4V9848RS5G; KX2391; KX2-391;KX-01; 2-[5-[4-(2-morpholin-4-ylethoxy)phenyl]pyridin-2-yl]-~{N}-(phenylmethyl)ethanamide; Klisyri; KX 01; Tirbanibulin [USAN]; Tirbanibulin (USAN/INN); KX2-391 (Tirbanibulin); Tirbanibulin (KX2-391); MLS006011272; SCHEMBL153779; GTPL7957; KX01; DTXSID30237862; HMS3656J15; HMS3673E15; BCP02845; EX-A2434; XKB01682; BDBM50303801; NSC756643; NSC800779; s2700; WHO 10864; ZINC43152787; N-benzyl-2-(5-{4-[2-(morpholin-4-yl)ethoxy]phenyl}pyridin-2-yl)acetamide; N-benzyl-2-[5-[4-(2-morpholin-4-ylethoxy)phenyl]pyridin-2-yl]acetamide; BCP9000828; CCG-264983; CS-0248; DB06137; KX2-391; KX-01; NSC-756643; NSC-800779; SB16619; 2-Pyridineacetamide, 5-(4-(2-(4-morpholinyl)ethoxy)phenyl)-n-(phenylmethyl)-; NCGC00346644-01; NCGC00346644-05; AS-73245; HY-10340; KX 2-391; SMR004703022; DB-119272; SW219670-1; X7515; C77028; D11691; A915990; BRD-K29968218-001-01-6; Q27888424; 2-(5-(4-(2-morpholinoethoxy)phenyl)pyridin-2-yl)-N-benzylacetamide; 2-(5-(4-(2-morpholinoethoxyl)phenyl)pyridin-2-yl)-N-benzylacetamide; 2-[5-[4-(2-morpholin-4-ylethoxy)phenyl]pyridin-2-yl]-N-(phenylmethyl)acetamide; 2-Pyridineacetamide,5-[4-[2-(4-morpholinyl)ethoxy]phenyl]-N-(phenylmethyl)-; 5-[4-[2-(4-Morpholinyl)ethoxy]phenyl]-N-(phenylmethyl)-2-pyridineacetamide; DN0
Click to Show/Hide
|
||||
| Indication |
In total 4 Indication(s)
|
||||
| Structure |
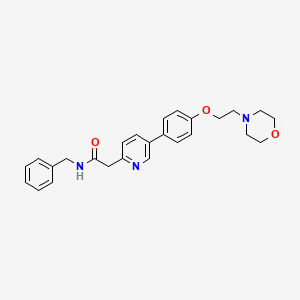
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Resistance Information Discovered by Cell Line Test for This Drug
(1 diseases)
[1]
|
||||
| Target | PI3-kinase gamma (PIK3CG) | PK3CG_HUMAN | [1] | ||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
9
|
||||
| IsoSMILES |
C1COCCN1CCOC2=CC=C(C=C2)C3=CN=C(C=C3)CC(=O)NCC4=CC=CC=C4
|
||||
| InChI |
InChI=1S/C26H29N3O3/c30-26(28-19-21-4-2-1-3-5-21)18-24-9-6-23(20-27-24)22-7-10-25(11-8-22)32-17-14-29-12-15-31-16-13-29/h1-11,20H,12-19H2,(H,28,30)
|
||||
| InChIKey |
HUNGUWOZPQBXGX-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
| TTD Drug ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-02: Benign/in-situ/malignant neoplasm
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Phosphatase and tensin homolog (PTEN) | [1] | |||
| Resistant Disease | Ovarian cancer [ICD-11: 2C73.0] | |||
| Molecule Alteration | Missense mutation | p.G129R (c.385G>A) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | RMUG-S | Ovary | Homo sapiens (Human) | CVCL_3158 |
| RMUG-L | Endometrium | Homo sapiens (Human) | CVCL_3157 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay; EdU assay; Annexin V and 7-aminoactinomycin D assay; Flow cytometry analysis | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
