Drug Information
Drug (ID: DG00286) and It's Reported Resistant Information
| Name |
Vinblastine
|
||||
|---|---|---|---|---|---|
| Synonyms |
Nincaluicolflastine; Rozevin; VLB; Vinblastin; Vinblastina; Vinblastinum; Vincaleucoblastin; Vincaleucoblastine; Vincaleukoblastine; Vincoblastine; Vinblastina [DCIT]; VR-8; Vinblastina (TN); Vinblastine (INN); Vinblastine [INN:BAN]; Vinblastinum [INN-Latin]; NDC 0002-1452-01; (2ALPHA,2'BETA,3BETA,4ALPHA,5BETA)-VINCALEUKOBLASTINE; (2xi,3beta,4'beta,19xi)-vincaleukoblastine; 1H-Indolizino(8,1-cd)carbazole-5-carboxylic acid
Click to Show/Hide
|
||||
| Indication |
In total 1 Indication(s)
|
||||
| Structure |
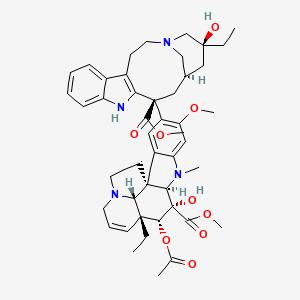
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Clinically Reported Resistance for This Drug
(1 diseases)
[2]
Disease(s) with Resistance Information Discovered by Cell Line Test for This Drug
(4 diseases)
[3]
[3]
[3]
[3]
|
||||
| Target | Tubulin beta-2 chain (TUBB2) | TBB2A_HUMAN | [1] | ||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
C46H58N4O9
|
||||
| IsoSMILES |
CC[C@@]1(C[C@H]2C[C@@](C3=C(CCN(C2)C1)C4=CC=CC=C4N3)(C5=C(C=C6C(=C5)[C@]78CCN9[C@H]7[C@@](C=CC9)([C@H]([C@@]([C@@H]8N6C)(C(=O)OC)O)OC(=O)C)CC)OC)C(=O)OC)O
|
||||
| InChI |
1S/C46H58N4O9/c1-8-42(54)23-28-24-45(40(52)57-6,36-30(15-19-49(25-28)26-42)29-13-10-11-14-33(29)47-36)32-21-31-34(22-35(32)56-5)48(4)38-44(31)17-20-50-18-12-16-43(9-2,37(44)50)39(59-27(3)51)46(38,55)41(53)58-7/h10-14,16,21-22,28,37-39,47,54-55H,8-9,15,17-20,23-26H2,1-7H3/t28-,37-,38+,39+,42-,43+,44+,45-,46-/m0/s1
|
||||
| InChIKey |
JXLYSJRDGCGARV-CFWMRBGOSA-N
|
||||
| PubChem CID | |||||
| TTD Drug ID | |||||
| VARIDT ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-02: Benign/in-situ/malignant neoplasm
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Multidrug resistance protein 1 (ABCB1) | [4] | |||
| Sensitive Disease | Renal cell carcinoma [ICD-11: 2C90.0] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Kidney cancer [ICD-11: 2C90] | |||
| The Specified Disease | Renal cell carcinoma | |||
| The Studied Tissue | Kidney | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 4.11E-46 Fold-change: -1.42E+00 Z-score: -1.91E+01 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Flp-In-293/Mock cells | Kidney | Homo sapiens (Human) | CVCL_U421 |
| Flp-In-293/ABCB1 cells | Kidney | Homo sapiens (Human) | CVCL_U421 | |
| Experiment for Molecule Alteration |
ATPase assay | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Through calcein assays, we found that epimagnolin A inhibited the ABCB1-mediated export of calcein. This result suggests that epimagnolin A behaved as inhibitor or substrate for ABCB1. In ATPase assays, epimagnolin A stimulated ABCB1-dependent ATPase activity. This result indicates that epimagnolin A was recognised as a substrate by ABCB1, since ABCB1 utilises energy derived from ATP hydrolysis for substrate transport. Furthermore, in MTT assays we found that the cytotoxicity of daunorubicin, doxorubicin, vinblastine, and vincristine was enhanced by epimagnolin A in a manner comparable to verapamil, a typical substrate for ABCB1. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Multidrug resistance protein 1 (ABCB1) | [3] | |||
| Resistant Disease | Glioma [ICD-11: 2A00.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | U87-MG cells | Brain | Homo sapiens (Human) | CVCL_0022 |
| In Vivo Model | Athymic nu/nu female mice xenograft model | Mus musculus | ||
| Experiment for Drug Resistance |
MTS assay | |||
| Mechanism Description | In a cell line expressing a high level of P-glycoprotein, the IC50 of TTI-237 increased 25-fold whereas those of paclitaxel and vincristine increased 806-fold and 925-fold. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Multidrug resistance protein 1 (ABCB1) | [5] | |||
| Sensitive Disease | Chronic myeloid leukemia [ICD-11: 2A20.0] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | NCI-H460 cells | Lung | Homo sapiens (Human) | CVCL_0459 |
| K562 cells | Blood | Homo sapiens (Human) | CVCL_0004 | |
| HEK293 cells | Kidney | Homo sapiens (Human) | CVCL_0045 | |
| K562-R cells | Pleural effusion | Homo sapiens (Human) | CVCL_5950 | |
| NCI-H460/VBL cells | Bone marrow | Homo sapiens (Human) | CVCL_0459 | |
| In Vivo Model | SCID beige mice | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | In ABCB1-overexpressing cell lines, HG-829 significantly enhanced cytotoxicity to daunorubicin, paclitaxel, vinblastine, vincristine, and etoposide. Coadministration of HG-829 fully restored in vivo antitumor activity of daunorubicin in mice without added toxicity. Functional assays showed that HG-829 is not a Pgp substrate or competitive inhibitor of Pgp-mediated drug efflux but rather acts as a noncompetitive modulator of P-glycoprotein transport function. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Multidrug resistance protein 1 (ABCB1) | [3] | |||
| Resistant Disease | Squamous cell carcinoma [ICD-11: 2B6E.3] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | KB-3-1 cells | Lung | Homo sapiens (Human) | CVCL_2088 |
| KB-8-5 cells | Mouth | Homo sapiens (Human) | CVCL_5994 | |
| KB-V1 cells | Mouth | Homo sapiens (Human) | CVCL_2089 | |
| In Vivo Model | Athymic nu/nu female mice xenograft model | Mus musculus | ||
| Experiment for Drug Resistance |
MTS assay | |||
| Mechanism Description | In a cell line expressing a high level of P-glycoprotein, the IC50 of TTI-237 increased 25-fold whereas those of paclitaxel and vincristine increased 806-fold and 925-fold. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Multidrug resistance protein 1 (ABCB1) | [3] | |||
| Resistant Disease | Colorectal carcinoma [ICD-11: 2B91.3] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | LOVO cells | Colon | Homo sapiens (Human) | CVCL_0399 |
| In Vivo Model | Athymic nu/nu female mice xenograft model | Mus musculus | ||
| Experiment for Drug Resistance |
MTS assay | |||
| Mechanism Description | In a cell line expressing a high level of P-glycoprotein, the IC50 of TTI-237 increased 25-fold whereas those of paclitaxel and vincristine increased 806-fold and 925-fold. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: ATP-binding cassette sub-family B5 (ABCB5) | [6] | |||
| Sensitive Disease | Colorectal carcinoma [ICD-11: 2B91.3] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | CaCo2 cells | Colon | Homo sapiens (Human) | CVCL_0025 |
| HCT-8 cells | Colon | Homo sapiens (Human) | CVCL_2478 | |
| NIH-G185 cells | Ovary | Homo sapiens (Human) | CVCL_L991 | |
| NIH 3T3 cells | Colon | Homo sapiens (Human) | CVCL_0594 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | G185 cells were 27-135 fold more resistant to the cytotoxic drugs doxorubicin, vinblastine, colchicine and paclitaxel than the parental NIH 3T3 cells. Co-administration of TPGS enhanced the cytotoxicity of doxorubicin, vinblastine, paclitaxel, and colchicine in the G185 cells to levels comparable to the parental. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: hsa-mir-335 | [1] | |||
| Sensitive Disease | Hepatocellular carcinoma [ICD-11: 2C12.2] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell invasion | Inhibition | hsa05200 | |
| Cell migration | Inhibition | hsa04670 | ||
| Cell proliferation | Inhibition | hsa05200 | ||
| miR335/SIAH2/HDAC3 signaling pathway | Regulation | N.A. | ||
| In Vitro Model | SNU387 cells | Liver | Homo sapiens (Human) | CVCL_0250 |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
Trypan blue exclusion assay; Transwell assay | |||
| Mechanism Description | miR-335-mediated increased sensitivity to anti-cancer drugs was associated with its effect on HDAC3 and SIAH2 expression. miR-335 exerted apoptotic effects and inhibited ubiquitination of HDAC3 in anti-cancer drug-resistant cancer cell lines. miR-335 negatively regulated the invasion, migration, and growth rate of cancer cells. The mouse xenograft model showed that miR-335 negatively regulated the tumorigenic potential of cancer cells. The down-regulation of SIAH2 conferred sensitivity to anti-cancer drugs. The results of the study indicated that the miR-335/SIAH2/HDAC3 axis regulates the response to anti-cancer drugs. | |||
|
|
||||
| Key Molecule: E3 ubiquitin-protein ligase SIAH2 (SIAH2) | [1] | |||
| Sensitive Disease | Hepatocellular carcinoma [ICD-11: 2C12.2] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell invasion | Inhibition | hsa05200 | |
| Cell migration | Inhibition | hsa04670 | ||
| Cell proliferation | Inhibition | hsa05200 | ||
| miR335/SIAH2/HDAC3 signaling pathway | Regulation | N.A. | ||
| In Vitro Model | SNU387 cells | Liver | Homo sapiens (Human) | CVCL_0250 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Trypan blue exclusion assay; Transwell assay | |||
| Mechanism Description | miR-335-mediated increased sensitivity to anti-cancer drugs was associated with its effect on HDAC3 and SIAH2 expression. miR-335 exerted apoptotic effects and inhibited ubiquitination of HDAC3 in anti-cancer drug-resistant cancer cell lines. miR-335 negatively regulated the invasion, migration, and growth rate of cancer cells. The mouse xenograft model showed that miR-335 negatively regulated the tumorigenic potential of cancer cells. The down-regulation of SIAH2 conferred sensitivity to anti-cancer drugs. The results of the study indicated that the miR-335/SIAH2/HDAC3 axis regulates the response to anti-cancer drugs. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: hsa-let-7g | [2] | |||
| Resistant Disease | Ovarian cancer [ICD-11: 2C73.0] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | T47D cells | Breast | Homo sapiens (Human) | CVCL_0553 |
| IGROV1 cells | Ovary | Homo sapiens (Human) | CVCL_1304 | |
| OVCAR8 cells | Ovary | Homo sapiens (Human) | CVCL_1629 | |
| LOX-IMVI cells | Ovary | Homo sapiens (Human) | CVCL_1381 | |
| NCI/ADR-RES cells | Ovary | Homo sapiens (Human) | CVCL_1452 | |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
SRB cytotoxicity assay | |||
| Mechanism Description | IMP-1 is an RNA binding protein that acts by stabilizing the mRNA of a number of target genes. In addition, IMP-1 was shown to protect the mRNA of MDR1 from endonucleolytic attack in an in vitro RNA stability assay. Introducing let-7g into ADR-RES cells expressing both IMP-1 and MDR1 reduced expression of both proteins rendering the cells more sensitive to treatment with either Taxol or vinblastine without affecting the sensitivity of the cells to carboplatin. | |||
|
|
||||
| Key Molecule: Insulin-like growth factor 2 mRNA-binding protein 1 (IGF2BP1) | [2] | |||
| Resistant Disease | Ovarian cancer [ICD-11: 2C73.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | T47D cells | Breast | Homo sapiens (Human) | CVCL_0553 |
| IGROV1 cells | Ovary | Homo sapiens (Human) | CVCL_1304 | |
| OVCAR8 cells | Ovary | Homo sapiens (Human) | CVCL_1629 | |
| LOX-IMVI cells | Ovary | Homo sapiens (Human) | CVCL_1381 | |
| NCI/ADR-RES cells | Ovary | Homo sapiens (Human) | CVCL_1452 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
SRB cytotoxicity assay | |||
| Mechanism Description | IMP-1 is an RNA binding protein that acts by stabilizing the mRNA of a number of target genes. In addition, IMP-1 was shown to protect the mRNA of MDR1 from endonucleolytic attack in an in vitro RNA stability assay. Introducing let-7g into ADR-RES cells expressing both IMP-1 and MDR1 reduced expression of both proteins rendering the cells more sensitive to treatment with either Taxol or vinblastine without affecting the sensitivity of the cells to carboplatin. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Multidrug resistance protein 1 (ABCB1) | [3] | |||
| Resistant Disease | Cervical carcinoma [ICD-11: 2C77.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Hela cells | Cervix uteri | Homo sapiens (Human) | CVCL_0030 |
| In Vivo Model | Athymic nu/nu female mice xenograft model | Mus musculus | ||
| Experiment for Drug Resistance |
MTS assay | |||
| Mechanism Description | In a cell line expressing a high level of P-glycoprotein, the IC50 of TTI-237 increased 25-fold whereas those of paclitaxel and vincristine increased 806-fold and 925-fold. | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
