Drug Information
Drug (ID: DG00337) and It's Reported Resistant Information
| Name |
Pirarubicin
|
||||
|---|---|---|---|---|---|
| Synonyms |
Pinorubicin (TN); Therarubucin (TN)
Click to Show/Hide
|
||||
| Indication |
In total 1 Indication(s)
|
||||
| Structure |
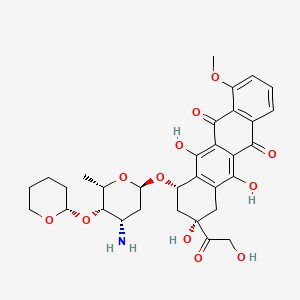
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Resistance Information Discovered by Cell Line Test for This Drug
(3 diseases)
[2]
[3]
[4]
|
||||
| Target | Human Deoxyribonucleic acid (hDNA) | NOUNIPROTAC | [1] | ||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
C32H37NO12
|
||||
| IsoSMILES |
C[C@H]1[C@H]([C@H](C[C@@H](O1)O[C@H]2C[C@@](CC3=C2C(=C4C(=C3O)C(=O)C5=C(C4=O)C(=CC=C5)OC)O)(C(=O)CO)O)N)O[C@H]6CCCCO6
|
||||
| InChI |
1S/C32H37NO12/c1-14-31(45-21-8-3-4-9-42-21)17(33)10-22(43-14)44-19-12-32(40,20(35)13-34)11-16-24(19)30(39)26-25(28(16)37)27(36)15-6-5-7-18(41-2)23(15)29(26)38/h5-7,14,17,19,21-22,31,34,37,39-40H,3-4,8-13,33H2,1-2H3/t14-,17-,19-,21-,22-,31+,32-/m0/s1
|
||||
| InChIKey |
KMSKQZKKOZQFFG-HSUXVGOQSA-N
|
||||
| PubChem CID | |||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
| VARIDT ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-02: Benign/in-situ/malignant neoplasm
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Ubiquitin carboxyl-terminal hydrolase 22 (USP22) | [4] | |||
| Resistant Disease | Hepatocellular carcinoma [ICD-11: 2C12.2] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Liver cancer [ICD-11: 2C12] | |||
| The Specified Disease | Liver cancer | |||
| The Studied Tissue | Liver tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 8.03E-01 Fold-change: 1.99E-03 Z-score: 2.50E-01 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Huh-7 cells | Liver | Homo sapiens (Human) | CVCL_0336 |
| HepG2 cells | Liver | Homo sapiens (Human) | CVCL_0027 | |
| Hep3B cells | Liver | Homo sapiens (Human) | CVCL_0326 | |
| SMMC7721 cells | Uterus | Homo sapiens (Human) | CVCL_0534 | |
| PLC cells | Liver | Homo sapiens (Human) | CVCL_0485 | |
| L02 cells | Liver | Homo sapiens (Human) | CVCL_6926 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | miR6825-5p, miR6845-5p and miR6886-3p could decrease the level of USP22 protein by binding to the 3'-untranlated region of USP22 mRNA. All the three microRNAs (miRNAs) were downregulated by HULC, which resulted in the elevation of USP22. The pathway 'HULC/USP22/Sirt1/ protective autophagy' attenuates the sensitivity of HCC cells to chemotherapeutic agents. | |||
|
|
||||
| Key Molecule: Hepatocellular carcinoma up-regulated long non-coding RNA (HULC) | [4] | |||
| Resistant Disease | Hepatocellular carcinoma [ICD-11: 2C12.2] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Huh-7 cells | Liver | Homo sapiens (Human) | CVCL_0336 |
| HepG2 cells | Liver | Homo sapiens (Human) | CVCL_0027 | |
| Hep3B cells | Liver | Homo sapiens (Human) | CVCL_0326 | |
| SMMC7721 cells | Uterus | Homo sapiens (Human) | CVCL_0534 | |
| PLC cells | Liver | Homo sapiens (Human) | CVCL_0485 | |
| L02 cells | Liver | Homo sapiens (Human) | CVCL_6926 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | LncRNA HULC triggers autophagy via stabilizing Sirt1 and attenuates the chemosensitivity of HCC cells. HULC inhibits the expression and activity of miR6825-5p, miR6845-5p and miR6886-3p. | |||
| Key Molecule: hsa-miR-6825-5p | [4] | |||
| Resistant Disease | Hepatocellular carcinoma [ICD-11: 2C12.2] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Huh-7 cells | Liver | Homo sapiens (Human) | CVCL_0336 |
| HepG2 cells | Liver | Homo sapiens (Human) | CVCL_0027 | |
| Hep3B cells | Liver | Homo sapiens (Human) | CVCL_0326 | |
| SMMC7721 cells | Uterus | Homo sapiens (Human) | CVCL_0534 | |
| PLC cells | Liver | Homo sapiens (Human) | CVCL_0485 | |
| L02 cells | Liver | Homo sapiens (Human) | CVCL_6926 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | miR6825-5p, miR6845-5p and miR6886-3p could decrease the level of USP22 protein by binding to the 3'-untranlated region of USP22 mRNA. All the three microRNAs (miRNAs) were downregulated by HULC, which resulted in the elevation of USP22. The pathway 'HULC/USP22/Sirt1/ protective autophagy' attenuates the sensitivity of HCC cells to chemotherapeutic agents. | |||
| Key Molecule: hsa-miR-6845-5p | [4] | |||
| Resistant Disease | Hepatocellular carcinoma [ICD-11: 2C12.2] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Huh-7 cells | Liver | Homo sapiens (Human) | CVCL_0336 |
| HepG2 cells | Liver | Homo sapiens (Human) | CVCL_0027 | |
| Hep3B cells | Liver | Homo sapiens (Human) | CVCL_0326 | |
| SMMC7721 cells | Uterus | Homo sapiens (Human) | CVCL_0534 | |
| PLC cells | Liver | Homo sapiens (Human) | CVCL_0485 | |
| L02 cells | Liver | Homo sapiens (Human) | CVCL_6926 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | miR6825-5p, miR6845-5p and miR6886-3p could decrease the level of USP22 protein by binding to the 3'-untranlated region of USP22 mRNA. All the three microRNAs (miRNAs) were downregulated by HULC, which resulted in the elevation of USP22. The pathway 'HULC/USP22/Sirt1/ protective autophagy' attenuates the sensitivity of HCC cells to chemotherapeutic agents. | |||
| Key Molecule: hsa-miR-6886-3p | [4] | |||
| Resistant Disease | Hepatocellular carcinoma [ICD-11: 2C12.2] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Huh-7 cells | Liver | Homo sapiens (Human) | CVCL_0336 |
| HepG2 cells | Liver | Homo sapiens (Human) | CVCL_0027 | |
| Hep3B cells | Liver | Homo sapiens (Human) | CVCL_0326 | |
| SMMC7721 cells | Uterus | Homo sapiens (Human) | CVCL_0534 | |
| PLC cells | Liver | Homo sapiens (Human) | CVCL_0485 | |
| L02 cells | Liver | Homo sapiens (Human) | CVCL_6926 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | miR6825-5p, miR6845-5p and miR6886-3p could decrease the level of USP22 protein by binding to the 3'-untranlated region of USP22 mRNA. All the three microRNAs (miRNAs) were downregulated by HULC, which resulted in the elevation of USP22. The pathway 'HULC/USP22/Sirt1/ protective autophagy' attenuates the sensitivity of HCC cells to chemotherapeutic agents. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: H19, imprinted maternally expressed transcript (H19) | [3] | |||
| Resistant Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cullin4A/MDR1 signaling pathway | Regulation | N.A. | |
| In Vitro Model | MCF-7 cells | Breast | Homo sapiens (Human) | CVCL_0031 |
| Experiment for Molecule Alteration |
Quantitative real-time RT-PCR | |||
| Experiment for Drug Resistance |
CellTiter AQueous One Solution Cell Proliferation Assay | |||
| Mechanism Description | LncRNA H19 is a major mediator of doxorubicin chemoresistance in breast cancer cells through a cullin4A-MDR1 pathway. H19 overexpression was contributed to cancer cell resistance to anthracyclines and paclitaxel as knockdown of H19 LncRNA by a specific H19 shRNA in Dox-resistant cells significantly improved the cell sensitivity to anthracyclines and paclitaxel. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: hsa-miR-34c-5p | [5] | |||
| Sensitive Disease | Cervical cancer [ICD-11: 2C77.0] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| miR34C-5p/ATG4B-autophagy signaling pathway | Regulation | N.A. | ||
| In Vitro Model | Hela cells | Cervix uteri | Homo sapiens (Human) | CVCL_0030 |
| Siha cells | Cervix uteri | Homo sapiens (Human) | CVCL_0032 | |
| C33A cells | Uterus | Homo sapiens (Human) | CVCL_1094 | |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | On the one side, THP induces apoptosis, which results in cell death. On the other side, THP activates the MIR34C-5p-ATG4B-autophagy signaling axis via the sequential triggering of MIR34C-5p downregulation, ATG4B mRNA stability enhancement, and ATG4B upregulation and autophagy induction. Moreover, autophagy protects cervical cancer cells from cell death. The autophagy inhibitor CQ sensitizes cervical cancer cells to THP treatment. | |||
|
|
||||
| Key Molecule: Cysteine protease ATG4B (ATG4B) | [5] | |||
| Sensitive Disease | Cervical cancer [ICD-11: 2C77.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| miR34C-5p/ATG4B-autophagy signaling pathway | Regulation | N.A. | ||
| In Vitro Model | Hela cells | Cervix uteri | Homo sapiens (Human) | CVCL_0030 |
| Siha cells | Cervix uteri | Homo sapiens (Human) | CVCL_0032 | |
| C33A cells | Uterus | Homo sapiens (Human) | CVCL_1094 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | On the one side, THP induces apoptosis, which results in cell death. On the other side, THP activates the MIR34C-5p-ATG4B-autophagy signaling axis via the sequential triggering of MIR34C-5p downregulation, ATG4B mRNA stability enhancement, and ATG4B upregulation and autophagy induction. Moreover, autophagy protects cervical cancer cells from cell death. The autophagy inhibitor CQ sensitizes cervical cancer cells to THP treatment. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: hsa-miR-34b-3p | [1] | |||
| Resistant Disease | Bladder cancer [ICD-11: 2C94.0] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell proliferation | Activation | hsa05200 | ||
| Notch/PkC/Ca++ signaling pathway | Inhibition | hsa04330 | ||
| In Vitro Model | 5637 cells | Bladder | Homo sapiens (Human) | CVCL_0126 |
| EJ cells | Bladder | Homo sapiens (Human) | CVCL_UI82 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
CCK8 assay; Flow cytometry assay | |||
| Mechanism Description | miR-34b-3p Represses the Multidrug-Chemoresistance of Bladder Cancer Cells by Regulating the CCND2 and P2RY1 Genes. | |||
| Key Molecule: hsa-miR-22-3p | [2] | |||
| Resistant Disease | Bladder cancer [ICD-11: 2C94.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell proliferation | Activation | hsa05200 | ||
| In Vitro Model | 5637 cells | Bladder | Homo sapiens (Human) | CVCL_0126 |
| T24 cells | Bladder | Homo sapiens (Human) | CVCL_0554 | |
| UM-UC-3 cells | Bladder | Homo sapiens (Human) | CVCL_1783 | |
| H-bc cells | Bladder | Homo sapiens (Human) | CVCL_BT00 | |
| HTB-1 cells | Bladder | Homo sapiens (Human) | CVCL_0359 | |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
Flow cytometry assay | |||
| Mechanism Description | miR 22 3p enhances multi chemoresistance by targeting NET1 in bladder cancer cells. | |||
|
|
||||
| Key Molecule: G1/S-specific cyclin-D2 (CCND2) | [1] | |||
| Resistant Disease | Bladder cancer [ICD-11: 2C94.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell proliferation | Activation | hsa05200 | ||
| Notch/PkC/Ca++ signaling pathway | Inhibition | hsa04330 | ||
| In Vitro Model | 5637 cells | Bladder | Homo sapiens (Human) | CVCL_0126 |
| EJ cells | Bladder | Homo sapiens (Human) | CVCL_UI82 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis; RT-qPCR | |||
| Experiment for Drug Resistance |
CCK8 assay; Flow cytometry assay | |||
| Mechanism Description | miR-34b-3p Represses the Multidrug-Chemoresistance of Bladder Cancer Cells by Regulating the CCND2 and P2RY1 Genes. | |||
| Key Molecule: P2Y purinoceptor 1 (P2RY1) | [1] | |||
| Resistant Disease | Bladder cancer [ICD-11: 2C94.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell proliferation | Activation | hsa05200 | ||
| Notch/PkC/Ca++ signaling pathway | Inhibition | hsa04330 | ||
| In Vitro Model | 5637 cells | Bladder | Homo sapiens (Human) | CVCL_0126 |
| EJ cells | Bladder | Homo sapiens (Human) | CVCL_UI82 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis; RT-qPCR | |||
| Experiment for Drug Resistance |
CCK8 assay; Flow cytometry assay | |||
| Mechanism Description | miR-34b-3p Represses the Multidrug-Chemoresistance of Bladder Cancer Cells by Regulating the CCND2 and P2RY1 Genes. | |||
| Key Molecule: Neuroepithelial cell-transforming gene 1 protein (NET1) | [2] | |||
| Resistant Disease | Bladder cancer [ICD-11: 2C94.0] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell proliferation | Activation | hsa05200 | ||
| In Vitro Model | 5637 cells | Bladder | Homo sapiens (Human) | CVCL_0126 |
| T24 cells | Bladder | Homo sapiens (Human) | CVCL_0554 | |
| UM-UC-3 cells | Bladder | Homo sapiens (Human) | CVCL_1783 | |
| H-bc cells | Bladder | Homo sapiens (Human) | CVCL_BT00 | |
| HTB-1 cells | Bladder | Homo sapiens (Human) | CVCL_0359 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Flow cytometry assay | |||
| Mechanism Description | miR 22 3p enhances multi chemoresistance by targeting NET1 in bladder cancer cells. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: hsa-miR-193a-3p | [6] | |||
| Sensitive Disease | Bladder cancer [ICD-11: 2C94.0] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| DNA damage response signaling pathway | Activation | hsa04218 | ||
| In Vitro Model | 5637 cells | Bladder | Homo sapiens (Human) | CVCL_0126 |
| H-bc cells | Bladder | Homo sapiens (Human) | CVCL_BT00 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
Flow cytometry assay | |||
| Mechanism Description | Among the differentially expressed genes between the chemosensitive (5637) and chemoresistant (H-bc) bladder cancer cell lines, the expression level of the PSEN1 gene (presenilin 1), a key component of the Gamma-secretase, is negatively correlated with chemoresistance. A small interfering RNA mediated repression of the PSEN1 gene suppresses cell apoptosis and de-sensitizes 5637 cells, while overexpression of the presenilin 1 sensitizes H-bc cells to the drug-triggered cell death. As a direct target of microRNA-193a-3p that promotes the multi-chemoresistance of the bladder cancer cell, PSEN1 acts as an important executor for the microRNA-193a-3p's positive impact on the multi-chemoresistance of bladder cancer, probably via its activating effect on DNA damage response pathway. In addition to the mechanistic insights, the key players in this microRNA-193a-3p/PSEN1 axis are likely the diagnostic and/or therapeutic targets for an effective chemotherapy of bladder cancer. | |||
|
|
||||
| Key Molecule: Presenilin-1 (PSEN1) | [6] | |||
| Sensitive Disease | Bladder cancer [ICD-11: 2C94.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| DNA damage response signaling pathway | Activation | hsa04218 | ||
| In Vitro Model | 5637 cells | Bladder | Homo sapiens (Human) | CVCL_0126 |
| H-bc cells | Bladder | Homo sapiens (Human) | CVCL_BT00 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Flow cytometry assay | |||
| Mechanism Description | Among the differentially expressed genes between the chemosensitive (5637) and chemoresistant (H-bc) bladder cancer cell lines, the expression level of the PSEN1 gene (presenilin 1), a key component of the Gamma-secretase, is negatively correlated with chemoresistance. A small interfering RNA mediated repression of the PSEN1 gene suppresses cell apoptosis and de-sensitizes 5637 cells, while overexpression of the presenilin 1 sensitizes H-bc cells to the drug-triggered cell death. As a direct target of microRNA-193a-3p that promotes the multi-chemoresistance of the bladder cancer cell, PSEN1 acts as an important executor for the microRNA-193a-3p's positive impact on the multi-chemoresistance of bladder cancer, probably via its activating effect on DNA damage response pathway. In addition to the mechanistic insights, the key players in this microRNA-193a-3p/PSEN1 axis are likely the diagnostic and/or therapeutic targets for an effective chemotherapy of bladder cancer. | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
