Molecule Information
General Information of the Molecule (ID: Mol00574)
| Name |
Presenilin-1 (PSEN1)
,Homo sapiens
|
||||
|---|---|---|---|---|---|
| Synonyms |
PS-1; Protein S182; PS1-CTF12; AD3; PS1; PSNL1
Click to Show/Hide
|
||||
| Molecule Type |
Protein
|
||||
| Gene Name |
PSEN1
|
||||
| Gene ID | |||||
| Location |
chr14:73136418-73223691[+]
|
||||
| Sequence |
MTELPAPLSYFQNAQMSEDNHLSNTVRSQNDNRERQEHNDRRSLGHPEPLSNGRPQGNSR
QVVEQDEEEDEELTLKYGAKHVIMLFVPVTLCMVVVVATIKSVSFYTRKDGQLIYTPFTE DTETVGQRALHSILNAAIMISVIVVMTILLVVLYKYRCYKVIHAWLIISSLLLLFFFSFI YLGEVFKTYNVAVDYITVALLIWNFGVVGMISIHWKGPLRLQQAYLIMISALMALVFIKY LPEWTAWLILAVISVYDLVAVLCPKGPLRMLVETAQERNETLFPALIYSSTMVWLVNMAE GDPEAQRRVSKNSKYNAESTERESQDTVAENDDGGFSEEWEAQRDSHLGPHRSTPESRAA VQELSSSILAGEDPEERGVKLGLGDFIFYSVLVGKASATASGDWNTTIACFVAILIGLCL TLLLLAIFKKALPALPISITFGLVFYFATDYLVQPFMDQLAFHQFYI Click to Show/Hide
|
||||
| 3D-structure |
|
||||
| Function |
Catalytic subunit of the gamma-secretase complex, an endoprotease complex that catalyzes the intramembrane cleavage of integral membrane proteins such as Notch receptors and APP (amyloid-beta precursor protein). Requires the presence of the other members of the gamma-secretase complex for protease activity. Plays a role in Notch and Wnt signaling cascades and regulation of downstream processes via its role in processing key regulatory proteins, and by regulating cytosolic CTNNB1 levels. Stimulates cell-cell adhesion via its interaction with CDH1; this stabilizes the complexes between CDH1 (E-cadherin) and its interaction partners CTNNB1 (beta-catenin), CTNND1 and JUP (gamma-catenin). Under conditions of apoptosis or calcium influx, cleaves CDH1. This promotes the disassembly of the complexes between CDH1 and CTNND1, JUP and CTNNB1, increases the pool of cytoplasmic CTNNB1, and thereby negatively regulates Wnt signaling. Required for normal embryonic brain and skeleton development, and for normal angiogenesis. Mediates the proteolytic cleavage of EphB2/CTF1 into EphB2/CTF2. The holoprotein functions as a calcium-leak channel that allows the passive movement of calcium from endoplasmic reticulum to cytosol and is therefore involved in calcium homeostasis. Involved in the regulation of neurite outgrowth. Is a regulator of presynaptic facilitation, spike transmission and synaptic vesicles replenishment in a process that depends on gamma-secretase activity. It acts through the control of SYT7 presynaptic expression.
Click to Show/Hide
|
||||
| Uniprot ID | |||||
| Ensembl ID | |||||
| HGNC ID | |||||
| Click to Show/Hide the Complete Species Lineage | |||||
Type(s) of Resistant Mechanism of This Molecule
Drug Resistance Data Categorized by Drug
Approved Drug(s)
8 drug(s) in total
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Bladder cancer [ICD-11: 2C94.0] | [1] | |||
| Sensitive Disease | Bladder cancer [ICD-11: 2C94.0] | |||
| Sensitive Drug | Cisplatin | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| DNA damage response signaling pathway | Activation | hsa04218 | ||
| In Vitro Model | 5637 cells | Bladder | Homo sapiens (Human) | CVCL_0126 |
| H-bc cells | Bladder | Homo sapiens (Human) | CVCL_BT00 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Flow cytometry assay | |||
| Mechanism Description | Among the differentially expressed genes between the chemosensitive (5637) and chemoresistant (H-bc) bladder cancer cell lines, the expression level of the PSEN1 gene (presenilin 1), a key component of the Gamma-secretase, is negatively correlated with chemoresistance. A small interfering RNA mediated repression of the PSEN1 gene suppresses cell apoptosis and de-sensitizes 5637 cells, while overexpression of the presenilin 1 sensitizes H-bc cells to the drug-triggered cell death. As a direct target of microRNA-193a-3p that promotes the multi-chemoresistance of the bladder cancer cell, PSEN1 acts as an important executor for the microRNA-193a-3p's positive impact on the multi-chemoresistance of bladder cancer, probably via its activating effect on DNA damage response pathway. In addition to the mechanistic insights, the key players in this microRNA-193a-3p/PSEN1 axis are likely the diagnostic and/or therapeutic targets for an effective chemotherapy of bladder cancer. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Esophageal cancer [ICD-11: 2B70.1] | [2] | |||
| Resistant Disease | Esophageal cancer [ICD-11: 2B70.1] | |||
| Resistant Drug | Docetaxel | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell proliferation | Activation | hsa05200 | ||
| In Vitro Model | KYSE150 cells | Esophagus | Homo sapiens (Human) | CVCL_1348 |
| KYSE510 cells | Esophagus | Homo sapiens (Human) | CVCL_1354 | |
| kYSE410 cells | Esophagus | Homo sapiens (Human) | CVCL_1352 | |
| kYSE450 cells | Esophagus | Homo sapiens (Human) | CVCL_1353 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Over-expression of miR-193a-3p increased the radioresistance and chemoresistance of oesophageal squamous cell carcinoma (ESCC) cells. In contrast, the down-regulation of miR-193a-3p decreased the radioresistance and chemoresistance of ESCC cells. In addition, miR-193a-3p inducing DNA damage has also been demonstrated through measuring the level of gamma-H2AX associated with miR-193a-3p. Moreover, a small interfering RNA(siRNA)-induced repression of the PSEN1 gene had an effect similar to that of miR-193a-3p up-regulation. The above processes also inhibited oesophageal cancer cells apoptosis. These findings suggest that miR-193a-3p contributes to the radiation and chemotherapy resistance of oesophageal carcinoma by down-regulating PSEN1. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Bladder cancer [ICD-11: 2C94.0] | [1] | |||
| Sensitive Disease | Bladder cancer [ICD-11: 2C94.0] | |||
| Sensitive Drug | Doxorubicin | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| DNA damage response signaling pathway | Activation | hsa04218 | ||
| In Vitro Model | 5637 cells | Bladder | Homo sapiens (Human) | CVCL_0126 |
| H-bc cells | Bladder | Homo sapiens (Human) | CVCL_BT00 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Flow cytometry assay | |||
| Mechanism Description | Among the differentially expressed genes between the chemosensitive (5637) and chemoresistant (H-bc) bladder cancer cell lines, the expression level of the PSEN1 gene (presenilin 1), a key component of the Gamma-secretase, is negatively correlated with chemoresistance. A small interfering RNA mediated repression of the PSEN1 gene suppresses cell apoptosis and de-sensitizes 5637 cells, while overexpression of the presenilin 1 sensitizes H-bc cells to the drug-triggered cell death. As a direct target of microRNA-193a-3p that promotes the multi-chemoresistance of the bladder cancer cell, PSEN1 acts as an important executor for the microRNA-193a-3p's positive impact on the multi-chemoresistance of bladder cancer, probably via its activating effect on DNA damage response pathway. In addition to the mechanistic insights, the key players in this microRNA-193a-3p/PSEN1 axis are likely the diagnostic and/or therapeutic targets for an effective chemotherapy of bladder cancer. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Bladder cancer [ICD-11: 2C94.0] | [1] | |||
| Sensitive Disease | Bladder cancer [ICD-11: 2C94.0] | |||
| Sensitive Drug | Epirubicin | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| DNA damage response signaling pathway | Activation | hsa04218 | ||
| In Vitro Model | 5637 cells | Bladder | Homo sapiens (Human) | CVCL_0126 |
| H-bc cells | Bladder | Homo sapiens (Human) | CVCL_BT00 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Flow cytometry assay | |||
| Mechanism Description | Among the differentially expressed genes between the chemosensitive (5637) and chemoresistant (H-bc) bladder cancer cell lines, the expression level of the PSEN1 gene (presenilin 1), a key component of the Gamma-secretase, is negatively correlated with chemoresistance. A small interfering RNA mediated repression of the PSEN1 gene suppresses cell apoptosis and de-sensitizes 5637 cells, while overexpression of the presenilin 1 sensitizes H-bc cells to the drug-triggered cell death. As a direct target of microRNA-193a-3p that promotes the multi-chemoresistance of the bladder cancer cell, PSEN1 acts as an important executor for the microRNA-193a-3p's positive impact on the multi-chemoresistance of bladder cancer, probably via its activating effect on DNA damage response pathway. In addition to the mechanistic insights, the key players in this microRNA-193a-3p/PSEN1 axis are likely the diagnostic and/or therapeutic targets for an effective chemotherapy of bladder cancer. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Esophageal cancer [ICD-11: 2B70.1] | [2] | |||
| Resistant Disease | Esophageal cancer [ICD-11: 2B70.1] | |||
| Resistant Drug | Fluorouracil | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell proliferation | Activation | hsa05200 | ||
| In Vitro Model | KYSE150 cells | Esophagus | Homo sapiens (Human) | CVCL_1348 |
| KYSE510 cells | Esophagus | Homo sapiens (Human) | CVCL_1354 | |
| kYSE410 cells | Esophagus | Homo sapiens (Human) | CVCL_1352 | |
| kYSE450 cells | Esophagus | Homo sapiens (Human) | CVCL_1353 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Over-expression of miR-193a-3p increased the radioresistance and chemoresistance of oesophageal squamous cell carcinoma (ESCC) cells. In contrast, the down-regulation of miR-193a-3p decreased the radioresistance and chemoresistance of ESCC cells. In addition, miR-193a-3p inducing DNA damage has also been demonstrated through measuring the level of gamma-H2AX associated with miR-193a-3p. Moreover, a small interfering RNA(siRNA)-induced repression of the PSEN1 gene had an effect similar to that of miR-193a-3p up-regulation. The above processes also inhibited oesophageal cancer cells apoptosis. These findings suggest that miR-193a-3p contributes to the radiation and chemotherapy resistance of oesophageal carcinoma by down-regulating PSEN1. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Esophageal cancer [ICD-11: 2B70.1] | [2] | |||
| Resistant Disease | Esophageal cancer [ICD-11: 2B70.1] | |||
| Resistant Drug | Paclitaxel | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell proliferation | Activation | hsa05200 | ||
| In Vitro Model | KYSE150 cells | Esophagus | Homo sapiens (Human) | CVCL_1348 |
| KYSE510 cells | Esophagus | Homo sapiens (Human) | CVCL_1354 | |
| kYSE410 cells | Esophagus | Homo sapiens (Human) | CVCL_1352 | |
| kYSE450 cells | Esophagus | Homo sapiens (Human) | CVCL_1353 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Over-expression of miR-193a-3p increased the radioresistance and chemoresistance of oesophageal squamous cell carcinoma (ESCC) cells. In contrast, the down-regulation of miR-193a-3p decreased the radioresistance and chemoresistance of ESCC cells. In addition, miR-193a-3p inducing DNA damage has also been demonstrated through measuring the level of gamma-H2AX associated with miR-193a-3p. Moreover, a small interfering RNA(siRNA)-induced repression of the PSEN1 gene had an effect similar to that of miR-193a-3p up-regulation. The above processes also inhibited oesophageal cancer cells apoptosis. These findings suggest that miR-193a-3p contributes to the radiation and chemotherapy resistance of oesophageal carcinoma by down-regulating PSEN1. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Bladder cancer [ICD-11: 2C94.0] | [1] | |||
| Sensitive Disease | Bladder cancer [ICD-11: 2C94.0] | |||
| Sensitive Drug | Paclitaxel | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| DNA damage response signaling pathway | Activation | hsa04218 | ||
| In Vitro Model | 5637 cells | Bladder | Homo sapiens (Human) | CVCL_0126 |
| H-bc cells | Bladder | Homo sapiens (Human) | CVCL_BT00 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Flow cytometry assay | |||
| Mechanism Description | Among the differentially expressed genes between the chemosensitive (5637) and chemoresistant (H-bc) bladder cancer cell lines, the expression level of the PSEN1 gene (presenilin 1), a key component of the Gamma-secretase, is negatively correlated with chemoresistance. A small interfering RNA mediated repression of the PSEN1 gene suppresses cell apoptosis and de-sensitizes 5637 cells, while overexpression of the presenilin 1 sensitizes H-bc cells to the drug-triggered cell death. As a direct target of microRNA-193a-3p that promotes the multi-chemoresistance of the bladder cancer cell, PSEN1 acts as an important executor for the microRNA-193a-3p's positive impact on the multi-chemoresistance of bladder cancer, probably via its activating effect on DNA damage response pathway. In addition to the mechanistic insights, the key players in this microRNA-193a-3p/PSEN1 axis are likely the diagnostic and/or therapeutic targets for an effective chemotherapy of bladder cancer. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Bladder cancer [ICD-11: 2C94.0] | [1] | |||
| Sensitive Disease | Bladder cancer [ICD-11: 2C94.0] | |||
| Sensitive Drug | Pirarubicin | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| DNA damage response signaling pathway | Activation | hsa04218 | ||
| In Vitro Model | 5637 cells | Bladder | Homo sapiens (Human) | CVCL_0126 |
| H-bc cells | Bladder | Homo sapiens (Human) | CVCL_BT00 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Flow cytometry assay | |||
| Mechanism Description | Among the differentially expressed genes between the chemosensitive (5637) and chemoresistant (H-bc) bladder cancer cell lines, the expression level of the PSEN1 gene (presenilin 1), a key component of the Gamma-secretase, is negatively correlated with chemoresistance. A small interfering RNA mediated repression of the PSEN1 gene suppresses cell apoptosis and de-sensitizes 5637 cells, while overexpression of the presenilin 1 sensitizes H-bc cells to the drug-triggered cell death. As a direct target of microRNA-193a-3p that promotes the multi-chemoresistance of the bladder cancer cell, PSEN1 acts as an important executor for the microRNA-193a-3p's positive impact on the multi-chemoresistance of bladder cancer, probably via its activating effect on DNA damage response pathway. In addition to the mechanistic insights, the key players in this microRNA-193a-3p/PSEN1 axis are likely the diagnostic and/or therapeutic targets for an effective chemotherapy of bladder cancer. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Esophageal cancer [ICD-11: 2B70.1] | [2] | |||
| Resistant Disease | Esophageal cancer [ICD-11: 2B70.1] | |||
| Resistant Drug | Vinorelbine | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell proliferation | Activation | hsa05200 | ||
| In Vitro Model | KYSE150 cells | Esophagus | Homo sapiens (Human) | CVCL_1348 |
| KYSE510 cells | Esophagus | Homo sapiens (Human) | CVCL_1354 | |
| kYSE410 cells | Esophagus | Homo sapiens (Human) | CVCL_1352 | |
| kYSE450 cells | Esophagus | Homo sapiens (Human) | CVCL_1353 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Over-expression of miR-193a-3p increased the radioresistance and chemoresistance of oesophageal squamous cell carcinoma (ESCC) cells. In contrast, the down-regulation of miR-193a-3p decreased the radioresistance and chemoresistance of ESCC cells. In addition, miR-193a-3p inducing DNA damage has also been demonstrated through measuring the level of gamma-H2AX associated with miR-193a-3p. Moreover, a small interfering RNA(siRNA)-induced repression of the PSEN1 gene had an effect similar to that of miR-193a-3p up-regulation. The above processes also inhibited oesophageal cancer cells apoptosis. These findings suggest that miR-193a-3p contributes to the radiation and chemotherapy resistance of oesophageal carcinoma by down-regulating PSEN1. | |||
Disease- and Tissue-specific Abundances of This Molecule
ICD Disease Classification 02

| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Esophagus | |
| The Specified Disease | Esophageal cancer | |
| The Expression Level of Disease Section Compare with the Adjacent Tissue | p-value: 1.49E-02; Fold-change: -6.23E-01; Z-score: -2.14E+00 | |
|
Molecule expression in the normal tissue adjacent to the diseased tissue of patients
Molecule expression in the diseased tissue of patients
|
||
| Disease-specific Molecule Abundances |
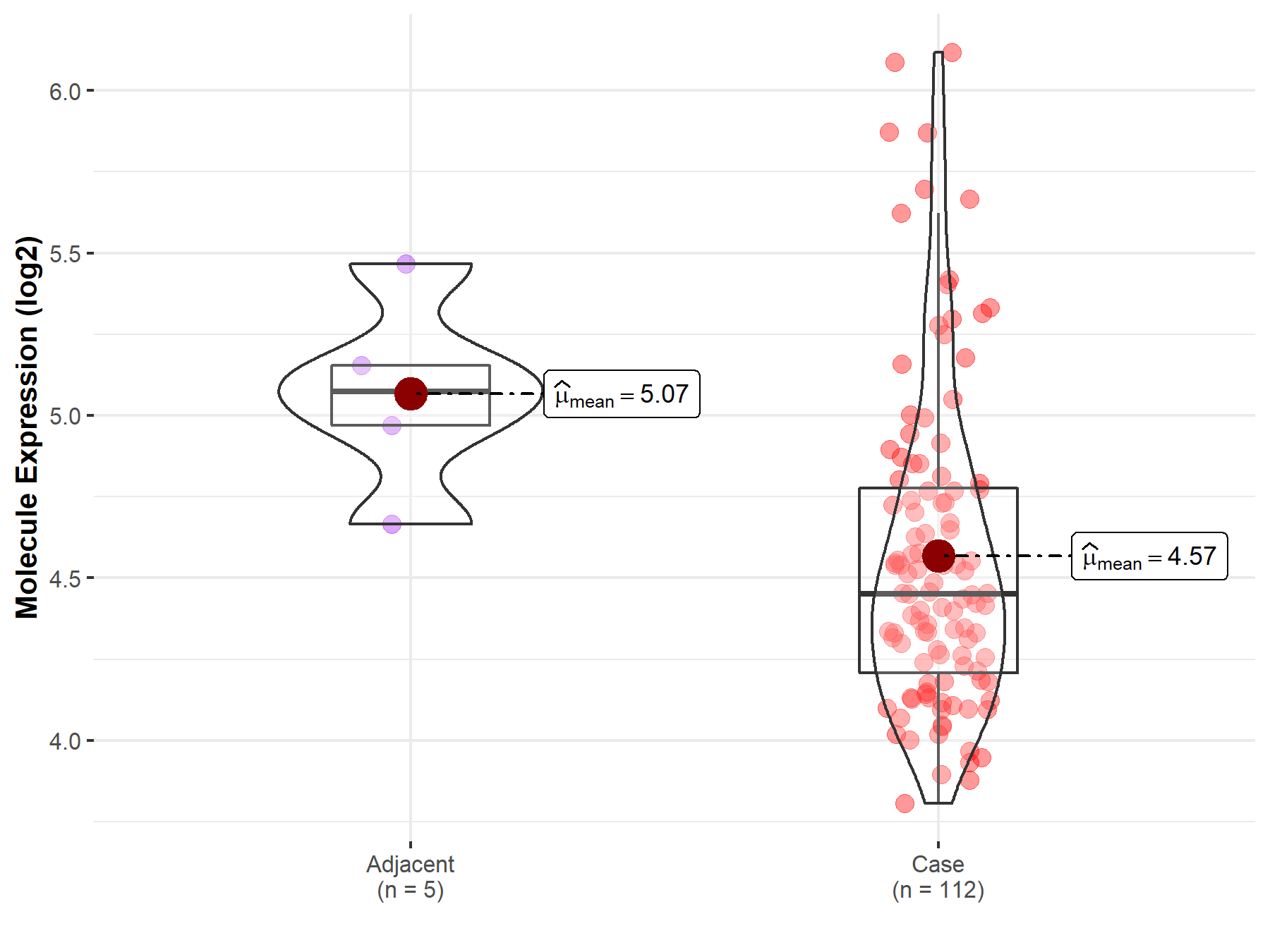
|
Click to View the Clearer Original Diagram |
| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Bladder tissue | |
| The Specified Disease | Bladder cancer | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 7.61E-01; Fold-change: -1.93E-01; Z-score: -6.14E-01 | |
|
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |

|
Click to View the Clearer Original Diagram |
Tissue-specific Molecule Abundances in Healthy Individuals

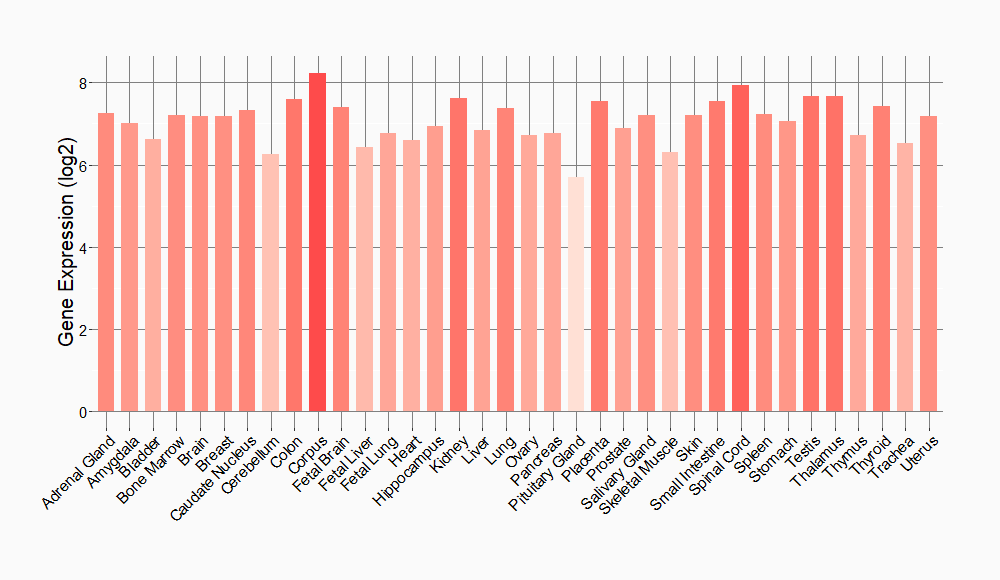
|
||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
