Molecule Information
General Information of the Molecule (ID: Mol00065)
| Name |
Receptor tyrosine-protein kinase erbB-2 (ERBB2)
,Homo sapiens
|
||||
|---|---|---|---|---|---|
| Molecule Type |
Protein
|
||||
| Gene Name |
ERBB2
|
||||
| Gene ID | |||||
| Location |
chr17:39687914-39730426[+]
|
||||
| Sequence |
MELAALCRWGLLLALLPPGAASTQVCTGTDMKLRLPASPETHLDMLRHLYQGCQVVQGNL
ELTYLPTNASLSFLQDIQEVQGYVLIAHNQVRQVPLQRLRIVRGTQLFEDNYALAVLDNG DPLNNTTPVTGASPGGLRELQLRSLTEILKGGVLIQRNPQLCYQDTILWKDIFHKNNQLA LTLIDTNRSRACHPCSPMCKGSRCWGESSEDCQSLTRTVCAGGCARCKGPLPTDCCHEQC AAGCTGPKHSDCLACLHFNHSGICELHCPALVTYNTDTFESMPNPEGRYTFGASCVTACP YNYLSTDVGSCTLVCPLHNQEVTAEDGTQRCEKCSKPCARVCYGLGMEHLREVRAVTSAN IQEFAGCKKIFGSLAFLPESFDGDPASNTAPLQPEQLQVFETLEEITGYLYISAWPDSLP DLSVFQNLQVIRGRILHNGAYSLTLQGLGISWLGLRSLRELGSGLALIHHNTHLCFVHTV PWDQLFRNPHQALLHTANRPEDECVGEGLACHQLCARGHCWGPGPTQCVNCSQFLRGQEC VEECRVLQGLPREYVNARHCLPCHPECQPQNGSVTCFGPEADQCVACAHYKDPPFCVARC PSGVKPDLSYMPIWKFPDEEGACQPCPINCTHSCVDLDDKGCPAEQRASPLTSIISAVVG ILLVVVLGVVFGILIKRRQQKIRKYTMRRLLQETELVEPLTPSGAMPNQAQMRILKETEL RKVKVLGSGAFGTVYKGIWIPDGENVKIPVAIKVLRENTSPKANKEILDEAYVMAGVGSP YVSRLLGICLTSTVQLVTQLMPYGCLLDHVRENRGRLGSQDLLNWCMQIAKGMSYLEDVR LVHRDLAARNVLVKSPNHVKITDFGLARLLDIDETEYHADGGKVPIKWMALESILRRRFT HQSDVWSYGVTVWELMTFGAKPYDGIPAREIPDLLEKGERLPQPPICTIDVYMIMVKCWM IDSECRPRFRELVSEFSRMARDPQRFVVIQNEDLGPASPLDSTFYRSLLEDDDMGDLVDA EEYLVPQQGFFCPDPAPGAGGMVHHRHRSSSTRSGGGDLTLGLEPSEEEAPRSPLAPSEG AGSDVFDGDLGMGAAKGLQSLPTHDPSPLQRYSEDPTVPLPSETDGYVAPLTCSPQPEYV NQPDVRPQPPSPREGPLPAARPAGATLERPKTLSPGKNGVVKDVFAFGGAVENPEYLTPQ GGAAPQPHPPPAFSPAFDNLYYWDQDPPERGAPPSTFKGTPTAENPEYLGLDVPV Click to Show/Hide
|
||||
| 3D-structure |
|
||||
| Function |
Protein tyrosine kinase that is part of several cell surface receptor complexes, but that apparently needs a coreceptor for ligand binding. Essential component of a neuregulin-receptor complex, although neuregulins do not interact with it alone. GP30 is a potential ligand for this receptor. Regulates outgrowth and stabilization of peripheral microtubules (MTs). Upon ERBB2 activation, the MEMO1-RHOA-DIAPH1 signaling pathway elicits the phosphorylation and thus the inhibition of GSK3B at cell membrane. This prevents the phosphorylation of APC and CLASP2, allowing its association with the cell membrane. In turn, membrane-bound APC allows the localization of MACF1 to the cell membrane, which is required for microtubule capture and stabilization.
Click to Show/Hide
|
||||
| Uniprot ID | |||||
| Ensembl ID | |||||
| HGNC ID | |||||
| Click to Show/Hide the Complete Species Lineage | |||||
Type(s) of Resistant Mechanism of This Molecule
Drug Resistance Data Categorized by Drug
Approved Drug(s)
16 drug(s) in total
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Breast cancer [ICD-11: 2C60.3] | [1] | |||
| Resistant Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Resistant Drug | Trastuzumab | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Breast cancer [ICD-11: 2C60] | |||
| The Specified Disease | Breast cancer | |||
| The Studied Tissue | Breast tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 3.50E-77 Fold-change: 2.67E-01 Z-score: 1.98E+01 |
|||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell invasion | Activation | hsa05200 | |
| Cell migration | Activation | hsa04670 | ||
| Cell viability | Inhibition | hsa05200 | ||
| miR125b/HER2/Snail1 signaling pathway | Regulation | N.A. | ||
| In Vitro Model | SkBR3 cells | Breast | Homo sapiens (Human) | CVCL_0033 |
| BT474 cells | Breast | Homo sapiens (Human) | CVCL_0179 | |
| In Vivo Model | BALB/c nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay; Wound-healing assay; Transwell assay | |||
| Mechanism Description | TINCR, which is transcriptionally activated by H3k27 acetylation, upregulates HER-2 expression by downregulating miR-125b and TINCR promotes trastuzumab resistance-induced EMT by directly targeting Snail-1. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: HER2 positive breast cancer [ICD-11: 2C60.8] | [17] | |||
| Sensitive Disease | HER2 positive breast cancer [ICD-11: 2C60.8] | |||
| Sensitive Drug | Trastuzumab | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell invasion | Inhibition | hsa05200 | |
| Cell proliferation | Inhibition | hsa05200 | ||
| HER2 signaling pathway | Activation | hsa04012 | ||
| In Vitro Model | SkBR3 cells | Breast | Homo sapiens (Human) | CVCL_0033 |
| BT474 cells | Breast | Homo sapiens (Human) | CVCL_0179 | |
| Experiment for Molecule Alteration |
Western blot analysis; RT-qPCR | |||
| Experiment for Drug Resistance |
WST1 assay | |||
| Mechanism Description | miR-770-5p overexpression downregulated HER2 and increased the effect of trastuzumab. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Colorectal cancer [ICD-11: 2B91.1] | [2] | |||
| Resistant Disease | Colorectal cancer [ICD-11: 2B91.1] | |||
| Resistant Drug | Cetuximab | |||
| Molecule Alteration | Structural variation | Amplification |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vivo Model | A retrospective survey in conducting clinical studies | Homo sapiens | ||
| Experiment for Molecule Alteration |
Next-generation sequencing assay | |||
| Experiment for Drug Resistance |
Liquid biopsy assay | |||
| Mechanism Description | Mechanisms of resistance to EGFR blockade include the emergence of kRAS, NRAS and EGFR extracellular domain mutations as well as HER2/MET alterations. | |||
| Disease Class: Colorectal cancer [ICD-11: 2B91.1] | [3] | |||
| Resistant Disease | Colorectal cancer [ICD-11: 2B91.1] | |||
| Resistant Drug | Cetuximab | |||
| Molecule Alteration | Structural variation | Amplification |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Experiment for Molecule Alteration |
Sanger sequencing assay; Next-generation sequencing assay | |||
| Mechanism Description | Mutations in kRAS, NRAS, and BRAF and amplification of ERBB2 and MET drive primary (de novo) resistance to anti-EGFR treatment. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Gastric cancer [ICD-11: 2B72.1] | [4] | |||
| Sensitive Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Sensitive Drug | Cisplatin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell proliferation | Inhibition | hsa05200 | ||
| mTOR signaling pathway | Inhibition | hsa04150 | ||
| In Vitro Model | SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay; Flow cytometry assay | |||
| Mechanism Description | The miR-495 exerts promotive effects on GC chemosensitivity via inactivation of the mTOR signaling pathway by suppressing ERBB2. | |||
| Disease Class: Gastric cancer [ICD-11: 2B72.1] | [5] | |||
| Sensitive Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Sensitive Drug | Cisplatin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell viability | Inhibition | hsa05200 | |
| In Vitro Model | BGC-823 cells | Gastric | Homo sapiens (Human) | CVCL_3360 |
| MGC-803 cells | Gastric | Homo sapiens (Human) | CVCL_5334 | |
| SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 | |
| AGS cells | Gastric | Homo sapiens (Human) | CVCL_0139 | |
| HGC27 cells | Gastric | Homo sapiens (Human) | CVCL_1279 | |
| NCI-N87 cells | Gastric | Homo sapiens (Human) | CVCL_1603 | |
| MkN-45 cells | Gastric | Homo sapiens (Human) | CVCL_0434 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | Overexpression of miR-125b improved the chemosensitivity of DDP in HGC-27 and MGC-803 cells and miR-125b obviously inhibited the expression of HER2 at protein level in HGC-27 and MGC-803 cells. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [6] | |||
| Resistant Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Resistant Drug | Dacomitinib | |||
| Molecule Alteration | Duplication | p.Y772_A775 (c.2314_2325) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 |
| Experiment for Molecule Alteration |
Sanger cDNA sequencing assay | |||
| Experiment for Drug Resistance |
CCK-8 assay | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [7] | |||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Sensitive Drug | Dacomitinib | |||
| Molecule Alteration | Complex-indel | p.M774_774delinsWLV (c.2320_2322delinsTGGCTTGTA) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | NSCLC cells | Lung | Homo sapiens (Human) | N.A. |
| Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 | |
| In Vivo Model | BALB/c nude mouse PDX model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis; SDS-PAGE assay | |||
| Experiment for Drug Resistance |
MTS assay; Crystal violet staining assay | |||
| Mechanism Description | Mutation in the covalent binding site of either EGFR or HER2 is sufficient to lead to drug resistance. | |||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [7] | |||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Sensitive Drug | Dacomitinib | |||
| Molecule Alteration | Duplication | p.G778_P780 (c.2332_2340) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | NSCLC cells | Lung | Homo sapiens (Human) | N.A. |
| Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 | |
| In Vivo Model | BALB/c nude mouse PDX model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis; SDS-PAGE assay | |||
| Experiment for Drug Resistance |
MTS assay; Crystal violet staining assay | |||
| Mechanism Description | Mutation in the covalent binding site of either EGFR or HER2 is sufficient to lead to drug resistance. | |||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [7] | |||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Sensitive Drug | Dacomitinib | |||
| Molecule Alteration | IF-insertion | p.G778_S779insCPG (c.2335_2336insGTCCTGGTT) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | NSCLC cells | Lung | Homo sapiens (Human) | N.A. |
| Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 | |
| In Vivo Model | BALB/c nude mouse PDX model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis; SDS-PAGE assay | |||
| Experiment for Drug Resistance |
MTS assay; Crystal violet staining assay | |||
| Mechanism Description | Mutation in the covalent binding site of either EGFR or HER2 is sufficient to lead to drug resistance. | |||
| Disease Class: Lung adenocarcinoma [ICD-11: 2C25.0] | [8] | |||
| Sensitive Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | |||
| Sensitive Drug | Dacomitinib | |||
| Molecule Alteration | Complex-indel | p.M774_774delinsWLV (c.2320_2322delinsTGGCTTGTA) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Lung | N.A. | ||
| Experiment for Molecule Alteration |
DNA sequencing assay | |||
| Experiment for Drug Resistance |
Clinical measurement assay | |||
| Disease Class: Lung adenocarcinoma [ICD-11: 2C25.0] | [8] | |||
| Sensitive Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | |||
| Sensitive Drug | Dacomitinib | |||
| Molecule Alteration | Duplication | p.G778_P780 (c.2332_2340) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Lung | N.A. | ||
| Experiment for Molecule Alteration |
DNA sequencing assay | |||
| Experiment for Drug Resistance |
Clinical measurement assay | |||
| Disease Class: Lung adenocarcinoma [ICD-11: 2C25.0] | [8] | |||
| Sensitive Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | |||
| Sensitive Drug | Dacomitinib | |||
| Molecule Alteration | IF-insertion | p.P780_Y781 (c.2340_2341) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Lung | N.A. | ||
| Experiment for Molecule Alteration |
DNA sequencing assay | |||
| Experiment for Drug Resistance |
Clinical measurement assay | |||
| Disease Class: Lung adenocarcinoma [ICD-11: 2C25.0] | [8] | |||
| Sensitive Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | |||
| Sensitive Drug | Dacomitinib | |||
| Molecule Alteration | Complex-indel | p.M774delinsWLV (c.2320delinsTGGCTGG) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Lung | N.A. | ||
| Experiment for Molecule Alteration |
DNA sequencing assay | |||
| Experiment for Drug Resistance |
Clinical measurement assay | |||
| Disease Class: Lung adenocarcinoma [ICD-11: 2C25.0] | [8] | |||
| Sensitive Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | |||
| Sensitive Drug | Dacomitinib | |||
| Molecule Alteration | Duplication | p.P780_Y781 (c.2340_2341) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Lung | N.A. | ||
| Experiment for Molecule Alteration |
DNA sequencing assay | |||
| Experiment for Drug Resistance |
Clinical measurement assay | |||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [6] | |||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Sensitive Drug | Dacomitinib | |||
| Molecule Alteration | Complex-indel | p.G776_776delinsVC (c.2326_2328delinsGTATGT) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 |
| Experiment for Molecule Alteration |
Sanger cDNA sequencing assay | |||
| Experiment for Drug Resistance |
CCK-8 assay | |||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [6] | |||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Sensitive Drug | Dacomitinib | |||
| Molecule Alteration | Duplication | p.G778_P780 (c.2332_2340)/p.780_Y781insGSP |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 |
| Experiment for Molecule Alteration |
Sanger cDNA sequencing assay | |||
| Experiment for Drug Resistance |
CCK-8 assay | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Breast cancer [ICD-11: 2C60.3] | [9] | |||
| Sensitive Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Sensitive Drug | Doxorubicin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| PI3K/AKT signaling pathway | Inhibition | hsa04151 | ||
| In Vitro Model | SkBR3 cells | Breast | Homo sapiens (Human) | CVCL_0033 |
| MDA-MB-231 cells | Breast | Homo sapiens (Human) | CVCL_0062 | |
| MDA-MB-453 cells | Breast | Homo sapiens (Human) | CVCL_0418 | |
| MDA-MB-468 cells | Breast | Homo sapiens (Human) | CVCL_0419 | |
| Experiment for Molecule Alteration |
Western blot analysis; Dual luciferase reporter assay | |||
| Experiment for Drug Resistance |
CCK8 assay; Flow cytometric analysis; Transwell invasion assay | |||
| Mechanism Description | miR1268b confers chemosensitivity in breast cancer by targeting ERBB2-mediated PI3k-AkT pathway. miR1268b could repress the PI3k-AkT signaling pathway by targeting ERBB2 and inhibit the anti-apoptosis protein Bcl2. | |||
| Disease Class: Gastric cancer [ICD-11: 2B72.1] | [4] | |||
| Sensitive Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Sensitive Drug | Doxorubicin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell proliferation | Inhibition | hsa05200 | ||
| mTOR signaling pathway | Inhibition | hsa04150 | ||
| In Vitro Model | SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay; Flow cytometry assay | |||
| Mechanism Description | The miR-495 exerts promotive effects on GC chemosensitivity via inactivation of the mTOR signaling pathway by suppressing ERBB2. | |||
| Disease Class: Chondrosarcoma [ICD-11: 2B50.0] | [10] | |||
| Sensitive Disease | Chondrosarcoma [ICD-11: 2B50.0] | |||
| Sensitive Drug | Doxorubicin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell proliferation | Inhibition | hsa05200 | ||
| Glucose metabolism signaling pathway | Regulation | N.A. | ||
| In Vitro Model | CH-2879 cells | Bone | Homo sapiens (Human) | CVCL_9921 |
| OUMS-27 cells | Bone | Homo sapiens (Human) | CVCL_3090 | |
| SW1353 cells | Bone | Homo sapiens (Human) | CVCL_0543 | |
| CS-1 cells | Bone | Homo sapiens (Human) | CVCL_T023 | |
| CSPG cells | Bone | Homo sapiens (Human) | N.A. | |
| JJ012 cells | Bone | Homo sapiens (Human) | CVCL_D605 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miR-125b was downregulated in chondrosarcoma cells compared with normal human chondrocytes. More importantly, miR-125b was downregulated in doxorubicin resistant cancer cells, with its overexpression enhancing doxorubicin-induced cytotoxicity and apoptosis, subsequently increasing the sensitivity of chondrosarcoma cells to doxorubicin. ErbB2 was a direct target of miR-125b in chondrosarcoma cells. The inhibition of ErbB2 by overexpression of miR-125b led to suppression of glucose metabolism, which rendered chondrosarcoma cells susceptible to doxorubicin. Restoring the expression of ErbB2 and glucose metabolic enzymes recovered doxorubicin resistance in counteracting miR-125b-mediated sensitivity. Taken together, miR-125b plays a critical role in doxorubicin resistance through suppression of ErbB2-induced glucose metabolism, and it may serve as a potential target for overcoming chemoresistance in human chondrosarcoma. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [6] | |||
| Resistant Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Resistant Drug | Erlotinib | |||
| Molecule Alteration | Duplication | p.Y772_A775 (c.2314_2325)/p.A775_G776insYVMA |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 |
| Experiment for Molecule Alteration |
Sanger cDNA sequencing assay | |||
| Experiment for Drug Resistance |
CCK-8 assay | |||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [6] | |||
| Resistant Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Resistant Drug | Erlotinib | |||
| Molecule Alteration | Complex-indel | p.G776_776delinsVC (c.2326_2328delinsGTATGT) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 |
| Experiment for Molecule Alteration |
Sanger cDNA sequencing assay | |||
| Experiment for Drug Resistance |
CCK-8 assay | |||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [6] | |||
| Resistant Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Resistant Drug | Erlotinib | |||
| Molecule Alteration | Duplication | p.G778_P780 (c.2332_2340)/p.780_Y781insGSP |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 |
| Experiment for Molecule Alteration |
Sanger cDNA sequencing assay | |||
| Experiment for Drug Resistance |
CCK-8 assay | |||
|
|
||||
| Disease Class: EGFR-mutant non-small cell lung cancer [ICD-11: 2C25.7] | [11] | |||
| Resistant Disease | EGFR-mutant non-small cell lung cancer [ICD-11: 2C25.7] | |||
| Resistant Drug | Erlotinib | |||
| Molecule Alteration | Structural variation | Copy number gain |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Experiment for Molecule Alteration |
Low throughput experiment assay | |||
| Experiment for Drug Resistance |
Progression-free survival assay | |||
| Mechanism Description | Acquired resistance can occur through failure of drug delivery to the target, as in isolated central nervous system (CNS) progression, or by selection of biological variants during TkI exposure. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Gastric cancer [ICD-11: 2B72.1] | [4] | |||
| Sensitive Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Sensitive Drug | Fluorouracil | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell proliferation | Inhibition | hsa05200 | ||
| mTOR signaling pathway | Inhibition | hsa04150 | ||
| In Vitro Model | SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay; Flow cytometry assay | |||
| Mechanism Description | The miR-495 exerts promotive effects on GC chemosensitivity via inactivation of the mTOR signaling pathway by suppressing ERBB2. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: EGFR-mutant non-small cell lung cancer [ICD-11: 2C25.7] | [11] | |||
| Resistant Disease | EGFR-mutant non-small cell lung cancer [ICD-11: 2C25.7] | |||
| Resistant Drug | Gefitinib | |||
| Molecule Alteration | Structural variation | Copy number gain |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Experiment for Molecule Alteration |
Low throughput experiment assay | |||
| Experiment for Drug Resistance |
Progression-free survival assay | |||
| Mechanism Description | Acquired resistance can occur through failure of drug delivery to the target, as in isolated central nervous system (CNS) progression, or by selection of biological variants during TkI exposure. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: HER2 positive breast cancer [ICD-11: 2C60.8] | [12] | |||
| Resistant Disease | HER2 positive breast cancer [ICD-11: 2C60.8] | |||
| Resistant Drug | Lapatinib | |||
| Molecule Alteration | Missense mutation | p.L755S (c.2263_2264delCTinsAG) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | HER2 signaling pathway | Activation | hsa04012 | |
| In Vitro Model | AU565 cells | Breast | Homo sapiens (Human) | CVCL_1074 |
| SkBR3 cells | Breast | Homo sapiens (Human) | CVCL_0033 | |
| BT474/AZ cells | Breast | Homo sapiens (Human) | CVCL_0179 | |
| In Vivo Model | Athymic mouse PDX model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Promega assay | |||
| Mechanism Description | HER2 reactivation through acquisition of the HER2L755S mutation was identified as a mechanism of acquired resistance to L-containing HER2-targeted therapy in preclinical HER2-amplified breast cancer models, which can be overcome by irreversible HER1/2 inhibitors. | |||
| Disease Class: HER2 positive breast cancer [ICD-11: 2C60.8] | [13] | |||
| Resistant Disease | HER2 positive breast cancer [ICD-11: 2C60.8] | |||
| Resistant Drug | Lapatinib | |||
| Molecule Alteration | Missense mutation | p.T798M (c.2393_2394delCAinsTG) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | BT474 cells | Breast | Homo sapiens (Human) | CVCL_0179 |
| MCF10A cells | Breast | Homo sapiens (Human) | CVCL_0598 | |
| In Vivo Model | Athymic female mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
HER2T798M sequencing assay | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | The missense mutation p.T798M (c.2393_2394delCAinsTG) in gene ERBB2 cause the resistance of Lapatinib by aberration of the drug's therapeutic target | |||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [6] | |||
| Resistant Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Resistant Drug | Lapatinib | |||
| Molecule Alteration | Duplication | p.Y772_A775 (c.2314_2325)/p.A775_G776insYVMA |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 |
| Experiment for Molecule Alteration |
Sanger cDNA sequencing assay | |||
| Experiment for Drug Resistance |
CCK-8 assay | |||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [6] | |||
| Resistant Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Resistant Drug | Lapatinib | |||
| Molecule Alteration | Complex-indel | p.G776_776delinsVC (c.2326_2328delinsGTATGT) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 |
| Experiment for Molecule Alteration |
Sanger cDNA sequencing assay | |||
| Experiment for Drug Resistance |
CCK-8 assay | |||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [6] | |||
| Resistant Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Resistant Drug | Lapatinib | |||
| Molecule Alteration | Duplication | p.G778_P780 (c.2332_2340)/p.780_Y781insGSP |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 |
| Experiment for Molecule Alteration |
Sanger cDNA sequencing assay | |||
| Experiment for Drug Resistance |
CCK-8 assay | |||
|
|
||||
| Disease Class: Breast cancer [ICD-11: 2C60.3] | [14] | |||
| Resistant Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Resistant Drug | Lapatinib | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell proliferation | Activation | hsa05200 | |
| ERRB2/3 signaling pathway | Activation | hsa04210 | ||
| In Vitro Model | ZR75-1 cells | Breast | Homo sapiens (Human) | CVCL_0588 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
WST-1 proliferation assay | |||
| Mechanism Description | Breast Cancer Anti-Estrogen Resistance 4 (BCAR4) Drives Proliferation of IPH-926 lobular Carcinoma Cells. Relative high BCAR4 mRNA expression was identified in IPH-926, a cell line derived from an endocrine-resistant lobular breast cancer. Moderate BCAR4 expression was evident in MDA-MB-134 and MDA-MB-453 breast cancer cells. BCAR4 protein was detected in breast cancer cells with ectopic (ZR-75-1-BCAR4) and endogenous (IPH-926, MDA-MB-453) BCAR4 mRNA expression. knockdown of BCAR4 inhibited cell proliferation. A similar effect was observed upon knockdown of ERBB2/3 and exposure to lapatinib, implying that BCAR4 acts in an ERBB2/3-dependent manner.BCAR4 encodes a functional protein, which drives proliferation of endocrine-resistant breast cancer cells. Lapatinib, a clinically approved EGFR/ERBB2 inhibitor, counteracts BCAR4-driven tumor cell growth, a clinical relevant observation. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Gastric cancer [ICD-11: 2B72.1] | [4] | |||
| Sensitive Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Sensitive Drug | Mitomycin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell proliferation | Inhibition | hsa05200 | ||
| mTOR signaling pathway | Inhibition | hsa04150 | ||
| In Vitro Model | SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay; Flow cytometry assay | |||
| Mechanism Description | The miR-495 exerts promotive effects on GC chemosensitivity via inactivation of the mTOR signaling pathway by suppressing ERBB2. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [6] | |||
| Resistant Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Resistant Drug | Neratinib | |||
| Molecule Alteration | Duplication | p.G778_P780 (c.2332_2340)/p.780_Y781insGSP |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 |
| Experiment for Molecule Alteration |
Sanger cDNA sequencing assay | |||
| Experiment for Drug Resistance |
CCK-8 assay | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | |||||||||||||
|
|
|||||||||||||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [6] | ||||||||||||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | ||||||||||||
| Sensitive Drug | Neratinib | ||||||||||||
| Molecule Alteration | Duplication | p.Y772_A775 (c.2314_2325)/p.A775_G776insYVMA |
|||||||||||
| Experimental Note | Revealed Based on the Cell Line Data | ||||||||||||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 | |||||||||
| Experiment for Molecule Alteration |
Sanger cDNA sequencing assay | ||||||||||||
| Experiment for Drug Resistance |
CCK-8 assay | ||||||||||||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [6] | ||||||||||||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | ||||||||||||
| Sensitive Drug | Neratinib | ||||||||||||
| Molecule Alteration | Complex-indel | p.G776_776delinsVC (c.2326_2328delinsGTATGT) |
|||||||||||
| Experimental Note | Revealed Based on the Cell Line Data | ||||||||||||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 | |||||||||
| Experiment for Molecule Alteration |
Sanger cDNA sequencing assay | ||||||||||||
| Experiment for Drug Resistance |
CCK-8 assay | ||||||||||||
|
|
|||||||||||||
| Disease Class: HER2 negative breast cancer [ICD-11: 2C60.11] | [15] | ||||||||||||
| Sensitive Disease | HER2 negative breast cancer [ICD-11: 2C60.11] | ||||||||||||
| Sensitive Drug | Neratinib | ||||||||||||
| Molecule Alteration | Missense mutation | p.S310F (c.929C>T) |
|||||||||||
| Wild Type Structure | Method: X-ray diffraction | Resolution: 3.09 Å | |||||||||||
| Mutant Type Structure | Method: Electron microscopy | Resolution: 3.09 Å | |||||||||||
| Download The Information of Sequence | Download The Structure File | ||||||||||||
-
T
M
Q
E
V
L
C
A
T
A
G
L
T
C
D
R
M
W
10
|
K
G
L
L
R
L
L
L
P
A
A
L
S
L
P
P
E
P
T
G
20
|
H
A
L
A
D
S
M
T
L
Q
R
V
H
C
L
T
Y
G
Q
T
30
|
G
D
C
M
Q
K
V
L
V
R
Q
L
G
P
N
A
L
S
E
P
40
|
L
E
T
T
Y
H
L
L
P
D
T
M
N
L
A
R
S
H
L
L
50
|
S
Y
F
Q
L
G
Q
C
D
Q
I
V
Q
V
E
Q
V
G
Q
N
60
|
G
L
Y
E
V
L
L
T
I
Y
A
L
H
P
N
T
Q
N
V
A
70
|
R
S
Q
L
V
S
P
F
L
L
Q
Q
R
D
L
I
R
Q
I
E
80
|
V
V
R
Q
G
G
T
Y
Q
V
L
L
F
I
E
A
D
H
N
N
90
|
Y
Q
A
V
L
R
A
Q
V
V
L
P
D
L
N
Q
G
R
D
L
100
|
P
R
L
I
N
V
N
R
T
G
T
T
P
Q
V
L
T
F
G
E
110
|
A
D
S
N
P
Y
G
A
G
L
L
A
R
V
E
L
L
D
Q
N
120
|
L
G
R
D
S
P
L
L
T
N
E
N
I
T
L
T
K
P
G
V
130
|
G
T
V
G
L
A
I
S
Q
P
R
G
N
G
P
L
Q
R
L
E
140
|
C
L
Y
Q
Q
L
D
R
T
S
I
L
L
T
W
E
K
I
D
L
150
|
I
K
F
G
H
G
K
V
N
L
N
I
Q
Q
L
R
A
N
L
P
160
|
T
Q
L
L
I
C
D
Y
T
Q
N
D
R
T
S
I
R
L
A
W
170
|
C
K
H
D
P
I
C
F
S
H
P
K
M
N
C
N
K
Q
G
L
180
|
S
A
R
L
C
T
W
L
G
I
E
D
S
T
S
N
E
R
D
S
190
|
C
R
Q
A
S
C
L
H
T
P
R
C
T
S
V
P
C
M
A
C
200
|
G
K
G
G
C
S
A
R
R
C
C
W
K
G
G
E
P
S
L
S
210
|
P
E
T
D
D
C
C
Q
C
S
H
L
E
T
Q
R
C
T
A
V
220
|
A
C
G
A
C
G
T
G
G
C
P
A
K
R
H
C
S
K
D
G
230
|
C
P
L
L
A
P
C
T
L
D
H
C
F
C
N
H
H
E
S
Q
240
|
G
C
I
A
C
A
E
G
L
C
H
T
C
G
P
P
A
K
L
H
250
|
V
S
T
D
Y
C
N
L
T
A
D
C
T
L
F
H
E
F
S
N
260
|
M
H
P
S
N
G
P
I
E
C
G
E
R
L
Y
H
T
C
F
P
270
|
G
A
A
L
S
V
C
T
V
Y
T
N
A
T
C
D
P
T
Y
F
280
|
N
E
Y
S
L
M
S
P
T
N
D
P
V
E
G
G
S
R
C
Y
290
|
T
T
L
F
V
G
C
A
P
S
L
C
H
V
N
T
Q
A
E
C
300
|
V
P
T
Y
A
N
E
Y
D
L
G
S
T
T
Q
D
R
V
C
G
310
|
E
F
K
C
C
T
S
L
K
V
P
C
C
P
A
L
R
H
V
N
320
|
C
Q
Y
E
G
V
L
T
G
A
M
E
E
D
H
G
L
T
R
Q
330
|
E
R
V
C
R
E
A
K
V
C
T
S
S
K
A
P
N
C
I
A
340
|
Q
R
E
V
F
C
A
Y
G
G
C
L
K
G
K
M
I
E
F
H
350
|
G
L
S
R
L
E
A
V
F
R
L
A
P
V
E
T
S
S
F
A
360
|
D
N
G
I
D
Q
P
E
A
F
S
A
N
G
T
C
A
K
P
K
370
|
L
I
Q
F
P
G
E
S
Q
L
L
A
Q
F
V
L
F
P
E
E
380
|
T
S
L
F
E
D
E
G
I
D
T
P
G
A
Y
S
L
N
Y
T
390
|
I
A
S
P
A
L
W
Q
P
P
D
E
S
Q
L
L
P
Q
D
V
400
|
L
F
S
E
V
T
F
L
Q
E
N
E
L
I
Q
T
V
G
I
Y
410
|
R
L
G
Y
R
I
I
S
L
A
H
W
N
P
G
D
A
S
Y
L
420
|
S
P
L
D
T
L
L
S
Q
V
G
F
L
Q
G
N
I
L
S
Q
430
|
W
V
L
I
G
R
L
G
R
R
S
I
L
L
R
H
E
N
L
G
440
|
G
A
S
Y
G
S
L
L
A
T
L
L
I
Q
H
G
H
L
N
G
450
|
T
I
H
S
L
W
C
L
F
G
V
L
H
R
T
S
V
L
P
R
460
|
W
E
D
L
Q
G
L
S
F
G
R
L
N
A
P
L
H
I
Q
H
470
|
A
H
L
N
L
T
H
H
T
L
A
C
N
F
R
V
P
H
E
T
480
|
D
V
E
P
C
W
V
D
G
Q
E
L
G
F
L
R
A
N
C
P
490
|
H
H
Q
Q
L
A
C
L
A
L
R
H
G
T
H
A
C
N
W
R
500
|
G
P
P
E
G
D
P
E
T
C
Q
V
C
G
V
E
N
G
C
L
510
|
S
A
Q
C
F
H
L
Q
R
L
G
C
Q
A
E
R
C
G
V
H
520
|
E
C
E
W
C
G
R
P
V
G
L
P
Q
T
G
Q
L
C
P
V
530
|
R
N
E
C
Y
S
V
Q
N
F
A
L
R
R
H
G
C
Q
L
E
540
|
P
C
C
V
H
E
P
E
E
C
C
R
Q
V
P
L
Q
Q
N
G
550
|
G
L
S
P
V
R
T
E
C
Y
F
V
G
N
P
A
E
R
A
H
560
|
D
C
Q
L
C
P
V
C
A
H
-
P
-
E
-
C
-
Q
-
P
570
|
-
Q
-
N
-
G
-
S
-
V
-
T
-
C
-
F
-
G
-
P
580
|
-
E
-
A
-
D
-
Q
-
C
-
V
-
A
-
C
-
A
-
H
590
|
-
Y
-
K
-
D
-
P
-
P
-
F
-
C
-
V
-
A
-
R
600
|
-
C
-
P
-
S
-
G
-
V
-
K
-
P
-
D
-
L
-
S
610
|
-
Y
-
M
-
P
-
I
-
W
-
K
-
F
-
P
-
D
-
E
620
|
-
E
-
G
-
A
-
C
-
Q
-
P
-
C
-
P
-
I
-
N
630
|
-
C
-
T
-
H
-
S
-
C
-
V
-
D
-
L
-
D
-
D
640
|
-
K
-
G
-
C
-
P
-
A
-
E
-
Q
-
R
-
A
-
S
650
|
-
P
-
L
-
T
-
S
-
I
-
I
-
S
-
A
-
V
-
V
660
|
-
G
-
I
-
L
-
L
-
V
-
V
-
V
-
L
-
G
-
V
670
|
-
V
-
F
-
G
-
I
-
L
-
I
-
K
-
R
-
R
-
Q
680
|
-
Q
-
K
-
I
-
R
-
K
-
Y
-
T
-
M
-
R
-
R
690
|
-
L
-
L
-
Q
-
E
-
T
-
E
-
L
-
V
-
E
-
P
700
|
-
L
-
T
-
P
-
S
-
G
-
A
-
M
-
P
-
N
-
Q
710
|
-
A
-
Q
-
M
-
R
-
I
-
L
-
K
-
E
-
T
-
E
720
|
-
L
-
R
-
K
-
V
-
K
-
V
-
L
-
G
-
S
-
G
730
|
-
A
-
F
-
G
-
T
-
V
-
Y
-
K
-
G
-
I
-
W
740
|
-
I
-
P
-
D
-
G
-
E
-
N
-
V
-
K
-
I
-
P
750
|
-
V
-
A
-
I
-
K
-
V
-
L
-
R
-
E
-
N
-
T
760
|
-
S
-
P
-
K
-
A
-
N
-
K
-
E
-
I
-
L
-
D
770
|
-
E
-
A
-
Y
-
V
-
M
-
A
-
G
-
V
-
D
-
S
780
|
-
P
-
Y
-
V
-
S
-
R
-
L
-
L
-
G
-
I
-
C
790
|
-
L
-
T
-
S
-
T
-
V
-
Q
-
L
-
V
-
T
-
Q
800
|
-
L
-
M
-
P
-
Y
-
G
-
C
-
L
-
L
-
D
-
H
810
|
-
V
-
R
-
E
-
N
-
R
-
G
-
R
-
L
-
G
-
S
820
|
-
Q
-
D
-
L
-
L
-
N
-
W
-
C
-
M
-
Q
-
I
830
|
-
A
-
K
-
G
-
M
-
S
-
Y
-
L
-
E
-
D
-
V
840
|
-
R
-
L
-
V
-
H
-
R
-
D
-
L
-
A
-
A
-
R
850
|
-
N
-
V
-
L
-
V
-
K
-
S
-
P
-
N
-
H
-
V
860
|
-
K
-
I
-
T
-
D
-
F
-
G
-
L
-
A
-
R
-
L
870
|
-
L
-
D
-
I
-
D
-
E
-
T
-
E
-
Y
-
H
-
A
880
|
-
D
-
G
-
G
-
K
-
V
-
P
-
I
-
K
-
W
-
M
890
|
-
A
-
L
-
E
-
S
-
I
-
L
-
R
-
R
-
R
-
F
900
|
-
T
-
H
-
Q
-
S
-
D
-
V
-
W
-
S
-
Y
-
G
910
|
-
V
-
T
-
V
-
W
-
E
-
L
-
M
-
T
-
F
-
G
920
|
-
A
-
K
-
P
-
Y
-
D
-
G
-
I
-
P
-
A
-
R
930
|
-
E
-
I
-
P
-
D
-
L
-
L
-
E
-
K
-
G
-
E
940
|
-
R
-
L
-
P
-
Q
-
P
-
P
-
I
-
C
-
T
-
I
950
|
-
D
-
V
-
Y
-
M
-
I
-
M
-
V
-
K
-
C
-
W
960
|
-
M
-
I
-
D
-
S
-
E
-
C
-
R
-
P
-
R
-
F
970
|
-
R
-
E
-
L
-
V
-
S
-
E
-
F
-
S
-
R
-
M
980
|
-
A
-
R
-
D
-
P
-
Q
-
R
-
F
-
V
-
V
-
I
990
|
-
Q
-
N
-
E
-
D
-
L
-
G
-
P
-
A
-
S
-
P
1000
|
-
L
-
D
-
S
-
T
-
F
-
Y
-
R
-
S
-
L
-
L
1010
|
-
E
-
D
-
D
-
D
-
M
-
G
-
D
-
L
-
V
-
D
1020
|
-
A
-
E
-
E
-
Y
-
L
-
V
-
P
-
Q
-
Q
-
G
1030
|
-
G
-
G
-
S
-
L
-
E
-
V
-
L
-
F
-
Q
-
G
1040
|
-
P
-
S
-
S
-
P
-
S
-
G
-
S
-
S
-
M
-
K
1050
|
-
I
-
E
-
E
-
G
-
K
-
L
-
V
-
I
-
W
-
I
1060
|
-
N
-
G
-
D
-
K
-
G
-
Y
-
N
-
G
-
L
-
A
1070
|
-
E
-
V
-
G
-
K
-
K
-
F
-
E
-
K
-
D
-
T
1080
|
-
G
-
I
-
K
-
V
-
T
-
V
-
E
-
H
-
P
-
D
1090
|
-
K
-
L
-
E
-
E
-
K
-
F
-
P
-
Q
-
V
-
A
1100
|
-
A
-
T
-
G
-
D
-
G
-
P
-
D
-
I
-
I
-
F
1110
|
-
W
-
A
-
H
-
D
-
R
-
F
-
G
-
G
-
Y
-
A
1120
|
-
Q
-
S
-
G
-
L
-
L
-
A
-
E
-
I
-
T
-
P
1130
|
-
D
-
K
-
A
-
F
-
Q
-
D
-
K
-
L
-
Y
-
P
1140
|
-
F
-
T
-
W
-
D
-
A
-
V
-
R
-
Y
-
N
-
G
1150
|
-
K
-
L
-
I
-
A
-
Y
-
P
-
I
-
A
-
V
-
E
1160
|
-
A
-
L
-
S
-
L
-
I
-
Y
-
N
-
K
-
D
-
L
1170
|
-
L
-
P
-
N
-
P
-
P
-
K
-
T
-
W
-
E
-
E
1180
|
-
I
-
P
-
A
-
L
-
D
-
K
-
E
-
L
-
K
-
A
1190
|
-
K
-
G
-
K
-
S
-
A
-
L
-
M
-
F
-
N
-
L
1200
|
-
Q
-
E
-
P
-
Y
-
F
-
T
-
W
-
P
-
L
-
I
1210
|
-
A
-
A
-
D
-
G
-
G
-
Y
-
A
-
F
-
K
-
Y
1220
|
-
E
-
N
-
G
-
K
-
Y
-
D
-
I
-
K
-
D
-
V
1230
|
-
G
-
V
-
D
-
N
-
A
-
G
-
A
-
K
-
A
-
G
1240
|
-
L
-
T
-
F
-
L
-
V
-
D
-
L
-
I
-
K
-
N
1250
|
-
K
-
H
-
M
-
N
-
A
-
D
-
T
-
D
-
Y
-
S
1260
|
-
I
-
A
-
E
-
A
-
A
-
F
-
N
-
K
-
G
-
E
1270
|
-
T
-
A
-
M
-
T
-
I
-
N
-
G
-
P
-
W
-
A
1280
|
-
W
-
S
-
N
-
I
-
D
-
T
-
S
-
K
-
V
-
N
1290
|
-
Y
-
G
-
V
-
T
-
V
-
L
-
P
-
T
-
F
-
K
1300
|
-
G
-
Q
-
P
-
S
-
K
-
P
-
F
-
V
-
G
-
V
1310
|
-
L
-
S
-
A
-
G
-
I
-
N
-
A
-
A
-
S
-
P
1320
|
-
N
-
K
-
E
-
L
-
A
-
K
-
E
-
F
-
L
-
E
1330
|
-
N
-
Y
-
L
-
L
-
T
-
D
-
E
-
G
-
L
-
E
1340
|
-
A
-
V
-
N
-
K
-
D
-
K
-
P
-
L
-
G
-
A
1350
|
-
V
-
A
-
L
-
K
-
S
-
Y
-
E
-
E
-
E
-
L
1360
|
-
A
-
K
-
D
-
P
-
R
-
I
-
A
-
A
-
T
-
M
1370
|
-
E
-
N
-
A
-
Q
-
K
-
G
-
E
-
I
-
M
-
P
1380
|
-
N
-
I
-
P
-
Q
-
M
-
S
-
A
-
F
-
W
-
Y
1390
|
-
A
-
V
-
R
-
T
-
A
-
V
-
I
-
N
-
A
-
A
1400
|
-
S
-
G
-
R
-
Q
-
T
-
V
-
D
-
E
-
A
-
L
1410
|
-
K
-
D
-
A
-
Q
-
T
-
N
-
S
-
S
-
S
-
S
1420
|
-
G
-
P
-
S
-
S
-
P
-
S
-
A
-
W
-
S
-
H
1430
|
-
P
-
Q
-
F
-
E
-
K
-
G
-
G
-
G
-
S
-
G
1440
|
-
G
-
G
-
S
-
G
-
G
-
S
-
S
-
A
-
W
-
S
1450
|
-
H
-
P
-
Q
-
F
-
E
-
K
|
|||||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| Disease Class: HER2 negative breast cancer [ICD-11: 2C60.11] | [15] | ||||||||||||
| Sensitive Disease | HER2 negative breast cancer [ICD-11: 2C60.11] | ||||||||||||
| Sensitive Drug | Neratinib | ||||||||||||
| Molecule Alteration | Missense mutation | p.L755S (c.2263_2264delCTinsAG) |
|||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| Disease Class: HER2 negative breast cancer [ICD-11: 2C60.11] | [15] | ||||||||||||
| Sensitive Disease | HER2 negative breast cancer [ICD-11: 2C60.11] | ||||||||||||
| Sensitive Drug | Neratinib | ||||||||||||
| Molecule Alteration | Complex-indel | p.L755_E757delinsS (c.2263_2271delinsAGC) |
|||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| Disease Class: HER2 negative breast cancer [ICD-11: 2C60.11] | [15] | ||||||||||||
| Sensitive Disease | HER2 negative breast cancer [ICD-11: 2C60.11] | ||||||||||||
| Sensitive Drug | Neratinib | ||||||||||||
| Molecule Alteration | Duplication | p.Y772_A775 (c.2314_2325) |
|||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| Disease Class: HER2 negative breast cancer [ICD-11: 2C60.11] | [15] | ||||||||||||
| Sensitive Disease | HER2 negative breast cancer [ICD-11: 2C60.11] | ||||||||||||
| Sensitive Drug | Neratinib | ||||||||||||
| Molecule Alteration | Missense mutation | p.V777L (c.2329G>C) |
|||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| Disease Class: HER2 negative breast cancer [ICD-11: 2C60.11] | [15] | ||||||||||||
| Sensitive Disease | HER2 negative breast cancer [ICD-11: 2C60.11] | ||||||||||||
| Sensitive Drug | Neratinib | ||||||||||||
| Molecule Alteration | Duplication | p.G778_P780 (c.2332_2340) |
|||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| Disease Class: HER2 negative breast cancer [ICD-11: 2C60.11] | [15] | ||||||||||||
| Sensitive Disease | HER2 negative breast cancer [ICD-11: 2C60.11] | ||||||||||||
| Sensitive Drug | Neratinib | ||||||||||||
| Molecule Alteration | Missense mutation | p.L869R (c.2606T>G) |
|||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [6] | |||
| Resistant Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Resistant Drug | Osimertinib | |||
| Molecule Alteration | Duplication | p.Y772_A775 (c.2314_2325)/p.A775_G776insYVMA |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 |
| Experiment for Molecule Alteration |
Sanger cDNA sequencing assay | |||
| Experiment for Drug Resistance |
CCK-8 assay | |||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [6] | |||
| Resistant Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Resistant Drug | Osimertinib | |||
| Molecule Alteration | Duplication | p.G778_P780 (c.2332_2340)/p.780_Y781insGSP |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 |
| Experiment for Molecule Alteration |
Sanger cDNA sequencing assay | |||
| Experiment for Drug Resistance |
CCK-8 assay | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [6] | |||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Sensitive Drug | Osimertinib | |||
| Molecule Alteration | Complex-indel | p.G776_776delinsVC (c.2326_2328delinsGTATGT) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 |
| Experiment for Molecule Alteration |
Sanger cDNA sequencing assay | |||
| Experiment for Drug Resistance |
CCK-8 assay | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Colorectal cancer [ICD-11: 2B91.1] | [2] | |||
| Resistant Disease | Colorectal cancer [ICD-11: 2B91.1] | |||
| Resistant Drug | Panitumumab | |||
| Molecule Alteration | Structural variation | Amplification |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vivo Model | A retrospective survey in conducting clinical studies | Homo sapiens | ||
| Experiment for Molecule Alteration |
Next-generation sequencing assay | |||
| Experiment for Drug Resistance |
Liquid biopsy assay | |||
| Mechanism Description | Mechanisms of resistance to EGFR blockade include the emergence of kRAS, NRAS and EGFR extracellular domain mutations as well as HER2/MET alterations. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Breast cancer [ICD-11: 2C60.3] | [16] | |||
| Sensitive Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Sensitive Drug | Tamoxifen | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | MCF-7 cells | Breast | Homo sapiens (Human) | CVCL_0031 |
| T47D cells | Breast | Homo sapiens (Human) | CVCL_0553 | |
| MCF7/TAMR cells | Breast | Homo sapiens (Human) | CVCL_EG55 | |
| T47D/TAMR cells | Breast | Homo sapiens (Human) | CVCL_1D36 | |
| Experiment for Molecule Alteration |
Western blot analysis; Luciferase reporter assay | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | The ERBB2 expression is regulated at the post-transcriptional level by miR26a/b and the RNA-binding protein human antigen R, miR26a/b inhibits the translation of ERBB2 mRNA, whereas HuR enhances the stability of the ERBB2 mRNA. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Lung adenocarcinoma [ICD-11: 2C25.0] | [18] | |||
| Sensitive Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | |||
| Sensitive Drug | Trastuzumab-based chemotherapy | |||
| Molecule Alteration | Missense mutation | p.G776L (c.2326_2327delGGinsCT) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Lung | N.A. | ||
| Mechanism Description | The missense mutation p.G776L (c.2326_2327delGGinsCT) in gene ERBB2 cause the sensitivity of Trastuzumab-based chemotherapy by aberration of the drug's therapeutic target | |||
| Disease Class: Head and neck cancer [ICD-11: 2D42.0] | [19] | |||
| Sensitive Disease | Head and neck cancer [ICD-11: 2D42.0] | |||
| Sensitive Drug | Trastuzumab-based chemotherapy | |||
| Molecule Alteration | Copy number gain | . |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Human salivary ductal carcinoma tissue | N.A. | ||
| Mechanism Description | The copy number gain in gene ERBB2 cause the sensitivity of Trastuzumab-based chemotherapy by aberration of the drug's therapeutic target. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [6] | |||
| Resistant Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Resistant Drug | Tucatinib | |||
| Molecule Alteration | Duplication | p.Y772_A775 (c.2314_2325) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 |
| Experiment for Molecule Alteration |
Sanger cDNA sequencing assay | |||
| Experiment for Drug Resistance |
CCK-8 assay | |||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [6] | |||
| Resistant Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Resistant Drug | Tucatinib | |||
| Molecule Alteration | Complex-indel | p.G776_776delinsVC (c.2326_2328delinsGTATGT) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 |
| Experiment for Molecule Alteration |
Sanger cDNA sequencing assay | |||
| Experiment for Drug Resistance |
CCK-8 assay | |||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [6] | |||
| Resistant Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Resistant Drug | Tucatinib | |||
| Molecule Alteration | Duplication | p.G778_P780 (c.2332_2340) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 |
| Experiment for Molecule Alteration |
Sanger cDNA sequencing assay | |||
| Experiment for Drug Resistance |
CCK-8 assay | |||
Clinical Trial Drug(s)
9 drug(s) in total
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Breast adenocarcinoma [ICD-11: 2C60.1] | [13] | |||
| Sensitive Disease | Breast adenocarcinoma [ICD-11: 2C60.1] | |||
| Sensitive Drug | Buparlisib | |||
| Molecule Alteration | Missense mutation | p.T798M (c.2393_2394delCAinsTG) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | BT474 cells | Breast | Homo sapiens (Human) | CVCL_0179 |
| MCF10A cells | Breast | Homo sapiens (Human) | CVCL_0598 | |
| In Vivo Model | Athymic female mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
HER2T798M sequencing assay | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | The missense mutation p.T798M (c.2393_2394delCAinsTG) in gene ERBB2 cause the sensitivity of Buparlisib by aberration of the drug's therapeutic target | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [6] | |||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Sensitive Drug | Ganetespib | |||
| Molecule Alteration | Complex-indel | p.G776_776delinsVC (c.2326_2328delinsGTATGT) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 |
| Experiment for Molecule Alteration |
Sanger cDNA sequencing assay | |||
| Experiment for Drug Resistance |
CCK-8 assay | |||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [6] | |||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Sensitive Drug | Ganetespib | |||
| Molecule Alteration | Duplication | p.Y772_A775 (c.2314_2325)/p.A775_G776insYVMA + p.C805S |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 |
| Experiment for Molecule Alteration |
Sanger cDNA sequencing assay | |||
| Experiment for Drug Resistance |
CCK-8 assay | |||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [6] | |||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Sensitive Drug | Ganetespib | |||
| Molecule Alteration | Duplication | p.Y772_A775 (c.2314_2325)/p.A775_G776insYVMA + p.C805S |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 |
| Experiment for Molecule Alteration |
Sanger cDNA sequencing assay | |||
| Experiment for Drug Resistance |
CCK-8 assay | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | |||||||||||||
|
|
|||||||||||||
| Disease Class: Bladder cancer [ICD-11: 2C94.0] | [20] | ||||||||||||
| Resistant Disease | Bladder cancer [ICD-11: 2C94.0] | ||||||||||||
| Resistant Drug | Selumetinib | ||||||||||||
| Molecule Alteration | Missense mutation | p.S310F (c.929C>T) |
|||||||||||
| Wild Type Structure | Method: X-ray diffraction | Resolution: 3.09 Å | |||||||||||
| Mutant Type Structure | Method: Electron microscopy | Resolution: 3.09 Å | |||||||||||
| Download The Information of Sequence | Download The Structure File | ||||||||||||
-
T
M
Q
E
V
L
C
A
T
A
G
L
T
C
D
R
M
W
10
|
K
G
L
L
R
L
L
L
P
A
A
L
S
L
P
P
E
P
T
G
20
|
H
A
L
A
D
S
M
T
L
Q
R
V
H
C
L
T
Y
G
Q
T
30
|
G
D
C
M
Q
K
V
L
V
R
Q
L
G
P
N
A
L
S
E
P
40
|
L
E
T
T
Y
H
L
L
P
D
T
M
N
L
A
R
S
H
L
L
50
|
S
Y
F
Q
L
G
Q
C
D
Q
I
V
Q
V
E
Q
V
G
Q
N
60
|
G
L
Y
E
V
L
L
T
I
Y
A
L
H
P
N
T
Q
N
V
A
70
|
R
S
Q
L
V
S
P
F
L
L
Q
Q
R
D
L
I
R
Q
I
E
80
|
V
V
R
Q
G
G
T
Y
Q
V
L
L
F
I
E
A
D
H
N
N
90
|
Y
Q
A
V
L
R
A
Q
V
V
L
P
D
L
N
Q
G
R
D
L
100
|
P
R
L
I
N
V
N
R
T
G
T
T
P
Q
V
L
T
F
G
E
110
|
A
D
S
N
P
Y
G
A
G
L
L
A
R
V
E
L
L
D
Q
N
120
|
L
G
R
D
S
P
L
L
T
N
E
N
I
T
L
T
K
P
G
V
130
|
G
T
V
G
L
A
I
S
Q
P
R
G
N
G
P
L
Q
R
L
E
140
|
C
L
Y
Q
Q
L
D
R
T
S
I
L
L
T
W
E
K
I
D
L
150
|
I
K
F
G
H
G
K
V
N
L
N
I
Q
Q
L
R
A
N
L
P
160
|
T
Q
L
L
I
C
D
Y
T
Q
N
D
R
T
S
I
R
L
A
W
170
|
C
K
H
D
P
I
C
F
S
H
P
K
M
N
C
N
K
Q
G
L
180
|
S
A
R
L
C
T
W
L
G
I
E
D
S
T
S
N
E
R
D
S
190
|
C
R
Q
A
S
C
L
H
T
P
R
C
T
S
V
P
C
M
A
C
200
|
G
K
G
G
C
S
A
R
R
C
C
W
K
G
G
E
P
S
L
S
210
|
P
E
T
D
D
C
C
Q
C
S
H
L
E
T
Q
R
C
T
A
V
220
|
A
C
G
A
C
G
T
G
G
C
P
A
K
R
H
C
S
K
D
G
230
|
C
P
L
L
A
P
C
T
L
D
H
C
F
C
N
H
H
E
S
Q
240
|
G
C
I
A
C
A
E
G
L
C
H
T
C
G
P
P
A
K
L
H
250
|
V
S
T
D
Y
C
N
L
T
A
D
C
T
L
F
H
E
F
S
N
260
|
M
H
P
S
N
G
P
I
E
C
G
E
R
L
Y
H
T
C
F
P
270
|
G
A
A
L
S
V
C
T
V
Y
T
N
A
T
C
D
P
T
Y
F
280
|
N
E
Y
S
L
M
S
P
T
N
D
P
V
E
G
G
S
R
C
Y
290
|
T
T
L
F
V
G
C
A
P
S
L
C
H
V
N
T
Q
A
E
C
300
|
V
P
T
Y
A
N
E
Y
D
L
G
S
T
T
Q
D
R
V
C
G
310
|
E
F
K
C
C
T
S
L
K
V
P
C
C
P
A
L
R
H
V
N
320
|
C
Q
Y
E
G
V
L
T
G
A
M
E
E
D
H
G
L
T
R
Q
330
|
E
R
V
C
R
E
A
K
V
C
T
S
S
K
A
P
N
C
I
A
340
|
Q
R
E
V
F
C
A
Y
G
G
C
L
K
G
K
M
I
E
F
H
350
|
G
L
S
R
L
E
A
V
F
R
L
A
P
V
E
T
S
S
F
A
360
|
D
N
G
I
D
Q
P
E
A
F
S
A
N
G
T
C
A
K
P
K
370
|
L
I
Q
F
P
G
E
S
Q
L
L
A
Q
F
V
L
F
P
E
E
380
|
T
S
L
F
E
D
E
G
I
D
T
P
G
A
Y
S
L
N
Y
T
390
|
I
A
S
P
A
L
W
Q
P
P
D
E
S
Q
L
L
P
Q
D
V
400
|
L
F
S
E
V
T
F
L
Q
E
N
E
L
I
Q
T
V
G
I
Y
410
|
R
L
G
Y
R
I
I
S
L
A
H
W
N
P
G
D
A
S
Y
L
420
|
S
P
L
D
T
L
L
S
Q
V
G
F
L
Q
G
N
I
L
S
Q
430
|
W
V
L
I
G
R
L
G
R
R
S
I
L
L
R
H
E
N
L
G
440
|
G
A
S
Y
G
S
L
L
A
T
L
L
I
Q
H
G
H
L
N
G
450
|
T
I
H
S
L
W
C
L
F
G
V
L
H
R
T
S
V
L
P
R
460
|
W
E
D
L
Q
G
L
S
F
G
R
L
N
A
P
L
H
I
Q
H
470
|
A
H
L
N
L
T
H
H
T
L
A
C
N
F
R
V
P
H
E
T
480
|
D
V
E
P
C
W
V
D
G
Q
E
L
G
F
L
R
A
N
C
P
490
|
H
H
Q
Q
L
A
C
L
A
L
R
H
G
T
H
A
C
N
W
R
500
|
G
P
P
E
G
D
P
E
T
C
Q
V
C
G
V
E
N
G
C
L
510
|
S
A
Q
C
F
H
L
Q
R
L
G
C
Q
A
E
R
C
G
V
H
520
|
E
C
E
W
C
G
R
P
V
G
L
P
Q
T
G
Q
L
C
P
V
530
|
R
N
E
C
Y
S
V
Q
N
F
A
L
R
R
H
G
C
Q
L
E
540
|
P
C
C
V
H
E
P
E
E
C
C
R
Q
V
P
L
Q
Q
N
G
550
|
G
L
S
P
V
R
T
E
C
Y
F
V
G
N
P
A
E
R
A
H
560
|
D
C
Q
L
C
P
V
C
A
H
-
P
-
E
-
C
-
Q
-
P
570
|
-
Q
-
N
-
G
-
S
-
V
-
T
-
C
-
F
-
G
-
P
580
|
-
E
-
A
-
D
-
Q
-
C
-
V
-
A
-
C
-
A
-
H
590
|
-
Y
-
K
-
D
-
P
-
P
-
F
-
C
-
V
-
A
-
R
600
|
-
C
-
P
-
S
-
G
-
V
-
K
-
P
-
D
-
L
-
S
610
|
-
Y
-
M
-
P
-
I
-
W
-
K
-
F
-
P
-
D
-
E
620
|
-
E
-
G
-
A
-
C
-
Q
-
P
-
C
-
P
-
I
-
N
630
|
-
C
-
T
-
H
-
S
-
C
-
V
-
D
-
L
-
D
-
D
640
|
-
K
-
G
-
C
-
P
-
A
-
E
-
Q
-
R
-
A
-
S
650
|
-
P
-
L
-
T
-
S
-
I
-
I
-
S
-
A
-
V
-
V
660
|
-
G
-
I
-
L
-
L
-
V
-
V
-
V
-
L
-
G
-
V
670
|
-
V
-
F
-
G
-
I
-
L
-
I
-
K
-
R
-
R
-
Q
680
|
-
Q
-
K
-
I
-
R
-
K
-
Y
-
T
-
M
-
R
-
R
690
|
-
L
-
L
-
Q
-
E
-
T
-
E
-
L
-
V
-
E
-
P
700
|
-
L
-
T
-
P
-
S
-
G
-
A
-
M
-
P
-
N
-
Q
710
|
-
A
-
Q
-
M
-
R
-
I
-
L
-
K
-
E
-
T
-
E
720
|
-
L
-
R
-
K
-
V
-
K
-
V
-
L
-
G
-
S
-
G
730
|
-
A
-
F
-
G
-
T
-
V
-
Y
-
K
-
G
-
I
-
W
740
|
-
I
-
P
-
D
-
G
-
E
-
N
-
V
-
K
-
I
-
P
750
|
-
V
-
A
-
I
-
K
-
V
-
L
-
R
-
E
-
N
-
T
760
|
-
S
-
P
-
K
-
A
-
N
-
K
-
E
-
I
-
L
-
D
770
|
-
E
-
A
-
Y
-
V
-
M
-
A
-
G
-
V
-
D
-
S
780
|
-
P
-
Y
-
V
-
S
-
R
-
L
-
L
-
G
-
I
-
C
790
|
-
L
-
T
-
S
-
T
-
V
-
Q
-
L
-
V
-
T
-
Q
800
|
-
L
-
M
-
P
-
Y
-
G
-
C
-
L
-
L
-
D
-
H
810
|
-
V
-
R
-
E
-
N
-
R
-
G
-
R
-
L
-
G
-
S
820
|
-
Q
-
D
-
L
-
L
-
N
-
W
-
C
-
M
-
Q
-
I
830
|
-
A
-
K
-
G
-
M
-
S
-
Y
-
L
-
E
-
D
-
V
840
|
-
R
-
L
-
V
-
H
-
R
-
D
-
L
-
A
-
A
-
R
850
|
-
N
-
V
-
L
-
V
-
K
-
S
-
P
-
N
-
H
-
V
860
|
-
K
-
I
-
T
-
D
-
F
-
G
-
L
-
A
-
R
-
L
870
|
-
L
-
D
-
I
-
D
-
E
-
T
-
E
-
Y
-
H
-
A
880
|
-
D
-
G
-
G
-
K
-
V
-
P
-
I
-
K
-
W
-
M
890
|
-
A
-
L
-
E
-
S
-
I
-
L
-
R
-
R
-
R
-
F
900
|
-
T
-
H
-
Q
-
S
-
D
-
V
-
W
-
S
-
Y
-
G
910
|
-
V
-
T
-
V
-
W
-
E
-
L
-
M
-
T
-
F
-
G
920
|
-
A
-
K
-
P
-
Y
-
D
-
G
-
I
-
P
-
A
-
R
930
|
-
E
-
I
-
P
-
D
-
L
-
L
-
E
-
K
-
G
-
E
940
|
-
R
-
L
-
P
-
Q
-
P
-
P
-
I
-
C
-
T
-
I
950
|
-
D
-
V
-
Y
-
M
-
I
-
M
-
V
-
K
-
C
-
W
960
|
-
M
-
I
-
D
-
S
-
E
-
C
-
R
-
P
-
R
-
F
970
|
-
R
-
E
-
L
-
V
-
S
-
E
-
F
-
S
-
R
-
M
980
|
-
A
-
R
-
D
-
P
-
Q
-
R
-
F
-
V
-
V
-
I
990
|
-
Q
-
N
-
E
-
D
-
L
-
G
-
P
-
A
-
S
-
P
1000
|
-
L
-
D
-
S
-
T
-
F
-
Y
-
R
-
S
-
L
-
L
1010
|
-
E
-
D
-
D
-
D
-
M
-
G
-
D
-
L
-
V
-
D
1020
|
-
A
-
E
-
E
-
Y
-
L
-
V
-
P
-
Q
-
Q
-
G
1030
|
-
G
-
G
-
S
-
L
-
E
-
V
-
L
-
F
-
Q
-
G
1040
|
-
P
-
S
-
S
-
P
-
S
-
G
-
S
-
S
-
M
-
K
1050
|
-
I
-
E
-
E
-
G
-
K
-
L
-
V
-
I
-
W
-
I
1060
|
-
N
-
G
-
D
-
K
-
G
-
Y
-
N
-
G
-
L
-
A
1070
|
-
E
-
V
-
G
-
K
-
K
-
F
-
E
-
K
-
D
-
T
1080
|
-
G
-
I
-
K
-
V
-
T
-
V
-
E
-
H
-
P
-
D
1090
|
-
K
-
L
-
E
-
E
-
K
-
F
-
P
-
Q
-
V
-
A
1100
|
-
A
-
T
-
G
-
D
-
G
-
P
-
D
-
I
-
I
-
F
1110
|
-
W
-
A
-
H
-
D
-
R
-
F
-
G
-
G
-
Y
-
A
1120
|
-
Q
-
S
-
G
-
L
-
L
-
A
-
E
-
I
-
T
-
P
1130
|
-
D
-
K
-
A
-
F
-
Q
-
D
-
K
-
L
-
Y
-
P
1140
|
-
F
-
T
-
W
-
D
-
A
-
V
-
R
-
Y
-
N
-
G
1150
|
-
K
-
L
-
I
-
A
-
Y
-
P
-
I
-
A
-
V
-
E
1160
|
-
A
-
L
-
S
-
L
-
I
-
Y
-
N
-
K
-
D
-
L
1170
|
-
L
-
P
-
N
-
P
-
P
-
K
-
T
-
W
-
E
-
E
1180
|
-
I
-
P
-
A
-
L
-
D
-
K
-
E
-
L
-
K
-
A
1190
|
-
K
-
G
-
K
-
S
-
A
-
L
-
M
-
F
-
N
-
L
1200
|
-
Q
-
E
-
P
-
Y
-
F
-
T
-
W
-
P
-
L
-
I
1210
|
-
A
-
A
-
D
-
G
-
G
-
Y
-
A
-
F
-
K
-
Y
1220
|
-
E
-
N
-
G
-
K
-
Y
-
D
-
I
-
K
-
D
-
V
1230
|
-
G
-
V
-
D
-
N
-
A
-
G
-
A
-
K
-
A
-
G
1240
|
-
L
-
T
-
F
-
L
-
V
-
D
-
L
-
I
-
K
-
N
1250
|
-
K
-
H
-
M
-
N
-
A
-
D
-
T
-
D
-
Y
-
S
1260
|
-
I
-
A
-
E
-
A
-
A
-
F
-
N
-
K
-
G
-
E
1270
|
-
T
-
A
-
M
-
T
-
I
-
N
-
G
-
P
-
W
-
A
1280
|
-
W
-
S
-
N
-
I
-
D
-
T
-
S
-
K
-
V
-
N
1290
|
-
Y
-
G
-
V
-
T
-
V
-
L
-
P
-
T
-
F
-
K
1300
|
-
G
-
Q
-
P
-
S
-
K
-
P
-
F
-
V
-
G
-
V
1310
|
-
L
-
S
-
A
-
G
-
I
-
N
-
A
-
A
-
S
-
P
1320
|
-
N
-
K
-
E
-
L
-
A
-
K
-
E
-
F
-
L
-
E
1330
|
-
N
-
Y
-
L
-
L
-
T
-
D
-
E
-
G
-
L
-
E
1340
|
-
A
-
V
-
N
-
K
-
D
-
K
-
P
-
L
-
G
-
A
1350
|
-
V
-
A
-
L
-
K
-
S
-
Y
-
E
-
E
-
E
-
L
1360
|
-
A
-
K
-
D
-
P
-
R
-
I
-
A
-
A
-
T
-
M
1370
|
-
E
-
N
-
A
-
Q
-
K
-
G
-
E
-
I
-
M
-
P
1380
|
-
N
-
I
-
P
-
Q
-
M
-
S
-
A
-
F
-
W
-
Y
1390
|
-
A
-
V
-
R
-
T
-
A
-
V
-
I
-
N
-
A
-
A
1400
|
-
S
-
G
-
R
-
Q
-
T
-
V
-
D
-
E
-
A
-
L
1410
|
-
K
-
D
-
A
-
Q
-
T
-
N
-
S
-
S
-
S
-
S
1420
|
-
G
-
P
-
S
-
S
-
P
-
S
-
A
-
W
-
S
-
H
1430
|
-
P
-
Q
-
F
-
E
-
K
-
G
-
G
-
G
-
S
-
G
1440
|
-
G
-
G
-
S
-
G
-
G
-
S
-
S
-
A
-
W
-
S
1450
|
-
H
-
P
-
Q
-
F
-
E
-
K
|
|||||||||||||
| Experimental Note | Revealed Based on the Cell Line Data | ||||||||||||
| In Vitro Model | 5637 cells | Bladder | Homo sapiens (Human) | CVCL_0126 | |||||||||
| J82 cells | Bladder | Homo sapiens (Human) | CVCL_0359 | ||||||||||
| RT4 cells | Bladder | Homo sapiens (Human) | CVCL_0036 | ||||||||||
| T24 cells | Bladder | Homo sapiens (Human) | CVCL_0554 | ||||||||||
| SW780 cells | Bladder | Homo sapiens (Human) | CVCL_1728 | ||||||||||
| HT1376 cells | Bladder | Homo sapiens (Human) | CVCL_1292 | ||||||||||
| RT112 cells | Bladder | Homo sapiens (Human) | CVCL_1670 | ||||||||||
| TCCSuP cells | Bladder | Homo sapiens (Human) | CVCL_1738 | ||||||||||
| UM-UC3 cells | Urinary bladder | Homo sapiens (Human) | CVCL_1783 | ||||||||||
| WH cells | Bladder | Homo sapiens (Human) | CVCL_0C39 | ||||||||||
| VM-CUBIII cells | Urinary bladder | Homo sapiens (Human) | CVCL_9830 | ||||||||||
| VM-CUBII cells | Urinary bladder | Homo sapiens (Human) | CVCL_9829 | ||||||||||
| VM-CUBI cells | Obturator lymph node | Homo sapiens (Human) | CVCL_1786 | ||||||||||
| UM-UC-14 cells | Kidney | Homo sapiens (Human) | CVCL_2747 | ||||||||||
| TSU-PR1 cells | Urinary bladder | Homo sapiens (Human) | CVCL_4014 | ||||||||||
| SW1710 cells | Bladder | Homo sapiens (Human) | CVCL_1721 | ||||||||||
| SD cells | Bladder | Homo sapiens (Human) | CVCL_W902 | ||||||||||
| KU-19 cells | Blood | Bos taurus (Bovine) | CVCL_VN09 | ||||||||||
| JO'N cells | Urinary bladder | Homo sapiens (Human) | CVCL_M891 | ||||||||||
| JMSU-1 cells | Ascites | Homo sapiens (Human) | CVCL_2081 | ||||||||||
| HT1197 cells | Urinary bladder | Homo sapiens (Human) | CVCL_1291 | ||||||||||
| DSH1 cells | Urinary bladder | Homo sapiens (Human) | CVCL_1182 | ||||||||||
| CAL-29 cells | Urinary bladder | Homo sapiens (Human) | CVCL_1808 | ||||||||||
| BFTC-905 cells | Urinary bladder | Homo sapiens (Human) | CVCL_1083 | ||||||||||
| BC-3C cells | Urinary bladder | Homo sapiens (Human) | CVCL_1958 | ||||||||||
| 97-7 cells | Bladder | Homo sapiens (Human) | CVCL_8625 | ||||||||||
| 97-24 cells | Bladder | Homo sapiens (Human) | CVCL_8621 | ||||||||||
| 97-18 cells | Bladder | Homo sapiens (Human) | CVCL_8619 | ||||||||||
| 97-1 cells | Bladder | Homo sapiens (Human) | CVCL_8616 | ||||||||||
| 96-1 cells | Bladder | Homo sapiens (Human) | CVCL_8609 | ||||||||||
| 94-10 cells | Bladder | Homo sapiens (Human) | CVCL_8608 | ||||||||||
| 647V cells | Urinary bladder | Homo sapiens (Human) | CVCL_1049 | ||||||||||
| 253J cells | Lymph node | Homo sapiens (Human) | CVCL_7935/CVCL_7938 | ||||||||||
| Experiment for Molecule Alteration |
Western blot analysis | ||||||||||||
| Experiment for Drug Resistance |
MTT assay | ||||||||||||
| Disease Class: Bladder cancer [ICD-11: 2C94.0] | [20] | ||||||||||||
| Resistant Disease | Bladder cancer [ICD-11: 2C94.0] | ||||||||||||
| Resistant Drug | Selumetinib | ||||||||||||
| Molecule Alteration | Missense mutation | p.S653C (c.1958C>G) |
|||||||||||
| Experimental Note | Revealed Based on the Cell Line Data | ||||||||||||
| In Vitro Model | 5637 cells | Bladder | Homo sapiens (Human) | CVCL_0126 | |||||||||
| J82 cells | Bladder | Homo sapiens (Human) | CVCL_0359 | ||||||||||
| RT4 cells | Bladder | Homo sapiens (Human) | CVCL_0036 | ||||||||||
| T24 cells | Bladder | Homo sapiens (Human) | CVCL_0554 | ||||||||||
| SW780 cells | Bladder | Homo sapiens (Human) | CVCL_1728 | ||||||||||
| HT1376 cells | Bladder | Homo sapiens (Human) | CVCL_1292 | ||||||||||
| RT112 cells | Bladder | Homo sapiens (Human) | CVCL_1670 | ||||||||||
| TCCSuP cells | Bladder | Homo sapiens (Human) | CVCL_1738 | ||||||||||
| UM-UC3 cells | Urinary bladder | Homo sapiens (Human) | CVCL_1783 | ||||||||||
| WH cells | Bladder | Homo sapiens (Human) | CVCL_0C39 | ||||||||||
| VM-CUBIII cells | Urinary bladder | Homo sapiens (Human) | CVCL_9830 | ||||||||||
| VM-CUBII cells | Urinary bladder | Homo sapiens (Human) | CVCL_9829 | ||||||||||
| VM-CUBI cells | Obturator lymph node | Homo sapiens (Human) | CVCL_1786 | ||||||||||
| UM-UC-14 cells | Kidney | Homo sapiens (Human) | CVCL_2747 | ||||||||||
| TSU-PR1 cells | Urinary bladder | Homo sapiens (Human) | CVCL_4014 | ||||||||||
| SW1710 cells | Bladder | Homo sapiens (Human) | CVCL_1721 | ||||||||||
| SD cells | Bladder | Homo sapiens (Human) | CVCL_W902 | ||||||||||
| KU-19 cells | Blood | Bos taurus (Bovine) | CVCL_VN09 | ||||||||||
| JO'N cells | Urinary bladder | Homo sapiens (Human) | CVCL_M891 | ||||||||||
| JMSU-1 cells | Ascites | Homo sapiens (Human) | CVCL_2081 | ||||||||||
| HT1197 cells | Urinary bladder | Homo sapiens (Human) | CVCL_1291 | ||||||||||
| DSH1 cells | Urinary bladder | Homo sapiens (Human) | CVCL_1182 | ||||||||||
| CAL-29 cells | Urinary bladder | Homo sapiens (Human) | CVCL_1808 | ||||||||||
| BFTC-905 cells | Urinary bladder | Homo sapiens (Human) | CVCL_1083 | ||||||||||
| BC-3C cells | Urinary bladder | Homo sapiens (Human) | CVCL_1958 | ||||||||||
| 97-7 cells | Bladder | Homo sapiens (Human) | CVCL_8625 | ||||||||||
| 97-24 cells | Bladder | Homo sapiens (Human) | CVCL_8621 | ||||||||||
| 97-18 cells | Bladder | Homo sapiens (Human) | CVCL_8619 | ||||||||||
| 97-1 cells | Bladder | Homo sapiens (Human) | CVCL_8616 | ||||||||||
| 96-1 cells | Bladder | Homo sapiens (Human) | CVCL_8609 | ||||||||||
| 94-10 cells | Bladder | Homo sapiens (Human) | CVCL_8608 | ||||||||||
| 647V cells | Urinary bladder | Homo sapiens (Human) | CVCL_1049 | ||||||||||
| 253J cells | Lymph node | Homo sapiens (Human) | CVCL_7935/CVCL_7938 | ||||||||||
| Experiment for Molecule Alteration |
Western blot analysis | ||||||||||||
| Experiment for Drug Resistance |
MTT assay | ||||||||||||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [6] | |||
| Resistant Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Resistant Drug | Afabicin | |||
| Molecule Alteration | Duplication | p.Y772_A775 (c.2314_2325)/p.A775_G776insYVMA |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 |
| Experiment for Molecule Alteration |
Sanger cDNA sequencing assay | |||
| Experiment for Drug Resistance |
CCK-8 assay | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [6] | |||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Sensitive Drug | Afabicin | |||
| Molecule Alteration | Complex-indel | p.G776_776delinsVC (c.2326_2328delinsGTATGT) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 |
| Experiment for Molecule Alteration |
Sanger cDNA sequencing assay | |||
| Experiment for Drug Resistance |
CCK-8 assay | |||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [6] | |||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Sensitive Drug | Afabicin | |||
| Molecule Alteration | Duplication | p.G778_P780 (c.2332_2340)/p.780_Y781insGSP |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 |
| Experiment for Molecule Alteration |
Sanger cDNA sequencing assay | |||
| Experiment for Drug Resistance |
CCK-8 assay | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [6] | |||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Sensitive Drug | AUY922 | |||
| Molecule Alteration | Duplication | p.Y772_A775 (c.2314_2325) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 |
| Experiment for Molecule Alteration |
Sanger cDNA sequencing assay | |||
| Experiment for Drug Resistance |
CCK-8 assay | |||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [6] | |||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Sensitive Drug | AUY922 | |||
| Molecule Alteration | Complex-indel | p.G776_776delinsVC (c.2326_2328delinsGTATGT) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 |
| Experiment for Molecule Alteration |
Sanger cDNA sequencing assay | |||
| Experiment for Drug Resistance |
CCK-8 assay | |||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [6] | |||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Sensitive Drug | AUY922 | |||
| Molecule Alteration | Duplication | p.Y772_A775 (c.2314_2325)/p.A775_G776insYVMA + p.C805S |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 |
| Experiment for Molecule Alteration |
Sanger cDNA sequencing assay | |||
| Experiment for Drug Resistance |
CCK-8 assay | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Lung adenocarcinoma [ICD-11: 2C25.0] | [21] | |||
| Sensitive Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | |||
| Sensitive Drug | Poziotinib | |||
| Molecule Alteration | Duplication | p.Y772_A775 (c.2314_2325) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 |
| CUTO14 cells | N.A. | N.A. | N.A. | |
| In Vivo Model | Female nu/nu nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Cell Titer Glo assay | |||
| Mechanism Description | The duplication p.Y772_A775 (c.2314_2325) in gene ERBB2 cause the sensitivity of Poziotinib by aberration of the drug's therapeutic target. | |||
| Disease Class: Lung adenocarcinoma [ICD-11: 2C25.0] | [6] | |||
| Sensitive Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | |||
| Sensitive Drug | Poziotinib | |||
| Molecule Alteration | Duplication | p.Y772_A775 (c.2314_2325) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 |
| Experiment for Molecule Alteration |
Sanger cDNA sequencing assay | |||
| Experiment for Drug Resistance |
CCK-8 assay | |||
| Disease Class: Lung adenocarcinoma [ICD-11: 2C25.0] | [22] | |||
| Sensitive Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | |||
| Sensitive Drug | Poziotinib | |||
| Molecule Alteration | Duplication | p.Y772_A775 (c.2314_2325) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Disease Class: Lung adenocarcinoma [ICD-11: 2C25.0] | [22] | |||
| Sensitive Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | |||
| Sensitive Drug | Poziotinib | |||
| Molecule Alteration | Complex-indel | p.G776_776delinsVC (c.2326_2328delinsGTATGT) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Disease Class: Lung adenocarcinoma [ICD-11: 2C25.0] | [22] | |||
| Sensitive Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | |||
| Sensitive Drug | Poziotinib | |||
| Molecule Alteration | Missense mutation | p.E812K (c.2434G>A) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [21] | |||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Sensitive Drug | Poziotinib | |||
| Molecule Alteration | Duplication | p.Y772_A775 (c.2314_2325) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 |
| CUTO14 cells | N.A. | N.A. | N.A. | |
| In Vivo Model | Female nu/nu nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Cell Titer Glo assay | |||
| Mechanism Description | The duplication p.Y772_A775 (c.2314_2325) in gene ERBB2 cause the sensitivity of Poziotinib by aberration of the drug's therapeutic target. | |||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [21] | |||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Sensitive Drug | Poziotinib | |||
| Molecule Alteration | Complex-indel | p.G776_776delinsVV (c.2326_2328delinsGTAGTA) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 |
| CUTO14 cells | N.A. | N.A. | N.A. | |
| In Vivo Model | Female nu/nu nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Cell Titer Glo assay | |||
| Mechanism Description | The complex-indel p.G776_776delinsVV (c.2326_2328delinsGTAGTA) in gene ERBB2 cause the sensitivity of Poziotinib by aberration of the drug's therapeutic target. | |||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [21] | |||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Sensitive Drug | Poziotinib | |||
| Molecule Alteration | Complex-indel | p.G776_776delinsVC (c.2326_2328delinsGTATGT) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 |
| CUTO14 cells | N.A. | N.A. | N.A. | |
| In Vivo Model | Female nu/nu nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Cell Titer Glo assay | |||
| Mechanism Description | The complex-indel p.G776_776delinsVC (c.2326_2328delinsGTATGT) in gene ERBB2 cause the sensitivity of Poziotinib by aberration of the drug's therapeutic target. | |||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [21] | |||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Sensitive Drug | Poziotinib | |||
| Molecule Alteration | Duplication | p.G778_P780 (c.2332_2340) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 |
| CUTO14 cells | N.A. | N.A. | N.A. | |
| In Vivo Model | Female nu/nu nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Cell Titer Glo assay | |||
| Mechanism Description | The duplication p.G778_P780 (c.2332_2340) in gene ERBB2 cause the sensitivity of Poziotinib by aberration of the drug's therapeutic target. | |||
| Disease Class: Lung adenocarcinoma [ICD-11: 2C25.0] | [6] | |||
| Sensitive Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | |||
| Sensitive Drug | Poziotinib | |||
| Molecule Alteration | Complex-indel | p.G776_776delinsVC (c.2326_2328delinsGTATGT) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 |
| Experiment for Molecule Alteration |
Sanger cDNA sequencing assay | |||
| Experiment for Drug Resistance |
CCK-8 assay | |||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [6] | |||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Sensitive Drug | Poziotinib | |||
| Molecule Alteration | Duplication | p.G778_P780 (c.2332_2340)/p.780_Y781insGSP |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 |
| Experiment for Molecule Alteration |
Sanger cDNA sequencing assay | |||
| Experiment for Drug Resistance |
CCK-8 assay | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [23] | |||
| Resistant Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Resistant Drug | Sapitinib | |||
| Molecule Alteration | Missense mutation | p.L869R (c.2606T>G) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | MCF10A cells | Breast | Homo sapiens (Human) | CVCL_0598 |
| CW-2 cells | Colon | Homo sapiens (Human) | CVCL_1151 | |
| Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 | |
| In Vivo Model | NOD.Cg-Prkdcscid IL2rtftmWjl/Szj xenograft model | Mus musculus | ||
| Experiment for Drug Resistance |
Cell Titer Glo assay | |||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [23] | |||
| Resistant Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Resistant Drug | Sapitinib | |||
| Molecule Alteration | Missense mutation | p.L755S (c.2263_2264delCTinsAG) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | MCF10A cells | Breast | Homo sapiens (Human) | CVCL_0598 |
| CW-2 cells | Colon | Homo sapiens (Human) | CVCL_1151 | |
| Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 | |
| In Vivo Model | NOD.Cg-Prkdcscid IL2rtftmWjl/Szj xenograft model | Mus musculus | ||
| Experiment for Drug Resistance |
Cell Titer Glo assay | |||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [23] | |||
| Resistant Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Resistant Drug | Sapitinib | |||
| Molecule Alteration | Missense mutation | p.L755P (c.2264T>C) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | MCF10A cells | Breast | Homo sapiens (Human) | CVCL_0598 |
| CW-2 cells | Colon | Homo sapiens (Human) | CVCL_1151 | |
| Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 | |
| In Vivo Model | NOD.Cg-Prkdcscid IL2rtftmWjl/Szj xenograft model | Mus musculus | ||
| Experiment for Drug Resistance |
Cell Titer Glo assay | |||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [23] | |||
| Resistant Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Resistant Drug | Sapitinib | |||
| Molecule Alteration | Missense mutation | p.D769N (c.2305G>A) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | MCF10A cells | Breast | Homo sapiens (Human) | CVCL_0598 |
| CW-2 cells | Colon | Homo sapiens (Human) | CVCL_1151 | |
| Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 | |
| In Vivo Model | NOD.Cg-Prkdcscid IL2rtftmWjl/Szj xenograft model | Mus musculus | ||
| Experiment for Drug Resistance |
Cell Titer Glo assay | |||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [23] | |||
| Resistant Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Resistant Drug | Sapitinib | |||
| Molecule Alteration | Missense mutation | p.D769H (c.2305G>C) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | MCF10A cells | Breast | Homo sapiens (Human) | CVCL_0598 |
| CW-2 cells | Colon | Homo sapiens (Human) | CVCL_1151 | |
| Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 | |
| In Vivo Model | NOD.Cg-Prkdcscid IL2rtftmWjl/Szj xenograft model | Mus musculus | ||
| Experiment for Drug Resistance |
Cell Titer Glo assay | |||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [23] | |||
| Resistant Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Resistant Drug | Sapitinib | |||
| Molecule Alteration | Missense mutation | p.D769Y (c.2305G>T) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | MCF10A cells | Breast | Homo sapiens (Human) | CVCL_0598 |
| CW-2 cells | Colon | Homo sapiens (Human) | CVCL_1151 | |
| Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 | |
| In Vivo Model | NOD.Cg-Prkdcscid IL2rtftmWjl/Szj xenograft model | Mus musculus | ||
| Experiment for Drug Resistance |
Cell Titer Glo assay | |||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [23] | |||
| Resistant Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Resistant Drug | Sapitinib | |||
| Molecule Alteration | Duplication | p.Y772_A775 (c.2314_2325) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | MCF10A cells | Breast | Homo sapiens (Human) | CVCL_0598 |
| CW-2 cells | Colon | Homo sapiens (Human) | CVCL_1151 | |
| Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 | |
| In Vivo Model | NOD.Cg-Prkdcscid IL2rtftmWjl/Szj xenograft model | Mus musculus | ||
| Experiment for Drug Resistance |
Cell Titer Glo assay | |||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [23] | |||
| Resistant Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Resistant Drug | Sapitinib | |||
| Molecule Alteration | Missense mutation | p.V773M (c.2317G>A) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | MCF10A cells | Breast | Homo sapiens (Human) | CVCL_0598 |
| CW-2 cells | Colon | Homo sapiens (Human) | CVCL_1151 | |
| Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 | |
| In Vivo Model | NOD.Cg-Prkdcscid IL2rtftmWjl/Szj xenograft model | Mus musculus | ||
| Experiment for Drug Resistance |
Cell Titer Glo assay | |||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [23] | |||
| Resistant Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Resistant Drug | Sapitinib | |||
| Molecule Alteration | Complex-indel | p.G776_776delinsLC (c.2326_2328delinsCTTTGT) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | MCF10A cells | Breast | Homo sapiens (Human) | CVCL_0598 |
| CW-2 cells | Colon | Homo sapiens (Human) | CVCL_1151 | |
| Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 | |
| In Vivo Model | NOD.Cg-Prkdcscid IL2rtftmWjl/Szj xenograft model | Mus musculus | ||
| Experiment for Drug Resistance |
Cell Titer Glo assay | |||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [23] | |||
| Resistant Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Resistant Drug | Sapitinib | |||
| Molecule Alteration | Complex-indel | p.G776_776delinsVV (c.2326_2328delinsGTAGTA) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | MCF10A cells | Breast | Homo sapiens (Human) | CVCL_0598 |
| CW-2 cells | Colon | Homo sapiens (Human) | CVCL_1151 | |
| Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 | |
| In Vivo Model | NOD.Cg-Prkdcscid IL2rtftmWjl/Szj xenograft model | Mus musculus | ||
| Experiment for Drug Resistance |
Cell Titer Glo assay | |||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [23] | |||
| Resistant Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Resistant Drug | Sapitinib | |||
| Molecule Alteration | Complex-indel | p.G776_776delinsVC (c.2326_2328delinsGTATGT) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | MCF10A cells | Breast | Homo sapiens (Human) | CVCL_0598 |
| CW-2 cells | Colon | Homo sapiens (Human) | CVCL_1151 | |
| Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 | |
| In Vivo Model | NOD.Cg-Prkdcscid IL2rtftmWjl/Szj xenograft model | Mus musculus | ||
| Experiment for Drug Resistance |
Cell Titer Glo assay | |||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [23] | |||
| Resistant Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Resistant Drug | Sapitinib | |||
| Molecule Alteration | Missense mutation | p.V777L (c.2329G>C) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | MCF10A cells | Breast | Homo sapiens (Human) | CVCL_0598 |
| CW-2 cells | Colon | Homo sapiens (Human) | CVCL_1151 | |
| Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 | |
| In Vivo Model | NOD.Cg-Prkdcscid IL2rtftmWjl/Szj xenograft model | Mus musculus | ||
| Experiment for Drug Resistance |
Cell Titer Glo assay | |||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [23] | |||
| Resistant Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Resistant Drug | Sapitinib | |||
| Molecule Alteration | Duplication | p.G778_P780 (c.2332_2340) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | MCF10A cells | Breast | Homo sapiens (Human) | CVCL_0598 |
| CW-2 cells | Colon | Homo sapiens (Human) | CVCL_1151 | |
| Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 | |
| In Vivo Model | NOD.Cg-Prkdcscid IL2rtftmWjl/Szj xenograft model | Mus musculus | ||
| Experiment for Drug Resistance |
Cell Titer Glo assay | |||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [23] | |||
| Resistant Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Resistant Drug | Sapitinib | |||
| Molecule Alteration | IF-insertion | p.G778_S779insLPS (c.2334_2335insCTTCCTAGC) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | MCF10A cells | Breast | Homo sapiens (Human) | CVCL_0598 |
| CW-2 cells | Colon | Homo sapiens (Human) | CVCL_1151 | |
| Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 | |
| In Vivo Model | NOD.Cg-Prkdcscid IL2rtftmWjl/Szj xenograft model | Mus musculus | ||
| Experiment for Drug Resistance |
Cell Titer Glo assay | |||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [23] | |||
| Resistant Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Resistant Drug | Sapitinib | |||
| Molecule Alteration | Missense mutation | p.L786V (c.2356C>G) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | MCF10A cells | Breast | Homo sapiens (Human) | CVCL_0598 |
| CW-2 cells | Colon | Homo sapiens (Human) | CVCL_1151 | |
| Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 | |
| In Vivo Model | NOD.Cg-Prkdcscid IL2rtftmWjl/Szj xenograft model | Mus musculus | ||
| Experiment for Drug Resistance |
Cell Titer Glo assay | |||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [23] | |||
| Resistant Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Resistant Drug | Sapitinib | |||
| Molecule Alteration | Missense mutation | p.V842I (c.2524G>A) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | MCF10A cells | Breast | Homo sapiens (Human) | CVCL_0598 |
| CW-2 cells | Colon | Homo sapiens (Human) | CVCL_1151 | |
| Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 | |
| In Vivo Model | NOD.Cg-Prkdcscid IL2rtftmWjl/Szj xenograft model | Mus musculus | ||
| Experiment for Drug Resistance |
Cell Titer Glo assay | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [24] | |||
| Resistant Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Resistant Drug | AEE-788 | |||
| Molecule Alteration | Missense mutation | p.L755S (c.2263_2264delCTinsAG) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | HEK293 cells | Kidney | Homo sapiens (Human) | CVCL_0045 |
| Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTS assay | |||
| Mechanism Description | The missense mutation p.L755S (c.2263_2264delCTinsAG) in gene ERBB2 cause the resistance of AEE-788 by aberration of the drug's therapeutic target | |||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [24] | |||
| Resistant Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Resistant Drug | AEE-788 | |||
| Molecule Alteration | Missense mutation | p.L755P (c.2264T>C) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | HEK293 cells | Kidney | Homo sapiens (Human) | CVCL_0045 |
| Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTS assay | |||
| Mechanism Description | The missense mutation p.L755P (c.2264T>C) in gene ERBB2 cause the resistance of AEE-788 by aberration of the drug's therapeutic target | |||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [24] | |||
| Resistant Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Resistant Drug | AEE-788 | |||
| Molecule Alteration | Missense mutation | p.T798M (c.2393_2394delCAinsTG) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | HEK293 cells | Kidney | Homo sapiens (Human) | CVCL_0045 |
| Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTS assay | |||
| Mechanism Description | The missense mutation p.T798M (c.2393_2394delCAinsTG) in gene ERBB2 cause the resistance of AEE-788 by aberration of the drug's therapeutic target | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [24] | |||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Sensitive Drug | AEE-788 | |||
| Molecule Alteration | Missense mutation | p.V773A (c.2318T>C) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | HEK293 cells | Kidney | Homo sapiens (Human) | CVCL_0045 |
| Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTS assay | |||
| Mechanism Description | The missense mutation p.V773A (c.2318T>C) in gene ERBB2 cause the sensitivity of AEE-788 by aberration of the drug's therapeutic target | |||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [24] | |||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Sensitive Drug | AEE-788 | |||
| Molecule Alteration | Missense mutation | p.V777L (c.2329G>C) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | HEK293 cells | Kidney | Homo sapiens (Human) | CVCL_0045 |
| Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTS assay | |||
| Mechanism Description | The missense mutation p.V777L (c.2329G>C) in gene ERBB2 cause the sensitivity of AEE-788 by aberration of the drug's therapeutic target | |||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [24] | |||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Sensitive Drug | AEE-788 | |||
| Molecule Alteration | Missense mutation | p.N857S (c.2570A>G) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | HEK293 cells | Kidney | Homo sapiens (Human) | CVCL_0045 |
| Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTS assay | |||
| Mechanism Description | The missense mutation p.N857S (c.2570A>G) in gene ERBB2 cause the sensitivity of AEE-788 by aberration of the drug's therapeutic target | |||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [24] | |||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Sensitive Drug | AEE-788 | |||
| Molecule Alteration | Missense mutation | p.T862A (c.2584A>G) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | HEK293 cells | Kidney | Homo sapiens (Human) | CVCL_0045 |
| Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTS assay | |||
| Mechanism Description | The missense mutation p.T862A (c.2584A>G) in gene ERBB2 cause the sensitivity of AEE-788 by aberration of the drug's therapeutic target | |||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [24] | |||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Sensitive Drug | AEE-788 | |||
| Molecule Alteration | Missense mutation | p.H878Y (c.2632C>T) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | HEK293 cells | Kidney | Homo sapiens (Human) | CVCL_0045 |
| Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTS assay | |||
| Mechanism Description | The missense mutation p.H878Y (c.2632C>T) in gene ERBB2 cause the sensitivity of AEE-788 by aberration of the drug's therapeutic target | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [6] | |||
| Resistant Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Resistant Drug | Pyrotinib | |||
| Molecule Alteration | Duplication | p.G778_P780 (c.2332_2340)/p.780_Y781insGSP |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 |
| Experiment for Molecule Alteration |
Sanger cDNA sequencing assay | |||
| Experiment for Drug Resistance |
CCK-8 assay | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [6] | |||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Sensitive Drug | Pyrotinib | |||
| Molecule Alteration | Duplication | p.Y772_A775 (c.2314_2325) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 |
| Experiment for Molecule Alteration |
Sanger cDNA sequencing assay | |||
| Experiment for Drug Resistance |
CCK-8 assay | |||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [6] | |||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Sensitive Drug | Pyrotinib | |||
| Molecule Alteration | Complex-indel | p.G776_776delinsVC (c.2326_2328delinsGTATGT) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 |
| Experiment for Molecule Alteration |
Sanger cDNA sequencing assay | |||
| Experiment for Drug Resistance |
CCK-8 assay | |||
Discontinued Drug(s)
1 drug(s) in total
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Non-small cell lung cancer [ICD-11: 2C25.Y] | [25], [26] | |||
| Resistant Disease | Non-small cell lung cancer [ICD-11: 2C25.Y] | |||
| Resistant Drug | Rociletinib | |||
| Molecule Alteration | Structural variation | Copy number gain |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Experiment for Molecule Alteration |
Next-generation sequencing analysis; Gene copy number analysis | |||
| Experiment for Drug Resistance |
Progression-free survival assay | |||
| Mechanism Description | Similarly,resistance to the third-generation inhibitor rociletinib may not only be mediated by EGFR (L798I, C797S) mutations, but also by alterations of MET, PIk3CA, ERRB2, and kRAS, and by the negative selection of T790M-mutant subclones. | |||
|
|
||||
| Disease Class: Non-small cell lung cancer [ICD-11: 2C25.Y] | [25] | |||
| Resistant Disease | Non-small cell lung cancer [ICD-11: 2C25.Y] | |||
| Resistant Drug | Rociletinib | |||
| Molecule Alteration | Structural variation | Amplification |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vivo Model | A retrospective survey in conducting clinical studies | Homo sapiens | ||
| Experiment for Molecule Alteration |
Circulating tumour DNA (ctDNA) analysis | |||
| Experiment for Drug Resistance |
Tissue biopsy assay; CT scan assay | |||
| Mechanism Description | Increased MET copy number is the most frequent rociletinib resistance mechanism in this cohort and patients with multiple pre-existing mechanisms (T790M and MET) experience inferior responses. | |||
Preclinical Drug(s)
6 drug(s) in total
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [6] | |||
| Resistant Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Resistant Drug | AZ5104 | |||
| Molecule Alteration | Duplication | p.Y772_A775 (c.2314_2325) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 |
| Experiment for Molecule Alteration |
Sanger cDNA sequencing assay | |||
| Experiment for Drug Resistance |
CCK-8 assay | |||
|
|
||||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [6] | |||
| Resistant Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Resistant Drug | AZ5104 | |||
| Molecule Alteration | Duplication | p.G778_P780 (c.2332_2340) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [6] | |||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Sensitive Drug | AZ5104 | |||
| Molecule Alteration | Complex-indel | p.G776_776delinsVC (c.2326_2328delinsGTATGT) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 |
| Experiment for Molecule Alteration |
Sanger cDNA sequencing assay | |||
| Experiment for Drug Resistance |
CCK-8 assay | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [24] | |||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Sensitive Drug | EKI-285 | |||
| Molecule Alteration | Missense mutation | p.T798M (c.2393_2394delCAinsTG) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | HEK293 cells | Kidney | Homo sapiens (Human) | CVCL_0045 |
| Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTS assay | |||
| Mechanism Description | The missense mutation p.T798M (c.2393_2394delCAinsTG) in gene ERBB2 cause the sensitivity of EKI-285 by aberration of the drug's therapeutic target | |||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [24] | |||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Sensitive Drug | EKI-285 | |||
| Molecule Alteration | Missense mutation | p.L755S (c.2263_2264delCTinsAG) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | HEK293 cells | Kidney | Homo sapiens (Human) | CVCL_0045 |
| Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTS assay | |||
| Mechanism Description | The missense mutation p.L755S (c.2263_2264delCTinsAG) in gene ERBB2 cause the sensitivity of EKI-285 by aberration of the drug's therapeutic target | |||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [24] | |||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Sensitive Drug | EKI-285 | |||
| Molecule Alteration | Missense mutation | p.L755P (c.2264T>C) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | HEK293 cells | Kidney | Homo sapiens (Human) | CVCL_0045 |
| Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTS assay | |||
| Mechanism Description | The missense mutation p.L755P (c.2264T>C) in gene ERBB2 cause the sensitivity of EKI-285 by aberration of the drug's therapeutic target | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | |||||||||||||
|
|
|||||||||||||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [27] | ||||||||||||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | ||||||||||||
| Sensitive Drug | ERBB2 TKIs | ||||||||||||
| Molecule Alteration | Missense mutation | p.S310F (c.929C>T) |
|||||||||||
| Wild Type Structure | Method: X-ray diffraction | Resolution: 3.09 Å | |||||||||||
| Mutant Type Structure | Method: Electron microscopy | Resolution: 3.09 Å | |||||||||||
| Download The Information of Sequence | Download The Structure File | ||||||||||||
-
T
M
Q
E
V
L
C
A
T
A
G
L
T
C
D
R
M
W
10
|
K
G
L
L
R
L
L
L
P
A
A
L
S
L
P
P
E
P
T
G
20
|
H
A
L
A
D
S
M
T
L
Q
R
V
H
C
L
T
Y
G
Q
T
30
|
G
D
C
M
Q
K
V
L
V
R
Q
L
G
P
N
A
L
S
E
P
40
|
L
E
T
T
Y
H
L
L
P
D
T
M
N
L
A
R
S
H
L
L
50
|
S
Y
F
Q
L
G
Q
C
D
Q
I
V
Q
V
E
Q
V
G
Q
N
60
|
G
L
Y
E
V
L
L
T
I
Y
A
L
H
P
N
T
Q
N
V
A
70
|
R
S
Q
L
V
S
P
F
L
L
Q
Q
R
D
L
I
R
Q
I
E
80
|
V
V
R
Q
G
G
T
Y
Q
V
L
L
F
I
E
A
D
H
N
N
90
|
Y
Q
A
V
L
R
A
Q
V
V
L
P
D
L
N
Q
G
R
D
L
100
|
P
R
L
I
N
V
N
R
T
G
T
T
P
Q
V
L
T
F
G
E
110
|
A
D
S
N
P
Y
G
A
G
L
L
A
R
V
E
L
L
D
Q
N
120
|
L
G
R
D
S
P
L
L
T
N
E
N
I
T
L
T
K
P
G
V
130
|
G
T
V
G
L
A
I
S
Q
P
R
G
N
G
P
L
Q
R
L
E
140
|
C
L
Y
Q
Q
L
D
R
T
S
I
L
L
T
W
E
K
I
D
L
150
|
I
K
F
G
H
G
K
V
N
L
N
I
Q
Q
L
R
A
N
L
P
160
|
T
Q
L
L
I
C
D
Y
T
Q
N
D
R
T
S
I
R
L
A
W
170
|
C
K
H
D
P
I
C
F
S
H
P
K
M
N
C
N
K
Q
G
L
180
|
S
A
R
L
C
T
W
L
G
I
E
D
S
T
S
N
E
R
D
S
190
|
C
R
Q
A
S
C
L
H
T
P
R
C
T
S
V
P
C
M
A
C
200
|
G
K
G
G
C
S
A
R
R
C
C
W
K
G
G
E
P
S
L
S
210
|
P
E
T
D
D
C
C
Q
C
S
H
L
E
T
Q
R
C
T
A
V
220
|
A
C
G
A
C
G
T
G
G
C
P
A
K
R
H
C
S
K
D
G
230
|
C
P
L
L
A
P
C
T
L
D
H
C
F
C
N
H
H
E
S
Q
240
|
G
C
I
A
C
A
E
G
L
C
H
T
C
G
P
P
A
K
L
H
250
|
V
S
T
D
Y
C
N
L
T
A
D
C
T
L
F
H
E
F
S
N
260
|
M
H
P
S
N
G
P
I
E
C
G
E
R
L
Y
H
T
C
F
P
270
|
G
A
A
L
S
V
C
T
V
Y
T
N
A
T
C
D
P
T
Y
F
280
|
N
E
Y
S
L
M
S
P
T
N
D
P
V
E
G
G
S
R
C
Y
290
|
T
T
L
F
V
G
C
A
P
S
L
C
H
V
N
T
Q
A
E
C
300
|
V
P
T
Y
A
N
E
Y
D
L
G
S
T
T
Q
D
R
V
C
G
310
|
E
F
K
C
C
T
S
L
K
V
P
C
C
P
A
L
R
H
V
N
320
|
C
Q
Y
E
G
V
L
T
G
A
M
E
E
D
H
G
L
T
R
Q
330
|
E
R
V
C
R
E
A
K
V
C
T
S
S
K
A
P
N
C
I
A
340
|
Q
R
E
V
F
C
A
Y
G
G
C
L
K
G
K
M
I
E
F
H
350
|
G
L
S
R
L
E
A
V
F
R
L
A
P
V
E
T
S
S
F
A
360
|
D
N
G
I
D
Q
P
E
A
F
S
A
N
G
T
C
A
K
P
K
370
|
L
I
Q
F
P
G
E
S
Q
L
L
A
Q
F
V
L
F
P
E
E
380
|
T
S
L
F
E
D
E
G
I
D
T
P
G
A
Y
S
L
N
Y
T
390
|
I
A
S
P
A
L
W
Q
P
P
D
E
S
Q
L
L
P
Q
D
V
400
|
L
F
S
E
V
T
F
L
Q
E
N
E
L
I
Q
T
V
G
I
Y
410
|
R
L
G
Y
R
I
I
S
L
A
H
W
N
P
G
D
A
S
Y
L
420
|
S
P
L
D
T
L
L
S
Q
V
G
F
L
Q
G
N
I
L
S
Q
430
|
W
V
L
I
G
R
L
G
R
R
S
I
L
L
R
H
E
N
L
G
440
|
G
A
S
Y
G
S
L
L
A
T
L
L
I
Q
H
G
H
L
N
G
450
|
T
I
H
S
L
W
C
L
F
G
V
L
H
R
T
S
V
L
P
R
460
|
W
E
D
L
Q
G
L
S
F
G
R
L
N
A
P
L
H
I
Q
H
470
|
A
H
L
N
L
T
H
H
T
L
A
C
N
F
R
V
P
H
E
T
480
|
D
V
E
P
C
W
V
D
G
Q
E
L
G
F
L
R
A
N
C
P
490
|
H
H
Q
Q
L
A
C
L
A
L
R
H
G
T
H
A
C
N
W
R
500
|
G
P
P
E
G
D
P
E
T
C
Q
V
C
G
V
E
N
G
C
L
510
|
S
A
Q
C
F
H
L
Q
R
L
G
C
Q
A
E
R
C
G
V
H
520
|
E
C
E
W
C
G
R
P
V
G
L
P
Q
T
G
Q
L
C
P
V
530
|
R
N
E
C
Y
S
V
Q
N
F
A
L
R
R
H
G
C
Q
L
E
540
|
P
C
C
V
H
E
P
E
E
C
C
R
Q
V
P
L
Q
Q
N
G
550
|
G
L
S
P
V
R
T
E
C
Y
F
V
G
N
P
A
E
R
A
H
560
|
D
C
Q
L
C
P
V
C
A
H
-
P
-
E
-
C
-
Q
-
P
570
|
-
Q
-
N
-
G
-
S
-
V
-
T
-
C
-
F
-
G
-
P
580
|
-
E
-
A
-
D
-
Q
-
C
-
V
-
A
-
C
-
A
-
H
590
|
-
Y
-
K
-
D
-
P
-
P
-
F
-
C
-
V
-
A
-
R
600
|
-
C
-
P
-
S
-
G
-
V
-
K
-
P
-
D
-
L
-
S
610
|
-
Y
-
M
-
P
-
I
-
W
-
K
-
F
-
P
-
D
-
E
620
|
-
E
-
G
-
A
-
C
-
Q
-
P
-
C
-
P
-
I
-
N
630
|
-
C
-
T
-
H
-
S
-
C
-
V
-
D
-
L
-
D
-
D
640
|
-
K
-
G
-
C
-
P
-
A
-
E
-
Q
-
R
-
A
-
S
650
|
-
P
-
L
-
T
-
S
-
I
-
I
-
S
-
A
-
V
-
V
660
|
-
G
-
I
-
L
-
L
-
V
-
V
-
V
-
L
-
G
-
V
670
|
-
V
-
F
-
G
-
I
-
L
-
I
-
K
-
R
-
R
-
Q
680
|
-
Q
-
K
-
I
-
R
-
K
-
Y
-
T
-
M
-
R
-
R
690
|
-
L
-
L
-
Q
-
E
-
T
-
E
-
L
-
V
-
E
-
P
700
|
-
L
-
T
-
P
-
S
-
G
-
A
-
M
-
P
-
N
-
Q
710
|
-
A
-
Q
-
M
-
R
-
I
-
L
-
K
-
E
-
T
-
E
720
|
-
L
-
R
-
K
-
V
-
K
-
V
-
L
-
G
-
S
-
G
730
|
-
A
-
F
-
G
-
T
-
V
-
Y
-
K
-
G
-
I
-
W
740
|
-
I
-
P
-
D
-
G
-
E
-
N
-
V
-
K
-
I
-
P
750
|
-
V
-
A
-
I
-
K
-
V
-
L
-
R
-
E
-
N
-
T
760
|
-
S
-
P
-
K
-
A
-
N
-
K
-
E
-
I
-
L
-
D
770
|
-
E
-
A
-
Y
-
V
-
M
-
A
-
G
-
V
-
D
-
S
780
|
-
P
-
Y
-
V
-
S
-
R
-
L
-
L
-
G
-
I
-
C
790
|
-
L
-
T
-
S
-
T
-
V
-
Q
-
L
-
V
-
T
-
Q
800
|
-
L
-
M
-
P
-
Y
-
G
-
C
-
L
-
L
-
D
-
H
810
|
-
V
-
R
-
E
-
N
-
R
-
G
-
R
-
L
-
G
-
S
820
|
-
Q
-
D
-
L
-
L
-
N
-
W
-
C
-
M
-
Q
-
I
830
|
-
A
-
K
-
G
-
M
-
S
-
Y
-
L
-
E
-
D
-
V
840
|
-
R
-
L
-
V
-
H
-
R
-
D
-
L
-
A
-
A
-
R
850
|
-
N
-
V
-
L
-
V
-
K
-
S
-
P
-
N
-
H
-
V
860
|
-
K
-
I
-
T
-
D
-
F
-
G
-
L
-
A
-
R
-
L
870
|
-
L
-
D
-
I
-
D
-
E
-
T
-
E
-
Y
-
H
-
A
880
|
-
D
-
G
-
G
-
K
-
V
-
P
-
I
-
K
-
W
-
M
890
|
-
A
-
L
-
E
-
S
-
I
-
L
-
R
-
R
-
R
-
F
900
|
-
T
-
H
-
Q
-
S
-
D
-
V
-
W
-
S
-
Y
-
G
910
|
-
V
-
T
-
V
-
W
-
E
-
L
-
M
-
T
-
F
-
G
920
|
-
A
-
K
-
P
-
Y
-
D
-
G
-
I
-
P
-
A
-
R
930
|
-
E
-
I
-
P
-
D
-
L
-
L
-
E
-
K
-
G
-
E
940
|
-
R
-
L
-
P
-
Q
-
P
-
P
-
I
-
C
-
T
-
I
950
|
-
D
-
V
-
Y
-
M
-
I
-
M
-
V
-
K
-
C
-
W
960
|
-
M
-
I
-
D
-
S
-
E
-
C
-
R
-
P
-
R
-
F
970
|
-
R
-
E
-
L
-
V
-
S
-
E
-
F
-
S
-
R
-
M
980
|
-
A
-
R
-
D
-
P
-
Q
-
R
-
F
-
V
-
V
-
I
990
|
-
Q
-
N
-
E
-
D
-
L
-
G
-
P
-
A
-
S
-
P
1000
|
-
L
-
D
-
S
-
T
-
F
-
Y
-
R
-
S
-
L
-
L
1010
|
-
E
-
D
-
D
-
D
-
M
-
G
-
D
-
L
-
V
-
D
1020
|
-
A
-
E
-
E
-
Y
-
L
-
V
-
P
-
Q
-
Q
-
G
1030
|
-
G
-
G
-
S
-
L
-
E
-
V
-
L
-
F
-
Q
-
G
1040
|
-
P
-
S
-
S
-
P
-
S
-
G
-
S
-
S
-
M
-
K
1050
|
-
I
-
E
-
E
-
G
-
K
-
L
-
V
-
I
-
W
-
I
1060
|
-
N
-
G
-
D
-
K
-
G
-
Y
-
N
-
G
-
L
-
A
1070
|
-
E
-
V
-
G
-
K
-
K
-
F
-
E
-
K
-
D
-
T
1080
|
-
G
-
I
-
K
-
V
-
T
-
V
-
E
-
H
-
P
-
D
1090
|
-
K
-
L
-
E
-
E
-
K
-
F
-
P
-
Q
-
V
-
A
1100
|
-
A
-
T
-
G
-
D
-
G
-
P
-
D
-
I
-
I
-
F
1110
|
-
W
-
A
-
H
-
D
-
R
-
F
-
G
-
G
-
Y
-
A
1120
|
-
Q
-
S
-
G
-
L
-
L
-
A
-
E
-
I
-
T
-
P
1130
|
-
D
-
K
-
A
-
F
-
Q
-
D
-
K
-
L
-
Y
-
P
1140
|
-
F
-
T
-
W
-
D
-
A
-
V
-
R
-
Y
-
N
-
G
1150
|
-
K
-
L
-
I
-
A
-
Y
-
P
-
I
-
A
-
V
-
E
1160
|
-
A
-
L
-
S
-
L
-
I
-
Y
-
N
-
K
-
D
-
L
1170
|
-
L
-
P
-
N
-
P
-
P
-
K
-
T
-
W
-
E
-
E
1180
|
-
I
-
P
-
A
-
L
-
D
-
K
-
E
-
L
-
K
-
A
1190
|
-
K
-
G
-
K
-
S
-
A
-
L
-
M
-
F
-
N
-
L
1200
|
-
Q
-
E
-
P
-
Y
-
F
-
T
-
W
-
P
-
L
-
I
1210
|
-
A
-
A
-
D
-
G
-
G
-
Y
-
A
-
F
-
K
-
Y
1220
|
-
E
-
N
-
G
-
K
-
Y
-
D
-
I
-
K
-
D
-
V
1230
|
-
G
-
V
-
D
-
N
-
A
-
G
-
A
-
K
-
A
-
G
1240
|
-
L
-
T
-
F
-
L
-
V
-
D
-
L
-
I
-
K
-
N
1250
|
-
K
-
H
-
M
-
N
-
A
-
D
-
T
-
D
-
Y
-
S
1260
|
-
I
-
A
-
E
-
A
-
A
-
F
-
N
-
K
-
G
-
E
1270
|
-
T
-
A
-
M
-
T
-
I
-
N
-
G
-
P
-
W
-
A
1280
|
-
W
-
S
-
N
-
I
-
D
-
T
-
S
-
K
-
V
-
N
1290
|
-
Y
-
G
-
V
-
T
-
V
-
L
-
P
-
T
-
F
-
K
1300
|
-
G
-
Q
-
P
-
S
-
K
-
P
-
F
-
V
-
G
-
V
1310
|
-
L
-
S
-
A
-
G
-
I
-
N
-
A
-
A
-
S
-
P
1320
|
-
N
-
K
-
E
-
L
-
A
-
K
-
E
-
F
-
L
-
E
1330
|
-
N
-
Y
-
L
-
L
-
T
-
D
-
E
-
G
-
L
-
E
1340
|
-
A
-
V
-
N
-
K
-
D
-
K
-
P
-
L
-
G
-
A
1350
|
-
V
-
A
-
L
-
K
-
S
-
Y
-
E
-
E
-
E
-
L
1360
|
-
A
-
K
-
D
-
P
-
R
-
I
-
A
-
A
-
T
-
M
1370
|
-
E
-
N
-
A
-
Q
-
K
-
G
-
E
-
I
-
M
-
P
1380
|
-
N
-
I
-
P
-
Q
-
M
-
S
-
A
-
F
-
W
-
Y
1390
|
-
A
-
V
-
R
-
T
-
A
-
V
-
I
-
N
-
A
-
A
1400
|
-
S
-
G
-
R
-
Q
-
T
-
V
-
D
-
E
-
A
-
L
1410
|
-
K
-
D
-
A
-
Q
-
T
-
N
-
S
-
S
-
S
-
S
1420
|
-
G
-
P
-
S
-
S
-
P
-
S
-
A
-
W
-
S
-
H
1430
|
-
P
-
Q
-
F
-
E
-
K
-
G
-
G
-
G
-
S
-
G
1440
|
-
G
-
G
-
S
-
G
-
G
-
S
-
S
-
A
-
W
-
S
1450
|
-
H
-
P
-
Q
-
F
-
E
-
K
|
|||||||||||||
| Experimental Note | Revealed Based on the Cell Line Data | ||||||||||||
| In Vitro Model | NIH 3T3 cells | Colon | Homo sapiens (Human) | CVCL_0594 | |||||||||
| Experiment for Drug Resistance |
WST-1 assay | ||||||||||||
| Mechanism Description | The missense mutation p.S310F (c.929C>T) in gene ERBB2 cause the sensitivity of ERBB2 TKIs by aberration of the drug's therapeutic target | ||||||||||||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [27] | ||||||||||||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | ||||||||||||
| Sensitive Drug | ERBB2 TKIs | ||||||||||||
| Molecule Alteration | Missense mutation | p.G309E (c.926G>A) |
|||||||||||
| Experimental Note | Revealed Based on the Cell Line Data | ||||||||||||
| In Vitro Model | NIH 3T3 cells | Colon | Homo sapiens (Human) | CVCL_0594 | |||||||||
| Experiment for Drug Resistance |
WST-1 assay | ||||||||||||
| Mechanism Description | The missense mutation p.G309E (c.926G>A) in gene ERBB2 cause the sensitivity of ERBB2 TKIs by aberration of the drug's therapeutic target | ||||||||||||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [27] | ||||||||||||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | ||||||||||||
| Sensitive Drug | ERBB2 TKIs | ||||||||||||
| Molecule Alteration | Missense mutation | p.S310Y (c.929C>A) |
|||||||||||
| Experimental Note | Revealed Based on the Cell Line Data | ||||||||||||
| In Vitro Model | NIH 3T3 cells | Colon | Homo sapiens (Human) | CVCL_0594 | |||||||||
| Experiment for Drug Resistance |
WST-1 assay | ||||||||||||
| Mechanism Description | The missense mutation p.S310Y (c.929C>A) in gene ERBB2 cause the sensitivity of ERBB2 TKIs by aberration of the drug's therapeutic target | ||||||||||||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [27] | ||||||||||||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | ||||||||||||
| Sensitive Drug | ERBB2 TKIs | ||||||||||||
| Molecule Alteration | Missense mutation | p.C311R (c.931T>C) |
|||||||||||
| Experimental Note | Revealed Based on the Cell Line Data | ||||||||||||
| In Vitro Model | NIH 3T3 cells | Colon | Homo sapiens (Human) | CVCL_0594 | |||||||||
| Experiment for Drug Resistance |
WST-1 assay | ||||||||||||
| Mechanism Description | The missense mutation p.C311R (c.931T>C) in gene ERBB2 cause the sensitivity of ERBB2 TKIs by aberration of the drug's therapeutic target | ||||||||||||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [27] | ||||||||||||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | ||||||||||||
| Sensitive Drug | ERBB2 TKIs | ||||||||||||
| Molecule Alteration | Missense mutation | p.E321G (c.962A>G) |
|||||||||||
| Experimental Note | Revealed Based on the Cell Line Data | ||||||||||||
| In Vitro Model | NIH 3T3 cells | Colon | Homo sapiens (Human) | CVCL_0594 | |||||||||
| Experiment for Drug Resistance |
WST-1 assay | ||||||||||||
| Mechanism Description | The missense mutation p.E321G (c.962A>G) in gene ERBB2 cause the sensitivity of ERBB2 TKIs by aberration of the drug's therapeutic target | ||||||||||||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [28] | |||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Sensitive Drug | JQ1/Osimertinib | |||
| Molecule Alteration | IF-insertion | p.Y772_A775dupYVMA (c.2325_2326insTATGTAATGGCA) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 |
| NCI-H1781 cells | Pleural effusion | Homo sapiens (Human) | CVCL_1494 | |
| Experiment for Molecule Alteration |
Western blot analysis; Immunohistochemistry; H&E staining assay | |||
| Experiment for Drug Resistance |
CCK-8 assay | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Breast adenocarcinoma [ICD-11: 2C60.1] | [13] | |||
| Sensitive Disease | Breast adenocarcinoma [ICD-11: 2C60.1] | |||
| Sensitive Drug | Pilaralisib | |||
| Molecule Alteration | Missense mutation | p.T798M (c.2393_2394delCAinsTG) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | BT474 cells | Breast | Homo sapiens (Human) | CVCL_0179 |
| MCF10A cells | Breast | Homo sapiens (Human) | CVCL_0598 | |
| In Vivo Model | Athymic female mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
HER2T798M sequencing assay | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | The missense mutation p.T798M (c.2393_2394delCAinsTG) in gene ERBB2 cause the sensitivity of Pilaralisib by aberration of the drug's therapeutic target | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [24] | |||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Sensitive Drug | WZ4002 | |||
| Molecule Alteration | Missense mutation | p.L755S (c.2263_2264delCTinsAG) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | HEK293 cells | Kidney | Homo sapiens (Human) | CVCL_0045 |
| Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTS assay | |||
| Mechanism Description | The missense mutation p.L755S (c.2263_2264delCTinsAG) in gene ERBB2 cause the sensitivity of WZ4002 by aberration of the drug's therapeutic target | |||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [24] | |||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Sensitive Drug | WZ4002 | |||
| Molecule Alteration | Missense mutation | p.L755P (c.2264T>C) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | HEK293 cells | Kidney | Homo sapiens (Human) | CVCL_0045 |
| Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTS assay | |||
| Mechanism Description | The missense mutation p.L755P (c.2264T>C) in gene ERBB2 cause the sensitivity of WZ4002 by aberration of the drug's therapeutic target | |||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [24] | |||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Sensitive Drug | WZ4002 | |||
| Molecule Alteration | Missense mutation | p.T798M (c.2393_2394delCAinsTG) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | HEK293 cells | Kidney | Homo sapiens (Human) | CVCL_0045 |
| Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTS assay | |||
| Mechanism Description | The missense mutation p.T798M (c.2393_2394delCAinsTG) in gene ERBB2 cause the sensitivity of WZ4002 by aberration of the drug's therapeutic target | |||
Investigative Drug(s)
1 drug(s) in total
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Breast adenocarcinoma [ICD-11: 2C60.1] | [13] | |||
| Resistant Disease | Breast adenocarcinoma [ICD-11: 2C60.1] | |||
| Resistant Drug | CI-1040 | |||
| Molecule Alteration | Missense mutation | p.T798M (c.2393_2394delCAinsTG) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | BT474 cells | Breast | Homo sapiens (Human) | CVCL_0179 |
| MCF10A cells | Breast | Homo sapiens (Human) | CVCL_0598 | |
| In Vivo Model | Athymic female mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
HER2T798M sequencing assay | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | The missense mutation p.T798M (c.2393_2394delCAinsTG) in gene ERBB2 cause the resistance of CI-1040 by aberration of the drug's therapeutic target | |||
Disease- and Tissue-specific Abundances of This Molecule
ICD Disease Classification 02

| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Gastric tissue | |
| The Specified Disease | Gastric cancer | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 2.78E-03; Fold-change: 5.85E-01; Z-score: 4.11E+00 | |
| The Expression Level of Disease Section Compare with the Adjacent Tissue | p-value: 1.49E-08; Fold-change: 2.05E-01; Z-score: 8.91E-01 | |
|
Molecule expression in the normal tissue adjacent to the diseased tissue of patients
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |
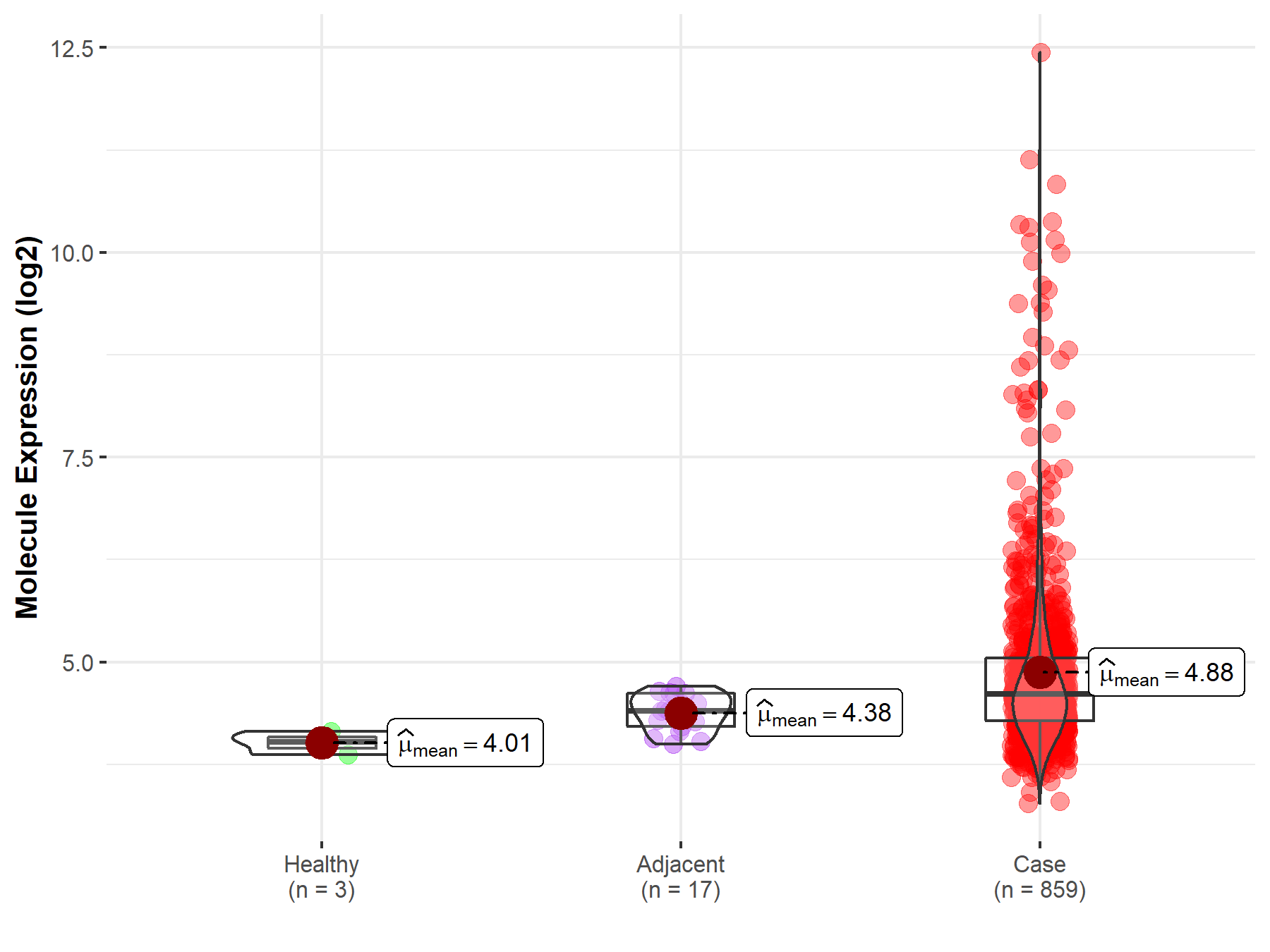
|
Click to View the Clearer Original Diagram |
| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Lung | |
| The Specified Disease | Lung cancer | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 7.14E-04; Fold-change: -1.26E-01; Z-score: -1.91E-01 | |
| The Expression Level of Disease Section Compare with the Adjacent Tissue | p-value: 3.52E-03; Fold-change: -1.10E-01; Z-score: -1.40E-01 | |
|
Molecule expression in the normal tissue adjacent to the diseased tissue of patients
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |
|
Click to View the Clearer Original Diagram |
| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Breast tissue | |
| The Specified Disease | Breast cancer | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 3.50E-77; Fold-change: 4.91E-01; Z-score: 1.12E+00 | |
| The Expression Level of Disease Section Compare with the Adjacent Tissue | p-value: 6.69E-13; Fold-change: 3.54E-01; Z-score: 6.25E-01 | |
|
Molecule expression in the normal tissue adjacent to the diseased tissue of patients
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |
|
Click to View the Clearer Original Diagram |
| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Bladder tissue | |
| The Specified Disease | Bladder cancer | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 5.67E-03; Fold-change: -1.26E+00; Z-score: -2.05E+00 | |
|
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |
|
Click to View the Clearer Original Diagram |
| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Head and neck tissue | |
| The Specified Disease | Head and neck cancer | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 3.48E-14; Fold-change: -6.47E-01; Z-score: -7.78E-01 | |
|
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |
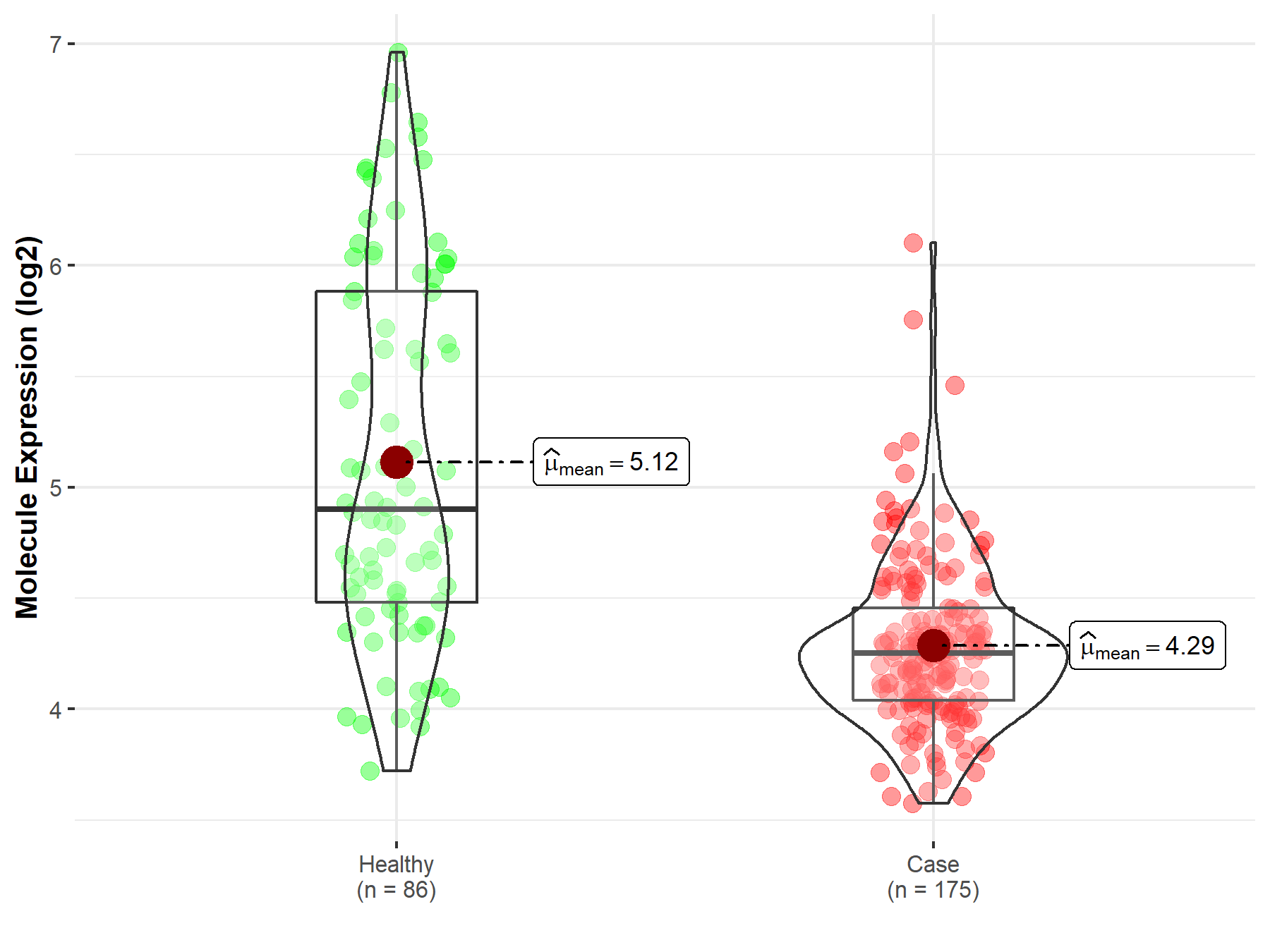
|
Click to View the Clearer Original Diagram |
Tissue-specific Molecule Abundances in Healthy Individuals

|
||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
