Drug Information
Drug (ID: DG01622) and It's Reported Resistant Information
| Name |
AZ5104
|
||||
|---|---|---|---|---|---|
| Synonyms |
AZ5104; 1421373-98-9; AZ-5104; N-(5-((4-(1H-indol-3-yl)pyrimidin-2-yl)amino)-2-((2-(dimethylamino)ethyl)(methyl)amino)-4-methoxyphenyl)acrylamide; UNII-2DWZ6SE1E1; 2DWZ6SE1E1; CHEMBL3353411; N-[2-[2-(dimethylamino)ethyl-methylamino]-5-[[4-(1H-indol-3-yl)pyrimidin-2-yl]amino]-4-methoxyphenyl]prop-2-enamide; AZD5104; AZ 5104; Osimertinib metabolite M6; SCHEMBL14663428; GTPL11580; EX-A824; AMY16678; AOB87166; BCP08428; HY-B0793; BDBM50029689; MFCD28899992; s7298; AKOS025404698; ZINC147059673; CCG-269587; CS-5192; NCGC00387805-01; AS-55926; DA-45003; QC-11825; B5836; FT-0733191; F14245; A885228; 2-Propenamide, N-(2-((2-(dimethylamino)ethyl)methylamino)-5-((4-(1H-indol-3-yl)-2-pyrimidinyl)amino)-4-methoxyphenyl)-; N-(2-((2-(Dimethylamino)ethyl)methylamino)-5-((4-(1H-indol-3-yl)-2-pyrimidinyl)amino)-4-methoxyphenyl)-2-propenamide; N-(2-[2-Dimethylaminoethyl-methylamino]-5-{[4-(1H-indol-3-yl)pyrimidin-2-yl]amino}-4-methoxyphenyl)prop-2-enamide; N-(2-{[2-(DIMETHYLAMINO)ETHYL](METHYL)AMINO}-5-{[4-(1H-INDOL-3-YL)PYRIMIDIN-2-YL]AMINO}-4-METHOXYPHENYL)PROP-2-ENAMIDE; N-[2-(2-Dimethylaminoethylmethylamino)-5-[[4-(1H-indol-3-yl)pyrimidin-2-yl]amino]-4-methoxyphenyl]prop-2-enamide
Click to Show/Hide
|
||||
| Structure |
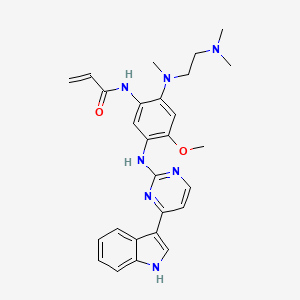
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Clinically Reported Resistance for This Drug
(1 diseases)
[1]
Disease(s) with Resistance Information Discovered by Cell Line Test for This Drug
(1 diseases)
[1]
|
||||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
10
|
||||
| IsoSMILES |
CN(C)CCN(C)C1=CC(=C(C=C1NC(=O)C=C)NC2=NC=CC(=N2)C3=CNC4=CC=CC=C43)OC
|
||||
| InChI |
InChI=1S/C27H31N7O2/c1-6-26(35)30-22-15-23(25(36-5)16-24(22)34(4)14-13-33(2)3)32-27-28-12-11-21(31-27)19-17-29-20-10-8-7-9-18(19)20/h6-12,15-17,29H,1,13-14H2,2-5H3,(H,30,35)(H,28,31,32)
|
||||
| InChIKey |
IQNVEOMHJHBNHC-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-02: Benign/in-situ/malignant neoplasm
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Receptor tyrosine-protein kinase erbB-2 (ERBB2) | [1] | |||
| Resistant Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Molecule Alteration | Duplication | p.Y772_A775 (c.2314_2325) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 |
| Experiment for Molecule Alteration |
Sanger cDNA sequencing assay | |||
| Experiment for Drug Resistance |
CCK-8 assay | |||
|
|
||||
| Key Molecule: Receptor tyrosine-protein kinase erbB-2 (ERBB2) | [1] | |||
| Resistant Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Molecule Alteration | Duplication | p.G778_P780 (c.2332_2340) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Receptor tyrosine-protein kinase erbB-2 (ERBB2) | [1] | |||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Molecule Alteration | Complex-indel | p.G776_776delinsVC (c.2326_2328delinsGTATGT) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 |
| Experiment for Molecule Alteration |
Sanger cDNA sequencing assay | |||
| Experiment for Drug Resistance |
CCK-8 assay | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
