Drug Information
Drug (ID: DG01054) and It's Reported Resistant Information
| Name |
Efinaconazole
|
||||
|---|---|---|---|---|---|
| Synonyms |
EFINACONAZOLE; 164650-44-6; (2R,3R)-2-(2,4-difluorophenyl)-3-(4-methylenepiperidin-1-yl)-1-(1H-1,2,4-triazol-1-yl)butan-2-ol; KP-103; JUBLIA; UNII-J82SB7FXWB; KP103; J82SB7FXWB; CHEBI:82718; 164905-19-5; (2R,3R)-2-(2,4-difluorophenyl)-3-(4-methylidenepiperidin-1-yl)-1-(1,2,4-triazol-1-yl)butan-2-ol; Kp 103; Efinaconazole [USAN:INN]; efinaconazol; efinaconazolum; Clenafin; IDP-108; Clenafin (TN); Jublia (TN); Efinaconazole(KP-103); Efinaconazole; KP-103; SCHEMBL300738; ZINC6251; CHEMBL2103877; Efinaconazole (JAN/USAN/INN); C18H22F2N4O; HSDB 8341; DTXSID40167787; BCP11665; EX-A2643; 2856AH; MFCD00936406; s5025; AKOS027323571; CCG-268012; CS-3500; DB09040; NCGC00390702-01; NCGC00390702-02; NCGC00390702-03; (2R,3R)-2-(2,4-Difluorofenil)-3-(4-metilenopiperidin-1-il)-1-(1H-1,2,4-triazin-1-il)butan-2-ol; AC-30630; AS-30126; HY-15660; D10021; A854585; Q21011225; (2R, 3R)-2-(2,4-Difluorophenyl)-3-(4-methylenepiperidyl)-1-(1,2,4-triazolyl)butan-2-ol; (2R,3R)-2-(2,4-Difluorophenyl)-3-(4-methylene-1-piperidinyl)-1-(1H-1,2,4-triazol-1-yl)-2-butanol; (2R,3R)-2-(2,4-difluorophenyl)-3-(4-methylene-1-piperidyl)-1-(1,2,4-triazol-1-yl)butan-2-ol; (2R,3R)-2-(2,4-difluorophenyl)-3-(4-methylenepiperidine-1-yl)-1-(1H-1,2,4-triazole-1-yl)butane-2-ol; (2R,3R)-2-(2,4-difluorophenyl)-3-(4-methylenepiperidino)-1-(1H-1,2,4-triazol-1-yl)butan-2-ol; (alphaR,betaR)-alpha-(2,4-Difluorophenyl)-beta-methyl-4-methylene-alpha-(1H-1,2,4-triazol-1-ylmethyl)-1-piperidineethanol; 1-Piperidineethanol, alpha-(2,4-difluorophenyl)-beta-methyl-4-methylene-alpha-(1H-1,2,4-triazol-1- ylmethyl)-, (alphaR,betaR)-; 1-Piperidineethanol, alpha-(2,4-difluorophenyl)-beta-methyl-4-methylene-alpha-(1H-1,2,4-triazol-1-ylmethyl)-, (alphaR,betaR)-
Click to Show/Hide
|
||||
| Indication |
In total 1 Indication(s)
|
||||
| Structure |
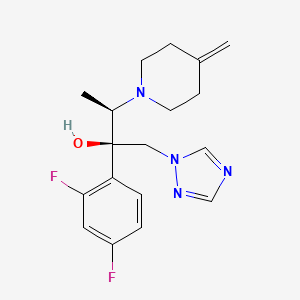
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Clinically Reported Resistance for This Drug
(1 diseases)
[1]
|
||||
| Target | Candida Cytochrome P450 51 (Candi ERG11) | CP51_CANAL | [1] | ||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
C18H22F2N4O
|
||||
| IsoSMILES |
C[C@H]([C@](CN1C=NC=N1)(C2=C(C=C(C=C2)F)F)O)N3CCC(=C)CC3
|
||||
| InChI |
1S/C18H22F2N4O/c1-13-5-7-23(8-6-13)14(2)18(25,10-24-12-21-11-22-24)16-4-3-15(19)9-17(16)20/h3-4,9,11-12,14,25H,1,5-8,10H2,2H3/t14-,18-/m1/s1
|
||||
| InChIKey |
NFEZZTICAUWDHU-RDTXWAMCSA-N
|
||||
| PubChem CID | |||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-01: Infectious/parasitic diseases
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Sterol 14-alpha demethylase cyp51A (CYP51A) | [2] | |||
| Sensitive Disease | Tinea unguium [ICD-11: 1F28.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
Antifungal susceptibility assay | |||
| Mechanism Description | The expression of?cyp51A?mRNA was induced by the addition of the azole antifungal drug efinaconazole, whereas no such induction was detected for?cyp51B, suggesting that Cyp51A functions as an azole-responsive Cyp51 isozyme. To explore the contribution of Cyp51A to susceptibility to azole drugs, the neomycin phosphotransferase (nptII) gene cassette was inserted into the?cyp51A?3'-untranslated region of deltaku80?to destabilize the mRNA of?cyp51A. In this mutant, the induction of?cyp51A?mRNA expression by efinaconazole was diminished. The minimum inhibitory concentration for several azole drugs of this strain was reduced, suggesting that dermatophyte Cyp51A contributes to the tolerance for azole drugs. | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
