Molecule Information
General Information of the Molecule (ID: Mol01252)
| Name |
X inactive specific transcript (XIST)
,Homo sapiens
|
||||
|---|---|---|---|---|---|
| Synonyms |
XIST
Click to Show/Hide
|
||||
| Molecule Type |
LncRNA
|
||||
| Gene Name |
LOC401093
|
||||
| Gene ID | |||||
| Location |
chrX:73820649-73852723[-]
|
||||
| Ensembl ID | |||||
| HGNC ID | |||||
| Click to Show/Hide the Complete Species Lineage | |||||
Type(s) of Resistant Mechanism of This Molecule
Drug Resistance Data Categorized by Drug
Approved Drug(s)
3 drug(s) in total
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Non-small cell lung cancer [ICD-11: 2C25.Y] | [1] | |||
| Sensitive Disease | Non-small cell lung cancer [ICD-11: 2C25.Y] | |||
| Sensitive Drug | Cisplatin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Lung cancer [ICD-11: 2C25] | |||
| The Specified Disease | Lung adenocarcinoma | |||
| The Studied Tissue | Lung | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.93E-01 Fold-change: -2.37E-01 Z-score: -1.30E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell autophagy | Activation | hsa04140 | |
| lncRNA-XIST/miR17 axis | Regulation | N.A. | ||
| In Vitro Model | A549 cells | Lung | Homo sapiens (Human) | CVCL_0023 |
| H1299 cells | Lung | Homo sapiens (Human) | CVCL_0060 | |
| Experiment for Molecule Alteration |
qPCR | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | Knockdown of LncRNA-XIST enhances the chemosensitivity of NSCLC cells via suppression of autophagy. LncRNA-XIST inhibits the expression of miR17 to modulate ATG7 and LncRNA-XIST regulates autophagy through ATG7. | |||
| Disease Class: Epithelial ovarian cancer [ICD-11: 2B5D.0] | [6] | |||
| Sensitive Disease | Epithelial ovarian cancer [ICD-11: 2B5D.0] | |||
| Sensitive Drug | Cisplatin | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell invasion | Inhibition | hsa05200 | |
| Cell proliferation | Inhibition | hsa05200 | ||
| In Vitro Model | OVCAR3 cells | Ovary | Homo sapiens (Human) | CVCL_0465 |
| CAOV3 cells | Ovary | Homo sapiens (Human) | CVCL_0201 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | Upregulation of long non-coding RNA XIST has anticancer effects on epithelial ovarian cancer cells through inverse downregulation of hsa-miR-214-3p. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Nasopharyngeal carcinoma [ICD-11: 2B6B.0] | [3] | |||
| Resistant Disease | Nasopharyngeal carcinoma [ICD-11: 2B6B.0] | |||
| Resistant Drug | Cisplatin | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell proliferation | Activation | hsa05200 | ||
| In Vitro Model | HNE1 cells | Nasopharynx | Homo sapiens (Human) | CVCL_0308 |
| Experiment for Molecule Alteration |
RT-PCR | |||
| Experiment for Drug Resistance |
MTT assay; Flow cytometry assay | |||
| Mechanism Description | Long non-coding RNA XIST modulates cisplatin resistance by altering PDCD4 and Fas-Lexpressions in human nasopharyngeal carcinoma HNE1 cells in vitro. XIST is up-regulated in HNE1/DDP cells, and down-regulation and up-regulation of XIST expression reduce and increase DDP resistance of the cells, respectively, possibly as a result of changes in the expressions of PDCD4 and Fas-L. | |||
| Disease Class: Non-small cell lung cancer [ICD-11: 2C25.Y] | [1] | |||
| Resistant Disease | Non-small cell lung cancer [ICD-11: 2C25.Y] | |||
| Resistant Drug | Cisplatin | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell autophagy | Inhibition | hsa04140 | |
| In Vitro Model | A549 cells | Lung | Homo sapiens (Human) | CVCL_0023 |
| H1299 cells | Lung | Homo sapiens (Human) | CVCL_0060 | |
| Experiment for Molecule Alteration |
qPCR | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | Knockdown of LncRNA-XIST enhances the chemosensitivity of NSCLC cells via suppression of autophagy. LncRNA-XIST inhibits the expression of miR17 to modulate ATG7 and LncRNA-XIST regulates autophagy through ATG7. | |||
| Disease Class: Lung adenocarcinoma [ICD-11: 2C25.0] | [4] | |||
| Resistant Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | |||
| Resistant Drug | Cisplatin | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | A549 cells | Lung | Homo sapiens (Human) | CVCL_0023 |
| A549/DDP cells | Lung | Homo sapiens (Human) | CVCL_0023 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
Flow cytometric analysis; TUNEL assay; MTT assay; Colony formation assay | |||
| Mechanism Description | LncRNA XIST overexpression in A549 cells increased their chemosensitivity to cisplatin both in vitro and in vivo by protecting cells from apoptosis and promoting cell proliferation. XIST functioned as competing endogenous RNA to repress let-7i, which controlled its down-stream target BAG-1. | |||
| Disease Class: Ovarian cancer [ICD-11: 2C73.0] | [5] | |||
| Resistant Disease | Ovarian cancer [ICD-11: 2C73.0] | |||
| Resistant Drug | Cisplatin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| In Vitro Model | CAOV3 cells | Ovary | Homo sapiens (Human) | CVCL_0201 |
| OVCA433 cells | Ovary | Homo sapiens (Human) | CVCL_0475 | |
| ALST cells | Ovary | Homo sapiens (Human) | CVCL_W778 | |
| OVCA432 cells | Ovary | Homo sapiens (Human) | CVCL_3769 | |
| OVCA 420 cells | Breast | Homo sapiens (Human) | CVCL_3935 | |
| OVCA3 cells | Ovary | Homo sapiens (Human) | CVCL_0465 | |
| OVCA429 cells | Ovary | Homo sapiens (Human) | CVCL_3936 | |
| OVCA633 cells | Ovary | Homo sapiens (Human) | CVCL_W776 | |
| OVCA680 cells | Ovary | Homo sapiens (Human) | CVCL_W781 | |
| OVCA702 cells | Ovary | Homo sapiens (Human) | CVCL_W782 | |
| OVCA810 cells | Ovary | Homo sapiens (Human) | CVCL_W783 | |
| Experiment for Molecule Alteration |
qPCR; Microarray assay | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | One possible down-stream candidate is XIAP, which is the most potent direct inhibitor of caspases and apoptosis among all human IAP family proteins. Down-regulated expression of XIAP has been shown to induce apoptosis in chemoresistant human ovarian cancer cells. Down-regulation of XIST might increase the expression level of XIAP and block drug-induced apoptosis to cause resistance phenotype. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Glioma [ICD-11: 2A00.1] | [2] | |||
| Sensitive Disease | Glioma [ICD-11: 2A00.1] | |||
| Sensitive Drug | Temozolomide | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Brain cancer [ICD-11: 2A00] | |||
| The Specified Disease | Glioblastoma multiforme | |||
| The Studied Tissue | Brain | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 4.93E-26 Fold-change: -1.80E+00 Z-score: -1.10E+01 |
|||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | DNA mismatch repair pathway | Regulation | N.A. | |
| In Vitro Model | U251 cells | Brain | Homo sapiens (Human) | CVCL_0021 |
| LN229 cells | Brain | Homo sapiens (Human) | CVCL_0393 | |
| U373 cells | Brain | Homo sapiens (Human) | CVCL_2219 | |
| U118 cells | Brain | Homo sapiens (Human) | CVCL_0633 | |
| NHA | Brain | Homo sapiens (Human) | N.A. | |
| Experiment for Molecule Alteration |
qPCR | |||
| Experiment for Drug Resistance |
MTT assay; BrdU incorporation assay | |||
| Mechanism Description | XIST can amplify the chemoresistance of glioma cell lines to TMZ through directly targetting miR29c via SP1 and MGMT. XIST/miR29c axis regulated glioma cell chemoresistance to TMZ through RNA mismatch repair pathway. XIST expression was up-regulated by miR29c inhibition while down-regulated by ectopic miR29, and XIST directly binds to miR29c to inhibit its expression, XIST and miR29c neatively regulates each other. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Malignant glioma [ICD-11: 2A00.2] | [2] | |||
| Resistant Disease | Malignant glioma [ICD-11: 2A00.2] | |||
| Resistant Drug | Temozolomide | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | DNA damage repair signaling pathway | Activation | hsa03410 | |
| In Vitro Model | U251 cells | Brain | Homo sapiens (Human) | CVCL_0021 |
| LN229 cells | Brain | Homo sapiens (Human) | CVCL_0393 | |
| U373 cells | Brain | Homo sapiens (Human) | CVCL_2219 | |
| U118 cells | Brain | Homo sapiens (Human) | CVCL_0633 | |
| NHA | Brain | Homo sapiens (Human) | N.A. | |
| Experiment for Molecule Alteration |
qPCR | |||
| Experiment for Drug Resistance |
MTT assay; BrdU incorporation assay | |||
| Mechanism Description | XIST was inversely correlated with miR29c, positively correlated with PS1, positively related with MGMT. XIST can inhibit miR29c expression by directly binding to miR29c and subsequently up-regulate the expression of SP1 and MGMT to promote the chemoresistance of glioma cells to TMZ. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Liver fibrogenesis [ICD-11: DB94.Y] | [7] | |||
| Resistant Disease | Liver fibrogenesis [ICD-11: DB94.Y] | |||
| Resistant Drug | Ethanol | |||
| Molecule Alteration | Up-regulation | Interaction |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | HSCs | N.A. | N.A. | N.A. |
| In Vivo Model | Male C57BL/6 mice model | Mus musculus | ||
| Experiment for Molecule Alteration |
Mimic assay; Luciferase assay; qRT-PCR; Western bloting analysis | |||
| Experiment for Drug Resistance |
MTT assay; Dual luciferase reporter assay | |||
| Mechanism Description | XIST enhances ethanol-induced HSCs autophagy and activation via miR-29b/HMGB1 axis. | |||
Clinical Trial Drug(s)
1 drug(s) in total
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Breast cancer [ICD-11: 2C60.3] | [8] | |||
| Resistant Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Resistant Drug | Abexinostat | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | MDA-MB-231 cells | Breast | Homo sapiens (Human) | CVCL_0062 |
| MDA-MB-436 cells | Breast | Homo sapiens (Human) | CVCL_0623 | |
| HCC1954 cells | Breast | Homo sapiens (Human) | CVCL_1259 | |
| BrCa-MZ-01 cells | Breast | Homo sapiens (Human) | CVCL_5495 | |
| CRCM226X cells | Breast | Homo sapiens (Human) | N.A. | |
| CRCM311X cells | Breast | Homo sapiens (Human) | N.A. | |
| CRCM389X cells | Breast | Homo sapiens (Human) | N.A. | |
| CRCM392X cells | Breast | Homo sapiens (Human) | N.A. | |
| S68 cells | Breast | Homo sapiens (Human) | CVCL_5585 | |
| Sk-BR-7 cells | Breast | Homo sapiens (Human) | CVCL_5218 | |
| SUM149 cells | Breast | Homo sapiens (Human) | CVCL_3422 | |
| SUM159 cells | Breast | Homo sapiens (Human) | CVCL_5423 | |
| Xist med cells | Breast | Homo sapiens (Human) | N.A. | |
| In Vivo Model | NOD/SCID nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
MTS assay | |||
| Mechanism Description | Abexinostat induced CSC differentiation in low-dose sensitive BCLs, whereas it did not have any effect on the CSC population from high-dose sensitive BCLs. Abexinostat effect is mediated at the cellular level through the modulation of the CSC pool. Only the PDX with low Xist expression displayed a significant decrease of its CSC population after abexinostat treatment, whereas HDACi treatment induced an increase of the CSC population in PDX with high Xist expression. | |||
Investigative Drug(s)
1 drug(s) in total
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Type 2 diabetes mellitus [ICD-11: 5A11.0] | [7] | |||
| Resistant Disease | Type 2 diabetes mellitus [ICD-11: 5A11.0] | |||
| Resistant Drug | D-Glucose | |||
| Molecule Alteration | Down-regulation | Interaction |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | ARPE-19 cells | Eye | Homo sapiens (Human) | CVCL_0145 |
| Experiment for Molecule Alteration |
Luciferase assay; qRT-PCR | |||
| Mechanism Description | XIST, likely through competitive binding of hsa-miR-21-5p, provides protection against hyperglycemia-associated injury in human retinal pigment epithelial cells. | |||
Disease- and Tissue-specific Abundances of This Molecule
ICD Disease Classification 02

| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Brain | |
| The Specified Disease | Brain lower grade glioma | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.03E-03; Fold-change: -8.69E-02 | |
|
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |
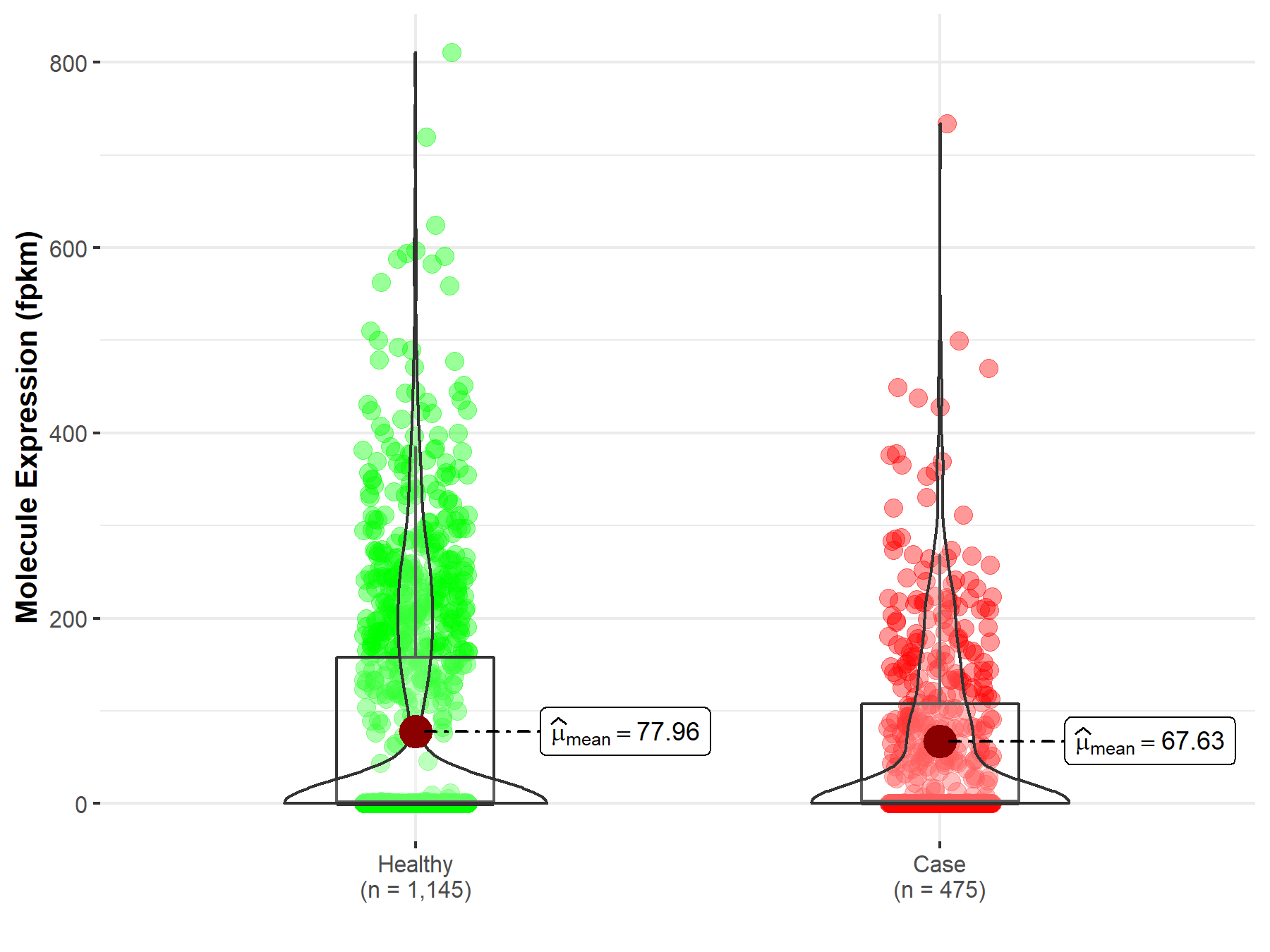
|
Click to View the Clearer Original Diagram |
| The Studied Tissue | Brain | |
| The Specified Disease | Glioblastoma multiforme | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.73E-02; Fold-change: 1.19E-01 | |
|
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |
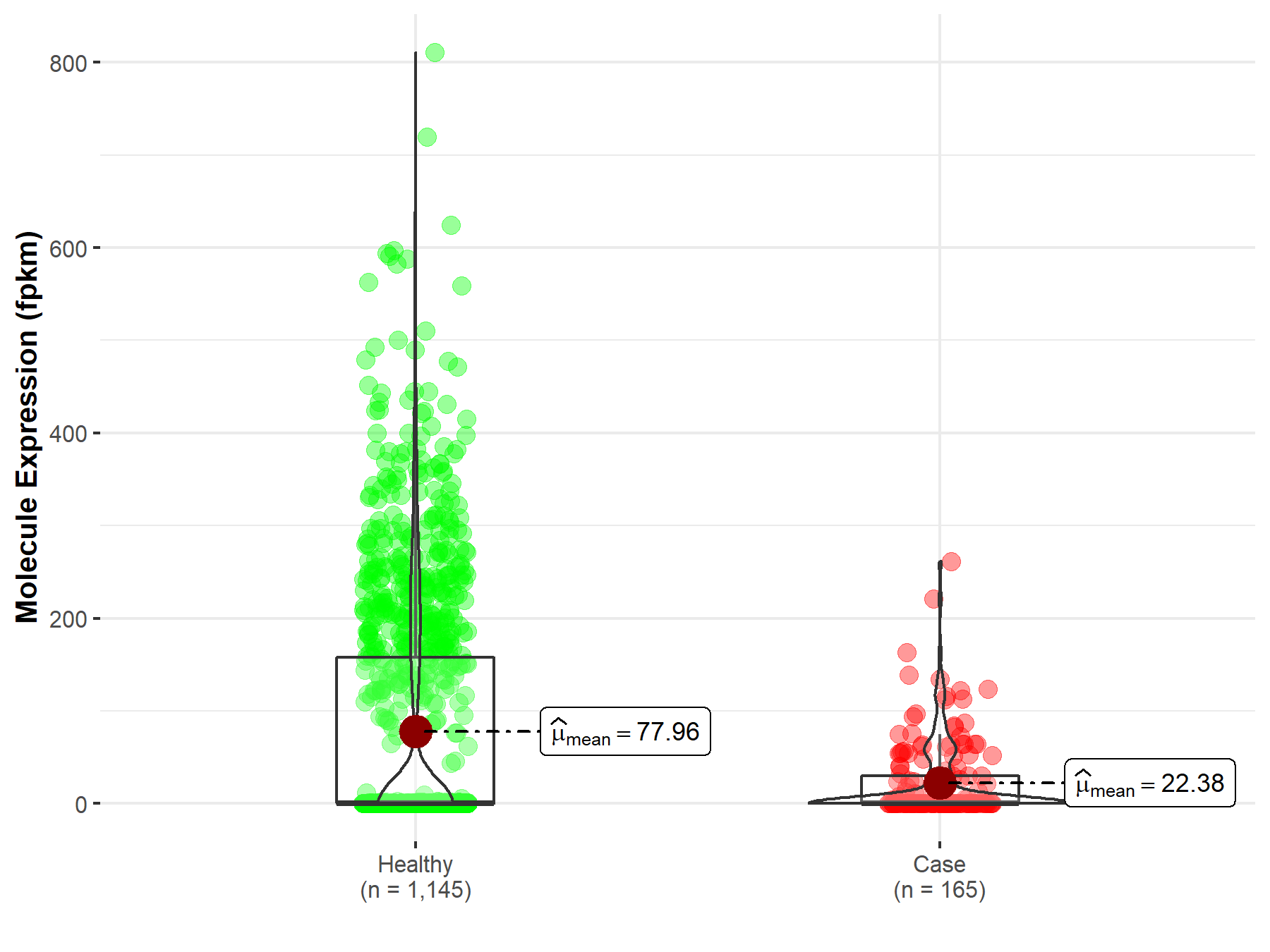
|
Click to View the Clearer Original Diagram |
| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Lung | |
| The Specified Disease | Lung adenocarcinoma | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 4.62E-04; Fold-change: -9.99E-02 | |
|
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |
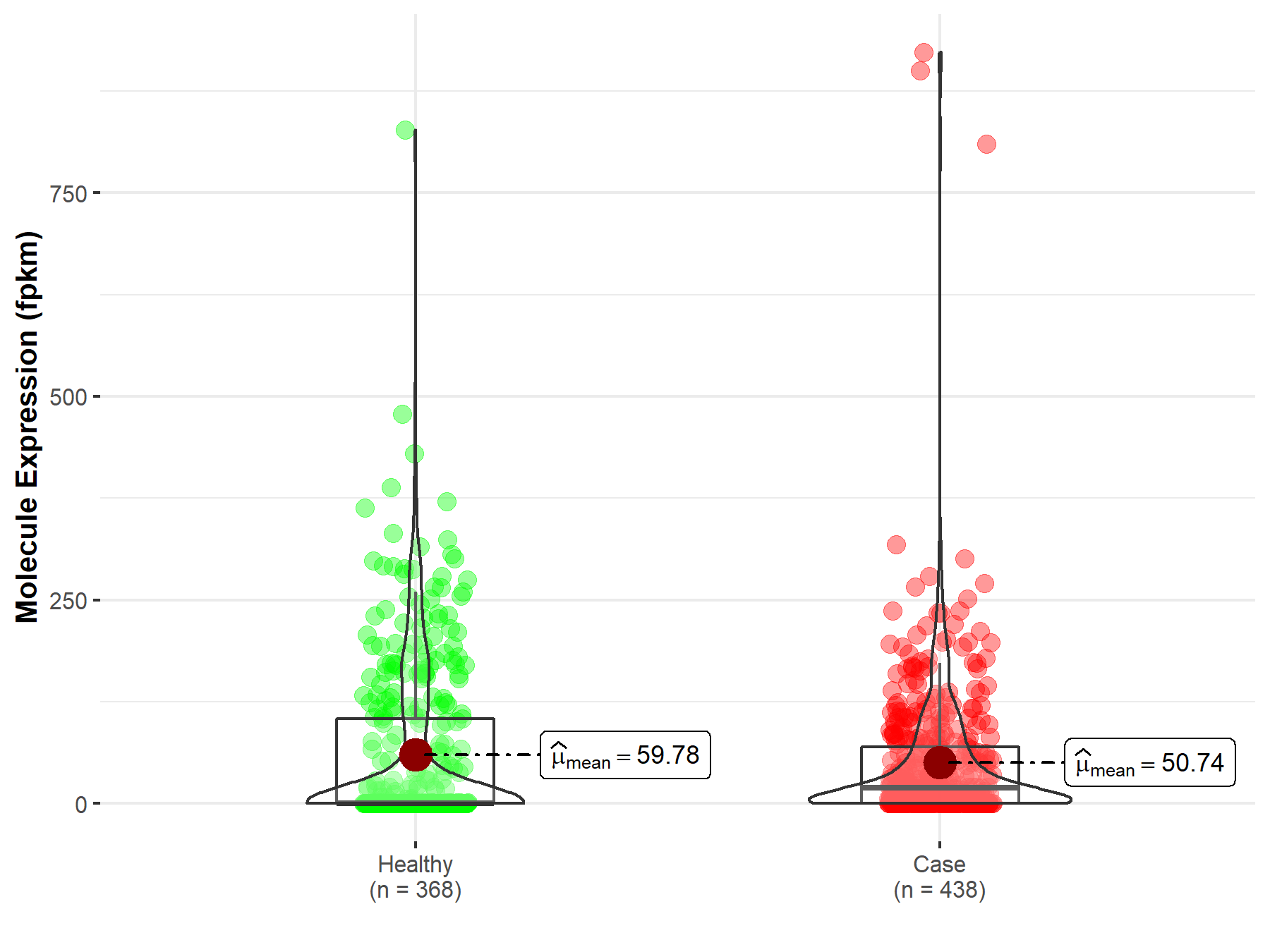
|
Click to View the Clearer Original Diagram |
| The Studied Tissue | Lung | |
| The Specified Disease | Lung squamous cell carcinoma | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.50E-03; Fold-change: 1.24E-01 | |
|
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |
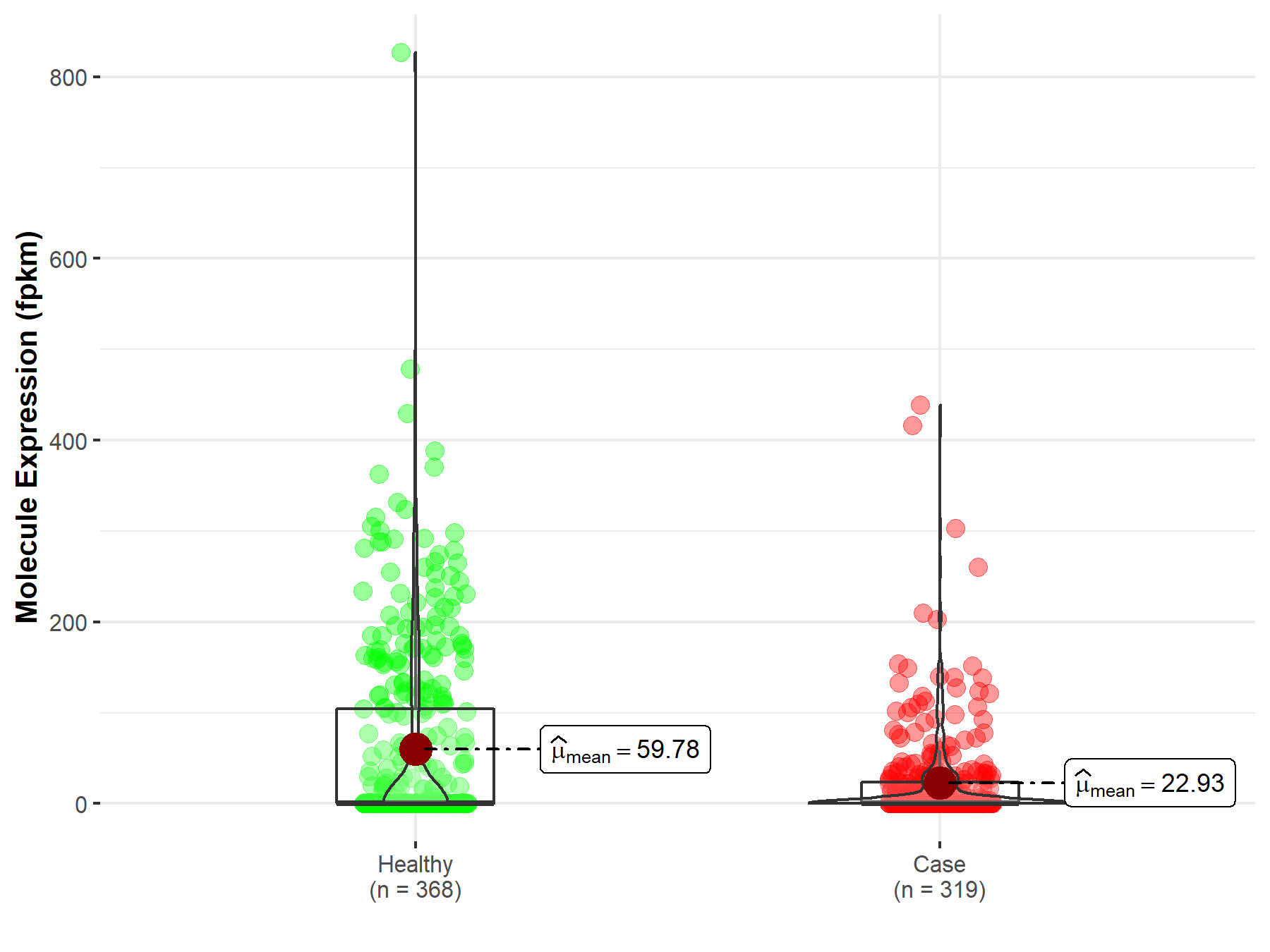
|
Click to View the Clearer Original Diagram |
| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Ovary | |
| The Specified Disease | Ovarian serous cystadenocarcinoma | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 3.14E-84; Fold-change: 3.41E-01 | |
|
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |
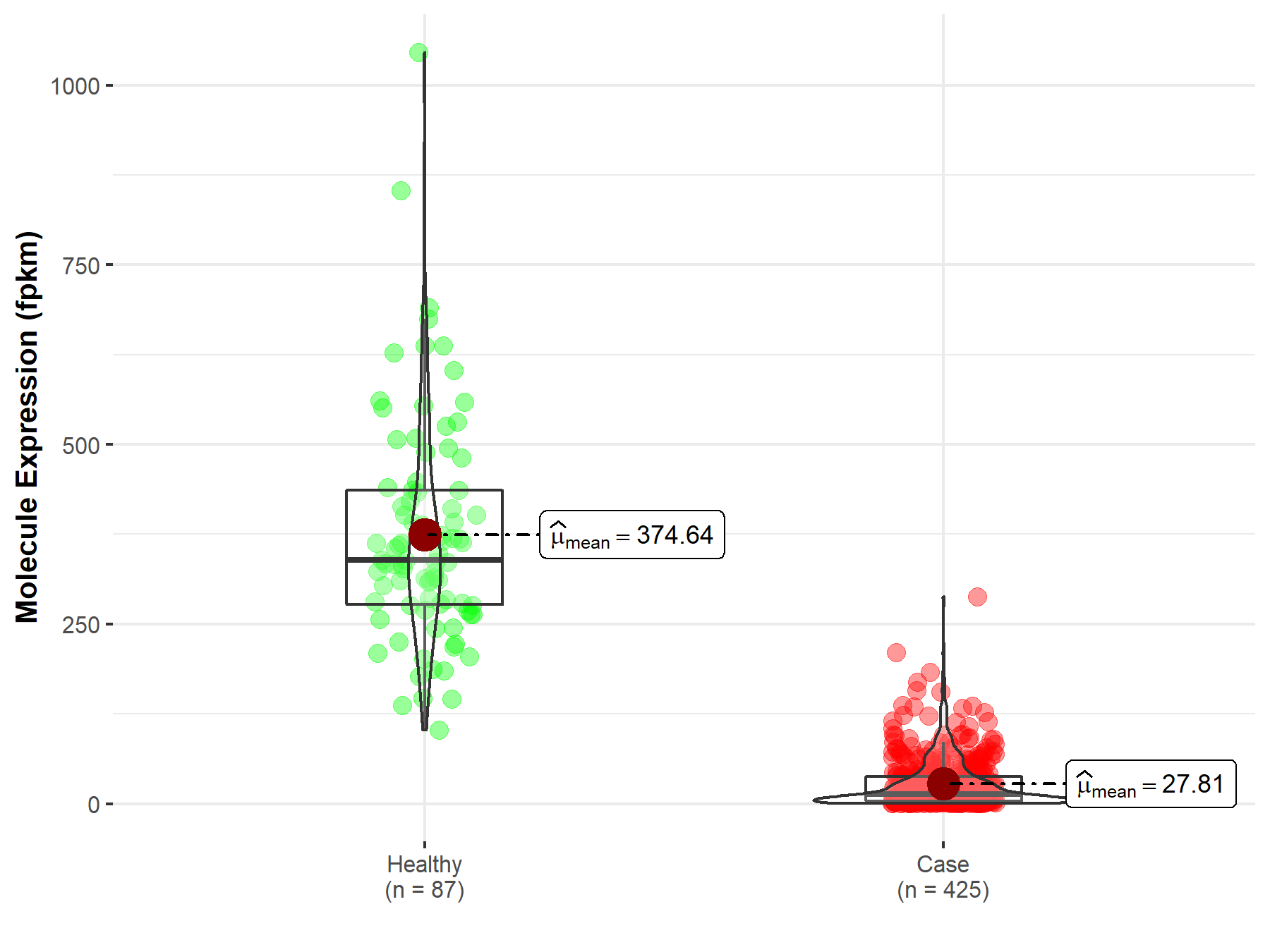
|
Click to View the Clearer Original Diagram |
Tissue-specific Molecule Abundances in Healthy Individuals

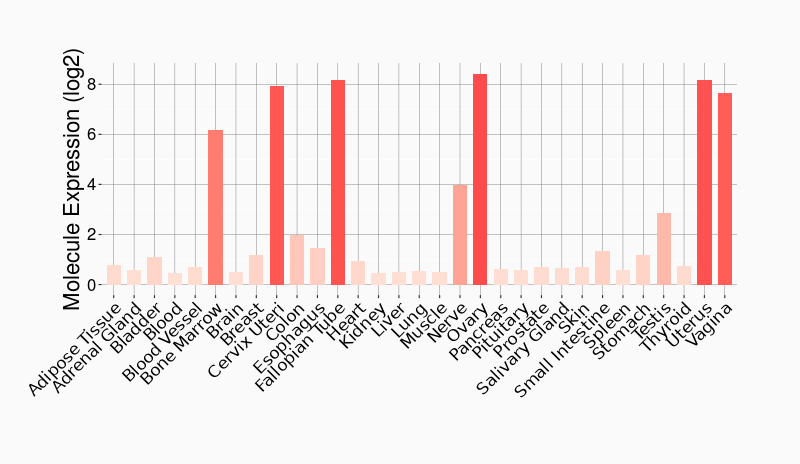
|
||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
