Drug Information
Drug (ID: DG01047) and It's Reported Resistant Information
| Name |
Octreotide
|
||||
|---|---|---|---|---|---|
| Synonyms |
Octreotide; Octreotide acetate; 83150-76-9; Sandostatin; SMS 201-995; UNII-RWM8CCW8GP; Longastatin; RWM8CCW8GP; 79517-01-4; SMS-201-995; CHEMBL1680; 83150-76-9 (free base); MFCD00871400; Octreotidum [Latin]; Octreotida [Spanish]; Octrotide; (4R,7S,10S,13R,16S,19R)-13-((1H-indol-3-yl)methyl)-19-((R)-2-amino-3-phenylpropanamido)-10-(4-aminobutyl)-16-benzyl-N-((2R,3R)-1,3-dihydroxybutan-2-yl)-7-((R)-1-hydroxyethyl)-6,9,12,15,18-pentaoxo-1,2-dithia-5,8,11,14,17-pentaazacycloicosane-4-carboxamide; [R-(R*,R*)]-D-Phenylalanyl-L-cysteinyl-L-phenylalanyl-D-tryptophyl-L-lysyl-L-threonyl-N-[2-hydroxy-1-(hydroxy-methyl)propyl]-cysteinamide cyclic(2-->7)-disulfide; Octreotida; Octreotidum; DRG-0115; Octreotide-LAR; SMS-995; Octreotide [USAN:INN:BAN]; Octreotode Acetate; (4R,7S,10S,13R,16S,19R)-10-(4-aminobutyl)-19-[[(2R)-2-amino-3-phenylpropanoyl]amino]-16-benzyl-N-[(2R,3R)-1,3-dihydroxybutan-2-yl]-7-[(1R)-1-hydroxyethyl]-13-(1H-indol-3-ylmethyl)-6,9,12,15,18-pentaoxo-1,2-dithia-5,8,11,14,17-pentazacycloicosane-4-carboxamide; SMS995; Octreotide, >=98% (HPLC); SCHEMBL10044649; HMS2090C09; 10-(4-Amino-butyl)-19-(2-amino-3-phenyl-propionylamino)-16-benzyl-7-(1-hydroxy-ethyl)-13-(1H-indol-3-ylmethyl)-6,9,12,15,18-pentaoxo-1,2-dithia-5,8,11,14,17-pentaaza-cycloicosane-4-carboxylic acid (2-hydroxy-1-hydroxymethyl-propyl)-amide; EX-A4865; BDBM50272772; AKOS015994656; CCG-270610; DB00104; HS-2020; AC-28733; L-Cysteinamide, D-phenylalanyl-L-cysteinyl-L-phenylalanyl-D-tryptophyl-L-lysyl-L-threonyl-N-(2-hydroxy-1-(hydroxymethyl)propyl)-, cyclic (2-7)-disulfide, (R-(R*,R*))-; 10-(4-Aminobutyl)-19-((2-amino-3-phenylpropanoyl)amino)-16-benzyl-7-(1-hydroxyethyl)-N-(2-hydroxy-1-(hydroxymethyl)propyl)-13-(1H-indol-3-ylmethyl)-6,9,12,15,18-pentaoxo-1,2-dithia-5,8,11,14,17-pentaazacycloicosane-4-carboxamide acetate; 17O014; 79517-01-4b; AB01275486-01; Q419935; Q-201501; H-D-Phe-Cys-Phe-D-Trp-Lys-Thr-Cys-L-threoninol disulfide bond acetate salt; (4R,7S,10S,13R,16S,19R)-10-(4-aminobutyl)-19-[[(2R)-2-amino-3-phenyl-propanoyl]amino]-16-benzyl-7-[(1R)-1-hydroxyethyl]-N-[(1R,2R)-2-hydroxy-1-(hydroxymethyl)propyl]-13-(1H-indol-3-ylmethyl)-6,9,12,15,18-pentaoxo-1,2-dithia-5,8,11,14,17-pentazacycloicosane-4-carboxamide; 10-(4-Amino-butyl)-19-(2-amino-3-phenyl-propionylamino)-16-benzyl-7-(1-hydroxy-ethyl)-13-(1H-indol-3-ylmethyl)-6,9,12,15,18-pentaoxo-1,2-dithia-5,8,11,14,17-pentaaza-cycloicosane-4-carboxylic acid (2-hydroxy-1-; 2-{[(13R,16S,19R)-10-(4-Amino-butyl)-19-((S)-2-amino-3-phenyl-propionylamino)-16-benzyl-7-(1-hydroxy-ethyl)-13-(1H-indol-3-ylmethyl)-6,9,12,15,18-pentaoxo-1,2-dithia-5,8,11,14,17-pentaaza-cycloicosane-4-carbonyl]-amino}-3-hydroxy-butyric acid; D-Phenylalanyl-L-cysteinyl-L-phenylalanyl-D-tryptophyl-L-lysyl-L-threonyl-L-cysteinyl-L-threoninol cyclic (2-7)-disulfide; D-Phenylalanyl-L-cysteinyl-L-phenylalanyl-D-tryptophyl-L-lysyl-L-threonyl-N-((1R,2R)-2-hydroxy-1-(hydroxymethyl)propyl)-L-cysteinamide cyclic (2-7)-disulfide; D-Phenylalanyl-L-cysteinyl-L-phenylalanyl-D-tryptophyl-L-lysyl-L-threonyl-N-[(1R,2R)-2-hydroxy-1-(hydroxymethyl)propyl]-L-cysteinamide Cyclic (2-7)-Disulfide Acetate; L-Cysteinamide, D-phenylalanyl-L-cysteinyl-L-phenylalanyl-D-tryptophyl-L-lysyl-L- threonyl-N-(2-hydroxy-1-(hydroxymethyl)propyl)-, cyclic (2->7)-disulfide, (R-(R*,R*))-
Click to Show/Hide
|
||||
| Indication |
In total 1 Indication(s)
|
||||
| Structure |
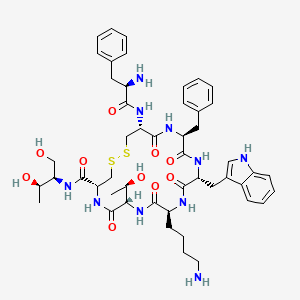
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Clinically Reported Resistance for This Drug
(1 diseases)
[1]
|
||||
| Target | Somatostatin receptor type 2 (SSTR2) | SSR2_HUMAN | [1] | ||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
C49H66N10O10S2
|
||||
| IsoSMILES |
C[C@H]([C@H]1C(=O)N[C@@H](CSSC[C@@H](C(=O)N[C@H](C(=O)N[C@@H](C(=O)N[C@H](C(=O)N1)CCCCN)CC2=CNC3=CC=CC=C32)CC4=CC=CC=C4)NC(=O)[C@@H](CC5=CC=CC=C5)N)C(=O)N[C@H](CO)[C@@H](C)O)O
|
||||
| InChI |
1S/C49H66N10O10S2/c1-28(61)39(25-60)56-48(68)41-27-71-70-26-40(57-43(63)34(51)21-30-13-5-3-6-14-30)47(67)54-37(22-31-15-7-4-8-16-31)45(65)55-38(23-32-24-52-35-18-10-9-17-33(32)35)46(66)53-36(19-11-12-20-50)44(64)59-42(29(2)62)49(69)58-41/h3-10,13-18,24,28-29,34,36-42,52,60-62H,11-12,19-23,25-27,50-51H2,1-2H3,(H,53,66)(H,54,67)(H,55,65)(H,56,68)(H,57,63)(H,58,69)(H,59,64)/t28-,29-,34-,36+,37+,38-,39-,40+,41+,42+/m1/s1
|
||||
| InChIKey |
DEQANNDTNATYII-OULOTJBUSA-N
|
||||
| PubChem CID | |||||
| TTD Drug ID | |||||
| VARIDT ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-02: Benign/in-situ/malignant neoplasm
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Forkhead box protein O3 (FOXO3) | [1] | |||
| Resistant Disease | Pituitary adenoma [ICD-11: 2F37.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| In Vitro Model | HCT8 cells | Colon | Homo sapiens (Human) | CVCL_2478 |
| LN-18 cells | Brain | Homo sapiens (Human) | CVCL_0392 | |
| ATCC 293T cells | Fetal kidney | Homo sapiens (Human) | CVCL_0063 | |
| SH-1-V3 cells | Esophagus | Homo sapiens (Human) | N.A. | |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
WST-1 assay | |||
| Mechanism Description | miR-34a upregulation leads not only to increased cell proliferation and GH secretion in vitro, but also induces resistance to the antiproliferative and hormonal effects of the first-generation somatostatin analog, octreotide. | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
