Drug Information
Drug (ID: DG01471) and It's Reported Resistant Information
| Name |
Idarubicin
|
||||
|---|---|---|---|---|---|
| Synonyms |
IDARUBICIN; 58957-92-9; 4-Demethoxydaunorubicin; 4-Demethoxydaunomycin; Idarubicine [INN-French]; Idarubicinum [INN-Latin]; Idarubicina [INN-Spanish]; UNII-ZRP63D75JW; NSC 256439; NSC-256439; ZRP63D75JW; (7S,9S)-9-acetyl-7-[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyloxan-2-yl]oxy-6,9,11-trihydroxy-8,10-dihydro-7H-tetracene-5,12-dione; Idarubicina; CHEBI:42068; (1S,3S)-3-acetyl-3,5,12-trihydroxy-6,11-dioxo-1,2,3,4,6,11-hexahydrotetracen-1-yl 3-amino-2,3,6-trideoxy-alpha-L-lyxo-hexopyranoside; 5,12-Naphthacenedione, 9-acetyl-7-((3-amino-2,3,6-trideoxy-alpha-L-lyxo-hexopyranosyl)oxy)-7,8,9,10-tetrahydro-6,9,11-trihydroxy-, (7S-cis)-; Idarubicine; Idarubicinum; Idarubicin [INN:BAN]; Idarubicinhydrochloride; DM5; MLS001401448; Daunomycin, 4-demethoxy-; NSC256439; (1S,3S)-3-acetyl-3,5,12-trihydroxy-6,11-dioxo-1,2,3,4,6,11-hexahydronaphthacen-1-yl 3-amino-2,3,6-trideoxy-alpha-L-lyxo-hexopyranoside; (7S,9S)-9-acetyl-7-{[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyloxan-2-yl]oxy}-6,9,11-trihydroxy-5,7,8,9,10,12-hexahydrotetracene-5,12-dione; Idarubicin (INN); Zavedos (TN); CCRIS 5083; NCGC00093976-03; SMR000466355; 4-DMD; SR-01000075934; I 1656; SCHEMBL3750; CHEMBL1117; Lopac0_000600; KBioSS_002388; Idarubicin hydrochloride, solid; cid_636362; GTPL7083; 4-DEMETHOXY-DAUNORUBICIN; DTXSID7023142; IDARUBICIN(Hydrochloride form); BDBM58490; BCPP000207; HMS2089D05; HMS3261H22; (7S,9S)-9-acetyl-7-[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyloxan-2-yl]oxy-6,9,11-trihydroxy-8,10-dihydro-7H-tetracene-5,12-dione;hydrochloride; ZINC3920266; Tox21_500600; AKOS015895563; AC-9384; BCP9000773; CCG-204689; DB01177; LP00600; SDCCGSBI-0050582.P002; NCGC00093976-01; NCGC00093976-02; NCGC00093976-04; NCGC00093976-05; NCGC00093976-18; NCGC00261285-01; (7S,9S)-9-acetyl-7-[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyl-tetrahydropyran-2-yl]oxy-6,9,11-trihydroxy-8,10-dihydro-7H-tetracene-5,12-dione; 5,12-Naphthacenedione, 7,8,9,10-tetrahydro-9-acetyl-7-((3-amino-2,3,6-trideoxy-alpha-L-lyxo-hexopyranosyl)oxy)-6,9,11-trihydroxy-, (7S-cis)-; EU-0100600; D08062; AB00698511-06; AB00698511-08; AB00698511-09; AB00698511-10; AB00698511_11; 957I929; A832088; A935911; Q1063862; SR-01000075934-1; BRD-K69650333-001-01-1; BRD-K69650333-001-02-9; BRD-K69650333-003-14-0; Idarubicin, United States Pharmacopeia (USP) Reference Standard; (7S,9S)-7-[(2R,4S,5S,6S)-4-azanyl-6-methyl-5-oxidanyl-oxan-2-yl]oxy-9-ethanoyl-6,9,11-tris(oxidanyl)-8,10-dihydro-7H-tetracene-5,12-dione; (7S,9S)-7-[(2R,4S,5S,6S)-4-azanyl-6-methyl-5-oxidanyl-oxan-2-yl]oxy-9-ethanoyl-6,9,11-tris(oxidanyl)-8,10-dihydro-7H-tetracene-5,12-dione;hydrochloride; (7S,9S)-9-Acetyl-7-(((2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)-6,9,11-trihydroxy-7,8,9,10-tetrahydrotetracene-5,12-dione; (7S,9S)-9-acetyl-7-((2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyltetrahydro-2H-pyran-2-yloxy)-6,9,11-trihydroxy-7,8,9,10-tetrahydrotetracene-5,12-dione; (7S,9S)-9-acetyl-7-[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyl-tetrahydropyran-2-yl]oxy-6,9,11-trihydroxy-8,10-dihydro-7H-tetracene-5,12-quinone;hydrochloride; (7S,9S)-9-acetyl-7-[[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyl-2-oxanyl]oxy]-6,9,11-trihydroxy-8,10-dihydro-7H-tetracene-5,12-dione; (7S,9S)-9-acetyl-7-[[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyl-2-oxanyl]oxy]-6,9,11-trihydroxy-8,10-dihydro-7H-tetracene-5,12-dione;hydrochloride; 4-Demethoxydaunorubicin; ; ; IMI-30; ; ; NSC-256439; ; ; (7S,9S)-9-Acetyl-7-[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyloxan-2-yl]oxy-6,9,11-trihydroxy-8,10-dihydro-7H-tetracene-5,12-dione; 5,12-Naphthacenedione, 7,8,9,10-tetrahydro-9-acetyl-7-((3-amino-2,3,6-trideoxy-.alpha.-L-lyxo-hexopyranosyl)oxy)-6,9,11-trihydroxy-, (7S-cis)-
Click to Show/Hide
|
||||
| Indication |
In total 1 Indication(s)
|
||||
| Structure |
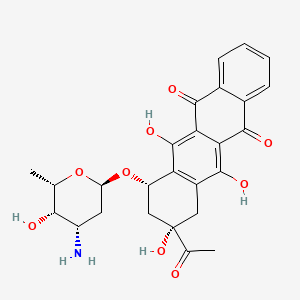
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Resistance Information Discovered by Cell Line Test for This Drug
(1 diseases)
[2]
|
||||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
3
|
||||
| IsoSMILES |
C[C@H]1[C@H]([C@H](C[C@@H](O1)O[C@H]2C[C@@](CC3=C2C(=C4C(=C3O)C(=O)C5=CC=CC=C5C4=O)O)(C(=O)C)O)N)O
|
||||
| InChI |
InChI=1S/C26H27NO9/c1-10-21(29)15(27)7-17(35-10)36-16-9-26(34,11(2)28)8-14-18(16)25(33)20-19(24(14)32)22(30)12-5-3-4-6-13(12)23(20)31/h3-6,10,15-17,21,29,32-34H,7-9,27H2,1-2H3/t10-,15-,16-,17-,21+,26-/m0/s1
|
||||
| InChIKey |
XDXDZDZNSLXDNA-TZNDIEGXSA-N
|
||||
| PubChem CID | |||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
| VARIDT ID | |||||
| INTEDE ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-02: Benign/in-situ/malignant neoplasm
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: ATP-binding cassette sub-family G2 (ABCG2) | [3] | |||
| Sensitive Disease | Chronic myeloid leukemia [ICD-11: 2A20.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | p-glycoprotein | Regulation | N.A. | |
| In Vitro Model | K562 cells | Blood | Homo sapiens (Human) | CVCL_0004 |
| K562 ABCG2 overexpression cells | Bone marrow | Homo sapiens (Human) | N.A. | |
| Experiment for Molecule Alteration |
Western blot assay | |||
| Experiment for Drug Resistance |
Cell viability assay; Flow Cytometry assay; DNA dye competition assay | |||
| Mechanism Description | Induction of DNA double-strand breaks and chromatin damage through histone eviction;Less affected by ABCG2-mediated drug export. | |||
| Key Molecule: Multidrug resistance protein 1 (ABCB1) | [3] | |||
| Sensitive Disease | Chronic myeloid leukemia [ICD-11: 2A20.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | p-glycoprotein | Regulation | N.A. | |
| In Vitro Model | K562 cells | Blood | Homo sapiens (Human) | CVCL_0004 |
| K562 ABCB1 overexpression cells | Bone marrow | Homo sapiens (Human) | N.A. | |
| Experiment for Molecule Alteration |
Western blot assay | |||
| Experiment for Drug Resistance |
Cell viability assay; Flow Cytometry assay; DNA dye competition assay | |||
| Mechanism Description | Induction of DNA double-strand breaks and chromatin damage through histone eviction. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Cyclin-dependent kinase inhibitor 1A (CDKN1A) | [2] | |||
| Resistant Disease | Acute myeloid leukemia [ICD-11: 2A60.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | DNA Damage Response Mechanism | Regulation | N.A. | |
| In Vitro Model | MV-4-11 cells | Peripheral blood | Homo sapiens (Human) | CVCL_0064 |
| MOLM-13 cells | Peripheral blood | Homo sapiens (Human) | CVCL_2119 | |
| Kasumi-1 cells | Peripheral blood | Homo sapiens (Human) | CVCL_0589 | |
| TF-1 cells | Blood | Homo sapiens (Human) | CVCL_0559 | |
| Experiment for Molecule Alteration |
RT-qPCR; Western blot assay | |||
| Experiment for Drug Resistance |
MTT assay; Trypan blue assay; Clonogenicity assay; IC50 assay; Flow cytometry assay | |||
| Mechanism Description | DNA Damage Response Mechanism (DDR) comprises numerous molecules and pathways intended to arrest the cell cycle until DNA damage is repaired or else drive the cell to apoptosis.DDR regulators demonstrate increased expression in patients with high cytogenetic risk possibly reflecting increased genotoxic stress.Using PCR arrays we observed an upregulation of of several DDR genes (CDKN1A, GADD45A, GADD45G, EXO1, and PPP1R15A) in KASUMI-1 and MV4-11 cell lines that survived following treatment with Idarubicin and Cytarabine. | |||
| Key Molecule: Acetyl-CoA acetyltransferase 2 (ACAT2) | [2] | |||
| Resistant Disease | Acute myeloid leukemia [ICD-11: 2A60.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | DNA Damage Response Mechanism | Regulation | N.A. | |
| In Vitro Model | MV-4-11 cells | Peripheral blood | Homo sapiens (Human) | CVCL_0064 |
| MOLM-13 cells | Peripheral blood | Homo sapiens (Human) | CVCL_2119 | |
| Kasumi-1 cells | Peripheral blood | Homo sapiens (Human) | CVCL_0589 | |
| TF-1 cells | Blood | Homo sapiens (Human) | CVCL_0559 | |
| Experiment for Molecule Alteration |
RT-qPCR; Western blot assay | |||
| Experiment for Drug Resistance |
MTT assay; Trypan blue assay; Clonogenicity assay; IC50 assay; Flow cytometry assay | |||
| Mechanism Description | DNA Damage Response Mechanism (DDR) comprises numerous molecules and pathways intended to arrest the cell cycle until DNA damage is repaired or else drive the cell to apoptosis.DDR regulators demonstrate increased expression in patients with high cytogenetic risk possibly reflecting increased genotoxic stress. Especially, PPP1R15A is mainly involved in the recovery of the cells from stress and it was the only DDR gene upregulated in AML patients. | |||
| Key Molecule: Growth arrest and DNA damage-inducible protein GADD45 gamma (GADD45G) | [2] | |||
| Resistant Disease | Acute myeloid leukemia [ICD-11: 2A60.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | DNA Damage Response Mechanism | Regulation | N.A. | |
| In Vitro Model | MV-4-11 cells | Peripheral blood | Homo sapiens (Human) | CVCL_0064 |
| MOLM-13 cells | Peripheral blood | Homo sapiens (Human) | CVCL_2119 | |
| Kasumi-1 cells | Peripheral blood | Homo sapiens (Human) | CVCL_0589 | |
| TF-1 cells | Blood | Homo sapiens (Human) | CVCL_0559 | |
| Experiment for Molecule Alteration |
RT-qPCR; Western blot assay | |||
| Experiment for Drug Resistance |
MTT assay; Trypan blue assay; Clonogenicity assay; IC50 assay; Flow cytometry assay | |||
| Mechanism Description | DNA Damage Response Mechanism (DDR) comprises numerous molecules and pathways intended to arrest the cell cycle until DNA damage is repaired or else drive the cell to apoptosis.DDR regulators demonstrate increased expression in patients with high cytogenetic risk possibly reflecting increased genotoxic stress.Using PCR arrays we observed an upregulation of of several DDR genes (CDKN1A, GADD45A, GADD45G, EXO1, and PPP1R15A) in KASUMI-1 and MV4-11 cell lines that survived following treatment with Idarubicin and Cytarabine. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | |||||||||||||
|
|
|||||||||||||
| Key Molecule: DNA (cytosine-5)-methyltransferase 3A (DNMT3A) | [1] | ||||||||||||
| Sensitive Disease | Acute myeloid leukemia [ICD-11: 2A60.0] | ||||||||||||
| Molecule Alteration | Missense mutation | p.R882H (c.2645G>A) |
|||||||||||
| Wild Type Structure | Method: X-ray diffraction | Resolution: 2.40 Å | |||||||||||
| Mutant Type Structure | Method: X-ray diffraction | Resolution: 2.44 Å | |||||||||||
| Download The Information of Sequence | Download The Structure File | ||||||||||||
-
A
A
E
E
630
|
K
K
R
R
K
K
P
P
I
I
R
R
V
V
L
L
S
S
L
L
640
|
F
F
D
D
G
G
I
I
A
A
T
T
G
G
L
L
L
L
V
V
650
|
L
L
K
K
D
D
L
L
G
G
I
I
Q
Q
V
V
D
D
R
R
660
|
Y
Y
I
I
A
A
S
S
E
E
V
V
C
C
E
E
D
D
S
S
670
|
I
I
T
T
V
V
G
G
M
M
V
V
R
R
H
H
Q
Q
G
G
680
|
K
K
I
I
M
M
Y
Y
V
V
G
G
D
D
V
V
R
R
S
S
690
|
V
V
T
T
Q
Q
K
K
H
H
I
I
Q
Q
E
E
W
W
G
G
700
|
P
P
F
F
D
D
L
L
V
V
I
I
G
G
G
G
S
S
P
P
710
|
C
C
N
N
D
D
L
L
S
S
I
I
V
V
N
N
P
P
A
A
720
|
R
R
K
K
G
G
L
L
Y
Y
E
E
G
G
T
T
G
G
R
R
730
|
L
L
F
F
F
F
E
E
F
F
Y
Y
R
R
L
L
L
L
H
H
740
|
D
D
A
A
R
R
P
P
K
K
E
E
G
G
D
D
D
D
R
R
750
|
P
P
F
F
F
F
W
W
L
L
F
F
E
E
N
N
V
V
V
V
760
|
A
A
M
M
G
G
V
V
S
S
D
D
K
K
R
R
D
D
I
I
770
|
S
S
R
R
F
F
L
L
E
E
S
S
N
N
P
P
V
V
M
M
780
|
I
I
D
D
A
A
K
K
E
E
V
V
S
S
A
A
A
A
H
H
790
|
R
R
A
A
R
R
Y
Y
F
F
W
W
G
G
N
N
L
L
P
P
800
|
G
G
M
M
N
N
R
R
P
P
L
L
A
A
S
S
T
T
V
V
810
|
N
N
D
D
K
K
L
L
E
E
L
L
Q
Q
E
E
C
C
L
L
820
|
E
E
H
H
G
G
R
R
I
I
A
A
K
K
F
F
S
S
K
K
830
|
V
V
R
R
T
T
I
I
T
T
T
T
R
R
S
S
N
N
S
S
840
|
I
I
K
K
Q
Q
G
G
K
K
D
D
Q
Q
H
H
F
F
P
P
850
|
V
V
F
F
M
M
N
N
E
E
K
K
E
E
D
D
I
I
L
L
860
|
W
W
C
C
T
T
E
E
M
M
E
E
R
R
V
V
F
F
G
G
870
|
F
F
P
P
V
V
H
H
Y
Y
T
T
D
D
V
V
S
S
N
N
880
|
M
M
S
S
R
H
L
L
A
A
R
R
Q
Q
R
R
L
L
L
L
890
|
G
G
R
R
S
S
W
W
S
S
V
V
P
P
V
V
I
I
R
R
900
|
H
H
L
L
F
F
A
A
P
P
L
L
K
K
E
E
Y
Y
F
F
910
|
A
A
C
C
V
V
|
|||||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| In Vitro Model | Bone marrow | N.A. | |||||||||||
| In Vivo Model | NOD/SCID mouse xenograft model | Mus musculus | |||||||||||
| Mechanism Description | The missense mutation p.R882H (c.2645G>A) in gene DNMT3A cause the sensitivity of Idarubicin by unusual activation of pro-survival pathway | ||||||||||||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
