Drug Information
Drug (ID: DG00985) and It's Reported Resistant Information
| Name |
Fotemustine
|
||||
|---|---|---|---|---|---|
| Synonyms |
Fotemustine; 92118-27-9; Diethyl (1-(3-(2-chloroethyl)-3-nitrosoureido)ethyl)phosphonate; Muphoran; Mustophorane; Fotemustina; Servier-10036; S 10036; Mustoforan; UNII-UPB2NN83AR; UPB2NN83AR; S-10036; UNII-QY93P3GN94; QY93P3GN94; (+-)-Diethyl (1-(3-(2-chloroethyl)-3-nitrosoureido)ethyl)phosphonate; MFCD00866278; Fotemustinum [Latin]; Diethyl-1-(3-(2-chloroethyl)-3-nitrosoureido)ethylphosphonate; Fotemustina [Spanish]; Fotemustinum; C9H19ClN3O5P; SMR002529685; CCRIS 6337; Fotemustine [INN:BAN]; HSDB 7762; Muphoran (TN); Fotemustine, (+)-; Fotemustine, (-)-; 1-(2-chloroethyl)-3-(1-diethoxyphosphorylethyl)-1-nitrosourea; Fotemustine (INN/BAN); SCHEMBL8880; MLS006010211; MLS006010716; CHEMBL549386; 6-Amino-3-hydroxy(1h)indazole; Fotemustine, >=98% (HPLC); DTXSID80869091; CHEBI:131852; BCP07342; HY-B0733; AKOS015920275; DS-1395; P-[1-[[[(2-Chloroethyl)nitrosoamino]carbonyl]amino]ethyl]-phosphonic acid diethyl ester; Phosphonic acid, (1-((((2-chloroethyl)nitrosoamino)carbonyl)amino)ethyl)-, diethyl ester; NCGC00346829-01; NCGC00346829-03; K679; DB-057290; FT-0630979; D07255; 118F279; A844148; SR-01000944936; Q1439555; SR-01000944936-1; diethyl 1-(3-(2-chloroethyl)-3-nitrosoureido)ethylphosphonate; 1-(2-chloroethyl)-3-(1-diethoxyphosphorylethyl)-1-nitroso-urea; diethyl (1-{[(2-chloroethyl)(nitroso)carbamoyl]amino}ethyl)phosphonate; diethyl (1-{[N-(2-chloroethyl)-N'-oxohydrazinecarbonyl]amino}ethyl)phosphonate; 191219-77-9; 191220-84-5; Muphoran; ; ; Mustoforan; ; ; S-10036; ; ; 1-(2-Chloroethyl)-3-(1-diethoxyphosphorylethyl)-1-nitrosourea; Phosphonic acid, (1-((((2-chloroethyl)nitrosoamino)carbonyl)amino)ethyl)-, diethyl ester, (+)-; Phosphonic acid, (1-((((2-chloroethyl)nitrosoamino)carbonyl)amino)ethyl)-, diethyl ester, (-)-
Click to Show/Hide
|
||||
| Indication |
In total 1 Indication(s)
|
||||
| Structure |
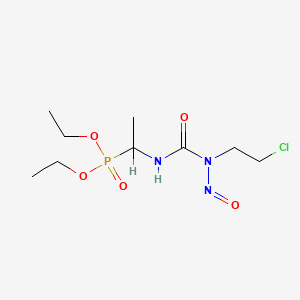
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Resistance Information Validated by in-vivo Model for This Drug
(1 diseases)
[1]
|
||||
| Target | Cytoplasmic thioredoxin reductase (TXNRD1) | TRXR1_HUMAN | [1] | ||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
C9H19ClN3O5P
|
||||
| IsoSMILES |
CCOP(=O)(C(C)NC(=O)N(CCCl)N=O)OCC
|
||||
| InChI |
1S/C9H19ClN3O5P/c1-4-17-19(16,18-5-2)8(3)11-9(14)13(12-15)7-6-10/h8H,4-7H2,1-3H3,(H,11,14)
|
||||
| InChIKey |
YAKWPXVTIGTRJH-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-16: Genitourinary system diseases
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Prostaglandin E2 receptor EP3 subtype (PE2R3) | [1] | |||
| Resistant Disease | Endometriosis [ICD-11: GA10.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Endometriosis [ICD-11: GA10] | |||
| The Specified Disease | Endometriosis | |||
| The Studied Tissue | Endometrium tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 8.85E-02 Fold-change: 3.20E-02 Z-score: 1.75E+00 |
|||
| Experimental Note | Discovered Using In-vivo Testing Model | |||
| Cell Pathway Regulation | MAPK signaling pathway | Activation | hsa04010 | |
| In Vivo Model | Female Sprague-Dawley rats model | Rattus norvegicus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Mechanism Description | Fotemustine and dexamethasone administration had anti-apoptotic activity, restoring the impaired mechanism (TUNEL assay and Western blot analysis of Bax and Bcl-2). Moreover, no gastric disfunction was detected (histological analysis of stomachs). Thus, our data showed that the combined therapy of fotemustine and dexamethasone reduced endometriosis-induced inflammation, hyperproliferation and apoptosis resistance. | |||
| Key Molecule: Prostaglandin G/H synthase 2 (PTGS2) | [1] | |||
| Resistant Disease | Endometriosis [ICD-11: GA10.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Discovered Using In-vivo Testing Model | |||
| Cell Pathway Regulation | MAPK signaling pathway | Activation | hsa04010 | |
| In Vivo Model | Female Sprague-Dawley rats model | Rattus norvegicus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Mechanism Description | Fotemustine and dexamethasone administration had anti-apoptotic activity, restoring the impaired mechanism (TUNEL assay and Western blot analysis of Bax and Bcl-2). Moreover, no gastric disfunction was detected (histological analysis of stomachs). Thus, our data showed that the combined therapy of fotemustine and dexamethasone reduced endometriosis-induced inflammation, hyperproliferation and apoptosis resistance. | |||
| Key Molecule: DNA-binding factor KBF1 (p105) (NFKB1) | [1] | |||
| Resistant Disease | Endometriosis [ICD-11: GA10.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Discovered Using In-vivo Testing Model | |||
| Cell Pathway Regulation | MAPK signaling pathway | Activation | hsa04010 | |
| In Vivo Model | Female Sprague-Dawley rats model | Rattus norvegicus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Mechanism Description | Fotemustine and dexamethasone administration had anti-apoptotic activity, restoring the impaired mechanism (TUNEL assay and Western blot analysis of Bax and Bcl-2). Moreover, no gastric disfunction was detected (histological analysis of stomachs). Thus, our data showed that the combined therapy of fotemustine and dexamethasone reduced endometriosis-induced inflammation, hyperproliferation and apoptosis resistance. | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
