Molecule Information
General Information of the Molecule (ID: Mol01908)
| Name |
Prostaglandin E2 receptor EP3 subtype (PE2R3)
,Homo sapiens
|
||||
|---|---|---|---|---|---|
| Synonyms |
PTGER3
Click to Show/Hide
|
||||
| Molecule Type |
Protein
|
||||
| Gene Name |
PE2R3
|
||||
| Gene ID | |||||
| Location |
chr1:70,852,353-71,047,816[-]
|
||||
| Sequence |
MKETRGYGGDAPFCTRLNHSYTGMWAPERSAEARGNLTRPPGSGEDCGSVSVAFPITMLL
TGFVGNALAMLLVSRSYRRRESKRKKSFLLCIGWLALTDLVGQLLTTPVVIVVYLSKQRW EHIDPSGRLCTFFGLTMTVFGLSSLFIASAMAVERALAIRAPHWYASHMKTRATRAVLLG VWLAVLAFALLPVLGVGQYTVQWPGTWCFISTGRGGNGTSSSHNWGNLFFASAFAFLGLL ALTVTFSCNLATIKALVSRCRAKATASQSSAQWGRITTETAIQLMGIMCVLSVCWSPLLI MMLKMIFNQTSVEHCKTHTEKQKECNFFLIAVRLASLNQILDPWVYLLLRKILLRKFCQI RYHTNNYASSSTSLPCQCSSTLMWSDHLER Click to Show/Hide
|
||||
| 3D-structure |
|
||||
| Function |
Receptor for prostaglandin E2 (PGE2). The activity of this receptor can couple to both the inhibition of adenylate cyclase mediated by G(i) proteins, and to an elevation of intracellular calcium. Required for normal development of fever in response to pyrinogens, including IL1B, prostaglandin E2 and bacterial lipopolysaccharide (LPS). Required for normal potentiation of platelet aggregation by prostaglandin E2, and thus plays a role in the regulation of blood coagulation. Required for increased HCO3(-) secretion in the duodenum in response to mucosal acidification, and thereby contributes to the protection of the mucosa against acid-induced ulceration. Not required for normal kidney function, normal urine volume and osmolality.
Click to Show/Hide
|
||||
| Uniprot ID | |||||
| Ensembl ID | |||||
| Click to Show/Hide the Complete Species Lineage | |||||
Type(s) of Resistant Mechanism of This Molecule
Drug Resistance Data Categorized by Drug
Approved Drug(s)
1 drug(s) in total
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Endometriosis [ICD-11: GA10.0] | [1] | |||
| Resistant Disease | Endometriosis [ICD-11: GA10.0] | |||
| Resistant Drug | Fotemustine | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Endometriosis [ICD-11: GA10] | |||
| The Specified Disease | Endometriosis | |||
| The Studied Tissue | Endometrium tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 8.85E-02 Fold-change: 3.20E-02 Z-score: 1.75E+00 |
|||
| Experimental Note | Discovered Using In-vivo Testing Model | |||
| Cell Pathway Regulation | MAPK signaling pathway | Activation | hsa04010 | |
| In Vivo Model | Female Sprague-Dawley rats model | Rattus norvegicus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Mechanism Description | Fotemustine and dexamethasone administration had anti-apoptotic activity, restoring the impaired mechanism (TUNEL assay and Western blot analysis of Bax and Bcl-2). Moreover, no gastric disfunction was detected (histological analysis of stomachs). Thus, our data showed that the combined therapy of fotemustine and dexamethasone reduced endometriosis-induced inflammation, hyperproliferation and apoptosis resistance. | |||
Disease- and Tissue-specific Abundances of This Molecule
ICD Disease Classification 16

| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Endometrium | |
| The Specified Disease | Endometriosis | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 8.85E-02; Fold-change: 1.20E-01; Z-score: 5.06E-01 | |
|
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |
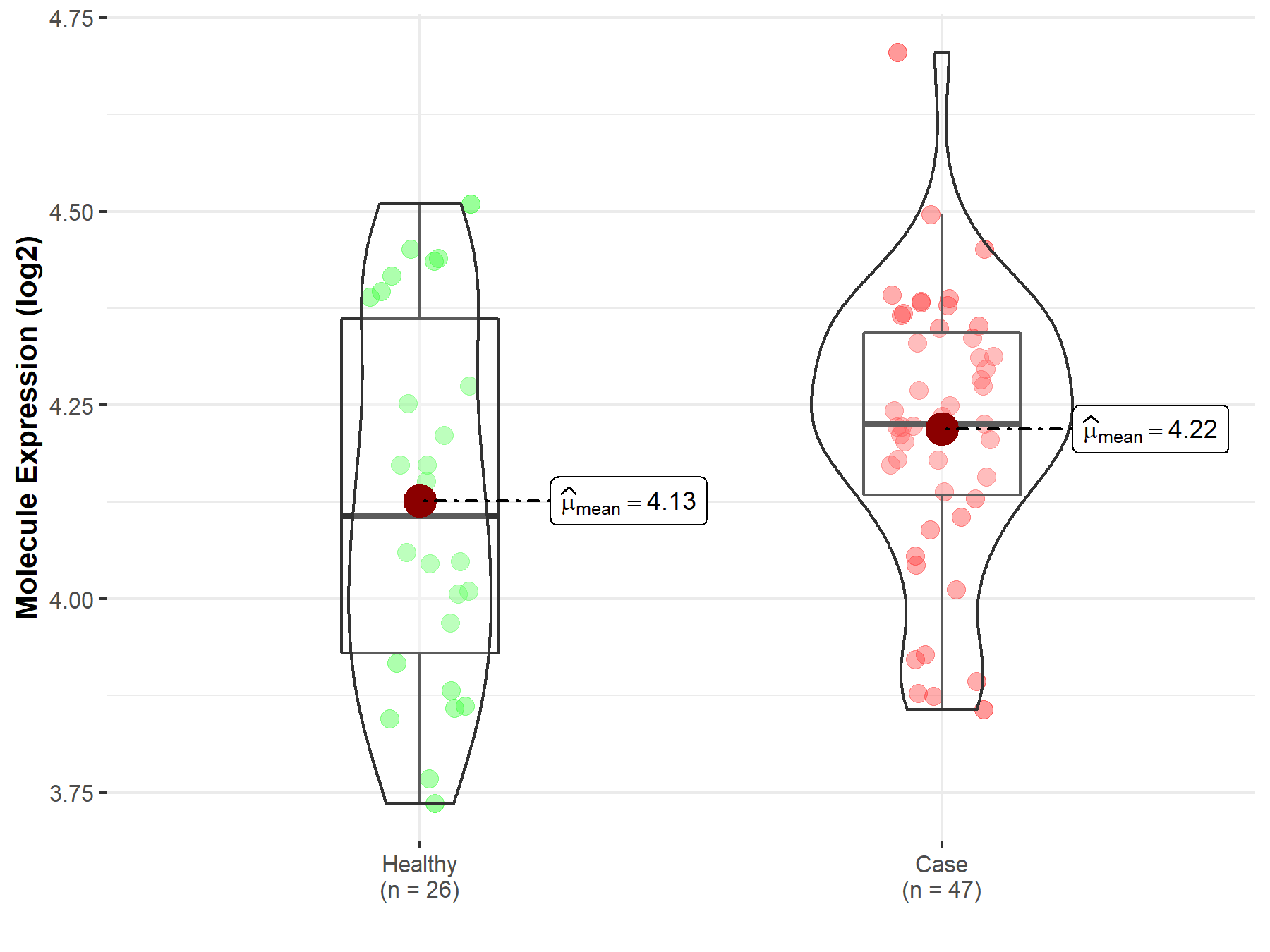
|
Click to View the Clearer Original Diagram |
Tissue-specific Molecule Abundances in Healthy Individuals

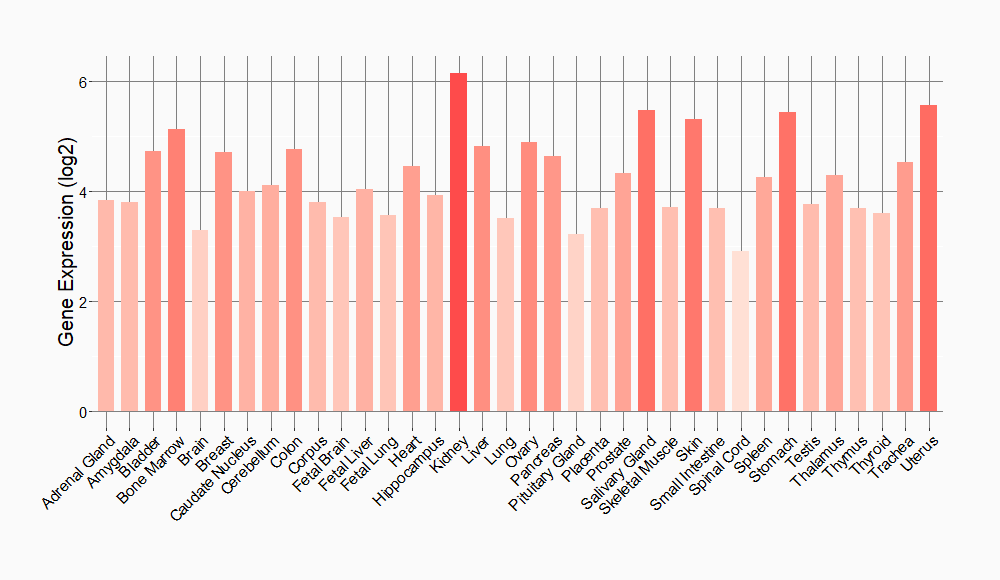
|
||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
