Drug Information
Drug (ID: DG00595) and It's Reported Resistant Information
| Name |
Phenobarbital
|
||||
|---|---|---|---|---|---|
| Synonyms |
Phenobarbital; Phenobarbitone; Phenobarbitol; Luminal; 50-06-6; Phenylethylbarbiturate; Phenobarbituric acid; Phenylethylmalonylurea; Fenobarbital; Phenemal; Adonal; Phenylethylbarbituric acid; Nunol; Neurobarb; Phenaemal; Dormiral; Gardenal; Hysteps; Aphenylbarbit; Aphenyletten; Dezibarbitur; Lepinaletten; Lumofridetten; Aephenal; Agrypnal; Amylofene; Barbenyl; Barbiphenyl; Barbipil; Barbivis; Barbonal; Barbophen; Bialminal; Cabronal; Calmetten; Calminal; Cardenal; Codibarbita; Coronaletta; Cratecil; Doscalun; Ensobarb; Ensodorm; Episedal; Epsylone; Eskabarb; Fenbital; Fenylettae; Gardepanyl; Glysoletten; Haplopan; Hennoletten; Hypnaletten; Hypnette; Hypnogen; Hypnolone; Hypnoltol; Liquital; Lixophen; Lubergal; Lubrokal; Lumesettes; Luphenil; Nirvonal; Parkotal; Pharmetten; Phenemalum; Phenobal; Phenobarbyl; Phenoluric; Phenolurio; Phenomet; Phenonyl; Phenoturic; Phenyletten; Phenyral; Polcominal; Promptonal; Sedizorin; Sedonettes; Solfoton; Sombutol; Somnolens; Somnoletten; Somnosan; Spasepilin; Starifen; Starilettae; Teolaxin; Barbita; Bardorm; Bartol; Chinoin; Duneryl; Epanal; Epidorm; Epilol; Etilfen; Euneryl; Fenemal; Fenosed; Haplos; Henotal; Leonal; Lepinal; Linasen; Lumesyn; Luramin; Molinal; Noptil; Sedabar; Sedicat; Sedlyn; Sedofen; Sedonal; Sevenal; Somonal; Lumen; Seda-Tablinen; Blu-phen; Nova-Pheno; Solu-Barb; Hypno-Tablinetten; Stental Extentabs; Phen-Bar; PHOB; Phenobarb; Sedophen; Talpheno; Triabarb; Versomnal; 5-Ethyl-5-phenylbarbituric acid; 5-Phenyl-5-ethylbarbituric acid; Tridezibarbitur; Triphenatol; Zadoletten; Barbinal; Barbiphen; Damoral; Dormina; Lefebar; Lephebar; Stental; Teoloxin; Theoloxin; Zadonal; SK-Phenobarbital; 5-ethyl-5-phenylpyrimidine-2,4,6(1H,3H,5H)-trione; Thenobarbital; 2,4,6(1H,3H,5H)-Pyrimidinetrione, 5-ethyl-5-phenyl-; 5-ethyl-5-phenyl-1,3-diazinane-2,4,6-trione; Dormital; Phenylethylbarbitursaeure; Phenylaethylbarbitursaeure; 5-Ethyl-5-phenyl-2,4,6(1H,3H,5H)-pyrimidinetrione; Barbituric acid, 5-ethyl-5-phenyl-; 5-Ethyl-5-phenyl-pyrimidine-2,4,6-trione; Barbilehae (barbilettae); UNII-YQE403BP4D; component of Tedral; Sedonal (sedative); component of Slowten; CHEBI:8069; component of Antrocol; component of Hecadrol; component of Bronkotabs; 5-Ethyl-5-phenyl-2,4,6-(1H,3H,5H)pyrimidinetrione; component of Primatene P; CHEMBL40; Phenobarbital (in methanol solution); component of Valpin 50-PB; YQE403BP4D; Austrominal; Phenobarbitalum; Phenobarbitonum; NSC-9848; NSC-128143; NCGC00159493-02; Fenobarbitale; Fenobarbitale [DCIT]; Elixir of phenobarbital; Phenobarbitalum [INN]; DSSTox_CID_1122; Chardonna-2; DSSTox_RID_75953; DSSTox_GSID_21122; Phenylethyl barbituric acid; Fenobarbital [INN-Spanish]; Phenyl-ethyl-barbituric acid; Phenobarbitalum [INN-Latin]; WLN: T6VMVMV FHJ F2 FR; Barbinol; Fenemal recip; CAS-50-06-6; Levsin PB Drops and Tablets; CCRIS 502; Luminal (TN); phenobarbital (PB); Acido 5-fenil-5-etilbarbiturico; HSDB 3157; Acido 5-fenil-5-etilbarbiturico [Italian]; 5-Ethyl-5-phenyl-2,6(1H,3H,5H)-pyrimidinetrione; EINECS 200-007-0; 2,6(1H,3H,5H)-Pyrimidinetrione, 5-ethyl-5-phenyl-; NSC 128143; Phenobar; AI3-02726; Phenobarbital [USP:INN:BAN:JAN]; Tedral (Salt/Mix); Primidone Impurity B; Kinesed (Salt/Mix); Antrocol (Salt/Mix); Donnatal (Salt/Mix); Donnazyme (Salt/Mix); Quadrinal (Salt/Mix); Mephobarbital M (nor); Barbidonna (Salt/Mix); Bronkotabs (Salt/Mix); PHENOBARBITAL CIV; Chardonna-2 (Salt/Mix); Epitope ID:116048; BIDD:PXR0061; Oprea1_384816; SCHEMBL16583; 5-ethyl-5-phenyl-hexahydropyrimidine-2,4,6-trione; Methylphenobarbital, M(nor-); MLS001240232; DivK1c_000987; GTPL2804; DTXSID5021122; SCHEMBL11114624; HMS503E15; KBio1_000987; NSC9848; Phenobarbital (JP17/USP/INN); NINDS_000987; HMS2272G06; Tox21_111713; Tox21_200510; BDBM50021437; NSC128143; STL367898; ZINC95588079; component of Primatene P (Salt/Mix); Phenobarbital 0.1 mg/ml in Methanol; Phenobarbital 1.0 mg/ml in Methanol; AKOS000605404; AKOS015964976; Barbituric acid, 5-ethyl-5-phenyl-,; AB02704; DB01174; MCULE-1782264021; component of Valpin 50-PB (Salt/Mix); IDI1_000987; NSC-128143-; Levsin PB Drops and Tablets (Salt/Mix); NCGC00159493-03; NCGC00159493-04; NCGC00258064-01; SMR000058986; DB-051722; C07434; D00506; Phenobarbital solution, 1.0 mg/mL in methanol; A827956; Q407241; SR-01000313151; SR-01000313151-1; Phenobarbital, United States Pharmacopeia (USP) Reference Standard; Phenobarbital solution, 1 mg/mL in methanol, ampule of 1 mL, certified reference material; 11097-06-6; UQA
Click to Show/Hide
|
||||
| Indication |
In total 1 Indication(s)
|
||||
| Structure |
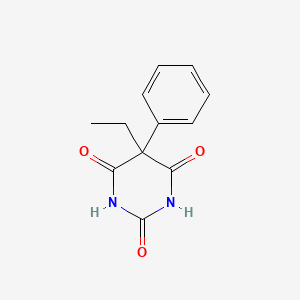
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Clinically Reported Resistance for This Drug
(1 diseases)
[2]
|
||||
| Target | Gamma-aminobutyric acid receptor (GAR) | NOUNIPROTAC | [1] | ||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
C12H12N2O3
|
||||
| IsoSMILES |
CCC1(C(=O)NC(=O)NC1=O)C2=CC=CC=C2
|
||||
| InChI |
1S/C12H12N2O3/c1-2-12(8-6-4-3-5-7-8)9(15)13-11(17)14-10(12)16/h3-7H,2H2,1H3,(H2,13,14,15,16,17)
|
||||
| InChIKey |
DDBREPKUVSBGFI-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
| VARIDT ID | |||||
| INTEDE ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-08: Nervous system diseases
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Potassium inwardly rectifying channel subfamily J member 10 (KCNJ10) | [1] | |||
| Sensitive Disease | Genetic generalized epilepsies [ICD-11: 8A61.0] | |||
| Molecule Alteration | SNP | rs12402969 C+ Genotypes CC+CT |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Experiment for Molecule Alteration |
Genotyping assay | |||
| Mechanism Description | By analyzing the association between KCNJ10 polymorphisms and anti-epileptic drug efficacy of GGEs we found the frequency of rs12402969 C allele and CC+CT genotypes were higher in GGEs drug responsive patients than that in drug resistant patients | |||
| Key Molecule: Potassium inwardly rectifying channel subfamily J member 10 (KCNJ10) | [1] | |||
| Sensitive Disease | Genetic generalized epilepsies [ICD-11: 8A61.0] | |||
| Molecule Alteration | SNP | rs12402969 C+ Genotypes CC+CT |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Experiment for Molecule Alteration |
Genotyping assay | |||
| Mechanism Description | By analyzing the association between KCNJ10 polymorphisms and anti-epileptic drug efficacy of GGEs we found the frequency of rs12402969 C allele and CC+CT genotypes were higher in GGEs drug responsive patients than that in drug resistant patients | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Multidrug resistance protein 1 (ABCB1) | [2] | |||
| Resistant Disease | Status epilepticus [ICD-11: 8A66.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Mechanism Description | Pgp is involved in the resistance to phenytoin and phenobarbital but not diazepam. | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
