Drug Information
Drug (ID: DG00305) and It's Reported Resistant Information
| Name |
Dacarbazine
|
||||
|---|---|---|---|---|---|
| Synonyms |
Biocarbazin; Biocarbazine; DTIC; DTICDome; DTIE; Dacarbazino; Dacarbazinum; Dacatic; Decarbazine; Deticene; Dimethyltriazenoimidazolecarboxamide; ICDMT; ICDT; Biocarbazine R; DTIC Dome; Dimethyl Imidazole Carboxamide; Dimethyl Triazeno Imidazole Carboxamide; Imidazole carboxamide; HE1150000; Carboxamide (TN); Carboxamide, Dimethyl Imidazole; DIC (TN); DTIC (TN); DTIC-Dome; Dacarbazino [INN-Spanish]; Dacarbazinum [INN-Latin]; Imidazole (TN); Imidazole Carboxamide, Dimethyl; NPFAPI-05; DTIC-Dome (TN); Di-me-triazenoimidazolecarboxamide; Di-methyl-triazenoimidazolecarboxamide; Dtic-Dome (TN); DTIC, DTIC-Dome, Dacarbazine; Dacarbazine (JAN/USP/INN); Dacarbazine [USAN:INN:BAN:JAN]; (5E)-5-(dimethylaminohydrazinylidene)imidazole-4-carboxamide; (5Z)-5-(dimethylaminohydrazinylidene)imidazole-4-carboxamide; (Dimethyltriazeno)imidazolecarboxamide; 4(5)-(3,3-Dimethyl-1-triazeno)imidazole-4-carboxamide; 4(5)-(3,3-Dimethyl-1-triazeno)imidazole-5(4)-carboxamide; 4-(3,3-Dimethyl-1-triazeno)imidazole-5-carboxamide; 4-(3,3-Dimethyltriazeno)imidazole-5-carboxamide; 4-(5)-(3,3-Dimethyl-1-triazeno)imidazole-5(4)-carboxamide; 4-(Dimethyltriazeno)imidazole-5-c arboxamide; 4-(Dimethyltriazeno)imidazole-5-carboxamide; 4-(or 5)-(3,3-Dimethyl-1-triazeno)imidazole-5(or 4)-carboxamide; 4-[(1E)-3,3-Dimethyltriaz-1-en-1-yl]-1H-imidazole-5-carboxamide; 4-[3,3-dimethyltriaz-1-en-1-yl]-1H-imidazole-5-carboxamide; 5(or 4)-(dimethyltriazeno)imidazol e-4(or 5)-carboxamide; 5(or 4)-(dimethyltriazeno)imidazole-4(or 5)-carboxamide; 5-(3,3-Dimethyl-1-triazeno)imidazole-4-carboxamide; 5-(3,3-Dimethyl-1-triazenyl)-1H-imidazole-4-carboxamide; 5-(3,3-Dimethyl-1-triazenyl)imidazole-4-carboxamide; 5-(3,3-Dimethyltri azeno)imidazole-4-carboxamide; 5-(3,3-Dimethyltriazeno)-imidazole-4-carbamide; 5-(3,3-Dimethyltriazeno)imidazole-4-carboxamide; 5-(3,3-dimethyltriaz-1-en-1-yl)-1H-imidazole-4-carboxamide; 5-(Dimethyltriazeno)-4-imidazolecarboxamide; 5-(Dimethyltriazeno)imidazole-4-carboxamide; 5-(Dimethyltriazeno)imidazole-4-carboximide; 5-(dimethylaminohydrazinylidene)imidazole-4-carboxamide; 5-[3,3-Dimethyl-1-triazenyl]imidazole-4-carboxamide
Click to Show/Hide
|
||||
| Indication |
In total 1 Indication(s)
|
||||
| Structure |
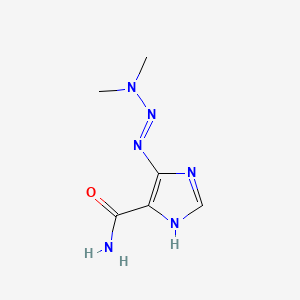
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Clinically Reported Resistance for This Drug
(1 diseases)
[2]
|
||||
| Target | Human Deoxyribonucleic acid (hDNA) | NOUNIPROTAC | [1] | ||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
C6H10N6O
|
||||
| IsoSMILES |
CN(C)/N=N/C1=C(NC=N1)C(=O)N
|
||||
| InChI |
1S/C6H10N6O/c1-12(2)11-10-6-4(5(7)13)8-3-9-6/h3H,1-2H3,(H2,7,13)(H,8,9)/b11-10+
|
||||
| InChIKey |
FDKXTQMXEQVLRF-ZHACJKMWSA-N
|
||||
| PubChem CID | |||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
| INTEDE ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-02: Benign/in-situ/malignant neoplasm
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Glutathione S-transferase P (GSTP1) | [2] | |||
| Resistant Disease | Malignant glioma [ICD-11: 2A00.2] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Brain cancer [ICD-11: 2A00] | |||
| The Specified Disease | Malignant glioma | |||
| The Studied Tissue | Blood | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 3.99E-02 Fold-change: 1.21E-01 Z-score: 2.07E+00 |
|||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Malignant gliomas tissue | N.A. | ||
| Experiment for Molecule Alteration |
Immunohistochemistry assay | |||
| Experiment for Drug Resistance |
EDR assay | |||
| Mechanism Description | In vitro drug resistance in malignant gliomas was independent of prior therapy. High-grade glioblastomas showed a lower level of extreme drug resistance than low-grade astrocytomas to cisplatin (11% versus 27%), temozolomide (14% versus 27%), irinotecan (33% versus 53%), and BCNU (29% versus 38%). A substantial percentage of brain tumors overexpressed biomarkers associated with drug resistance, including MGMT (67%), GSTP1 (49%), and mutant p53 (41%). MGMT and GSTP1 overexpression was independently associated with in vitro resistance to BCNU, whereas coexpression of these two markers was associated with the greatest degree of BCNU resistance. | |||
|
|
||||
| Key Molecule: Multidrug resistance protein 1 (ABCB1) | [2] | |||
| Resistant Disease | Malignant glioma [ICD-11: 2A00.2] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Malignant gliomas tissue | N.A. | ||
| Experiment for Molecule Alteration |
Immunohistochemistry assay | |||
| Experiment for Drug Resistance |
EDR assay | |||
| Mechanism Description | In vitro drug resistance in malignant gliomas was independent of prior therapy. High-grade glioblastomas showed a lower level of extreme drug resistance than low-grade astrocytomas to cisplatin (11% versus 27%), temozolomide (14% versus 27%), irinotecan (33% versus 53%), and BCNU (29% versus 38%). A substantial percentage of brain tumors overexpressed biomarkers associated with drug resistance, including MGMT (67%), GSTP1 (49%), and mutant p53 (41%). MGMT and GSTP1 overexpression was independently associated with in vitro resistance to BCNU, whereas coexpression of these two markers was associated with the greatest degree of BCNU resistance. | |||
|
|
||||
| Key Molecule: Methylated-DNA--protein-cysteine methyltransferase (MGMT) | [2] | |||
| Resistant Disease | Malignant glioma [ICD-11: 2A00.2] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Malignant gliomas tissue | N.A. | ||
| Experiment for Molecule Alteration |
Immunohistochemistry assay | |||
| Experiment for Drug Resistance |
EDR assay | |||
| Mechanism Description | In vitro drug resistance in malignant gliomas was independent of prior therapy. High-grade glioblastomas showed a lower level of extreme drug resistance than low-grade astrocytomas to cisplatin (11% versus 27%), temozolomide (14% versus 27%), irinotecan (33% versus 53%), and BCNU (29% versus 38%). A substantial percentage of brain tumors overexpressed biomarkers associated with drug resistance, including MGMT (67%), GSTP1 (49%), and mutant p53 (41%). MGMT and GSTP1 overexpression was independently associated with in vitro resistance to BCNU, whereas coexpression of these two markers was associated with the greatest degree of BCNU resistance. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: hsa-mir-31 | [1] | |||
| Sensitive Disease | Melanoma [ICD-11: 2C30.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell colony | Inhibition | hsa05200 | |
| Cell proliferation | Inhibition | hsa05200 | ||
| PI3K/AKT signaling pathway | Inhibition | hsa04151 | ||
| In Vitro Model | A375 cells | Skin | Homo sapiens (Human) | CVCL_0132 |
| 293T cells | Breast | Homo sapiens (Human) | CVCL_0063 | |
| HT144 cells | Skin | Homo sapiens (Human) | CVCL_0318 | |
| SkMEL5 cells | Skin | Homo sapiens (Human) | CVCL_0527 | |
| SkMEL1 cells | Skin | Homo sapiens (Human) | CVCL_0068 | |
| A2058 cells | Skin | Homo sapiens (Human) | CVCL_1059 | |
| A875 cells | Skin | Homo sapiens (Human) | CVCL_4733 | |
| M21 cells | Skin | Homo sapiens (Human) | CVCL_D031 | |
| SkMEL13 cells | Skin | Homo sapiens (Human) | CVCL_6022 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
MTS assay | |||
| Mechanism Description | miR-31 could suppress tumor growth and enhance sensitivity to dacarbazine (DTIC) by down-regulating SOX10 mainly via inhibiting PI3k/AkT signaling pathway in melanoma. | |||
|
|
||||
| Key Molecule: Transcription factor SOX-10 (SOX10) | [1] | |||
| Sensitive Disease | Melanoma [ICD-11: 2C30.0] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell colony | Inhibition | hsa05200 | |
| Cell proliferation | Inhibition | hsa05200 | ||
| PI3K/AKT signaling pathway | Inhibition | hsa04151 | ||
| In Vitro Model | A375 cells | Skin | Homo sapiens (Human) | CVCL_0132 |
| 293T cells | Breast | Homo sapiens (Human) | CVCL_0063 | |
| HT144 cells | Skin | Homo sapiens (Human) | CVCL_0318 | |
| SkMEL5 cells | Skin | Homo sapiens (Human) | CVCL_0527 | |
| SkMEL1 cells | Skin | Homo sapiens (Human) | CVCL_0068 | |
| A2058 cells | Skin | Homo sapiens (Human) | CVCL_1059 | |
| A875 cells | Skin | Homo sapiens (Human) | CVCL_4733 | |
| M21 cells | Skin | Homo sapiens (Human) | CVCL_D031 | |
| SkMEL13 cells | Skin | Homo sapiens (Human) | CVCL_6022 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTS assay | |||
| Mechanism Description | miR-31 could suppress tumor growth and enhance sensitivity to dacarbazine (DTIC) by down-regulating SOX10 mainly via inhibiting PI3k/AkT signaling pathway in melanoma. | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
