Molecule Information
General Information of the Molecule (ID: Mol00498)
| Name |
Methylated-DNA--protein-cysteine methyltransferase (MGMT)
,Homo sapiens
|
||||
|---|---|---|---|---|---|
| Synonyms |
6-O-methylguanine-DNA methyltransferase; MGMT; O-6-methylguanine-DNA-alkyltransferase
Click to Show/Hide
|
||||
| Molecule Type |
Protein
|
||||
| Gene Name |
MGMT
|
||||
| Gene ID | |||||
| Location |
chr10:129467190-129770983[+]
|
||||
| Sequence |
MDKDCEMKRTTLDSPLGKLELSGCEQGLHEIKLLGKGTSAADAVEVPAPAAVLGGPEPLM
QCTAWLNAYFHQPEAIEEFPVPALHHPVFQQESFTRQVLWKLLKVVKFGEVISYQQLAAL AGNPKAARAVGGAMRGNPVPILIPCHRVVCSSGAVGNYSGGLAVKEWLLAHEGHRLGKPG LGGSSGLAGAWLKGAGATSGSPPAGRN Click to Show/Hide
|
||||
| 3D-structure |
|
||||
| Function |
Involved in the cellular defense against the biological effects of O6-methylguanine (O6-MeG) and O4-methylthymine (O4-MeT) in DNA. Repairs the methylated nucleobase in DNA by stoichiometrically transferring the methyl group to a cysteine residue in the enzyme. This is a suicide reaction: the enzyme is irreversibly inactivated.
Click to Show/Hide
|
||||
| Uniprot ID | |||||
| Ensembl ID | |||||
| HGNC ID | |||||
| Click to Show/Hide the Complete Species Lineage | |||||
Type(s) of Resistant Mechanism of This Molecule
Drug Resistance Data Categorized by Drug
Approved Drug(s)
7 drug(s) in total
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Anaplastic astrocytoma [ICD-11: 2A00.04] | [1] | |||
| Resistant Disease | Anaplastic astrocytoma [ICD-11: 2A00.04] | |||
| Resistant Drug | Temozolomide | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Brain cancer [ICD-11: 2A00] | |||
| The Specified Disease | Glioma | |||
| The Studied Tissue | White matter | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 3.65E-01 Fold-change: 4.99E-02 Z-score: 9.29E-01 |
|||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Malignant gliomas tissue | N.A. | ||
| Experiment for Molecule Alteration |
Immunohistochemistry assay | |||
| Experiment for Drug Resistance |
Oncotech EDR assay | |||
| Mechanism Description | For drugs that have evaded cytosolic mechanisms of drug resistance, the nucleus is equipped with the capacity to remove BCNU or temozolomide alkyl groups from the O6-position of guanine via a reaction catalyzed by MGMT. Repair occurs before cross-link formation and involves an irreversible stoichiometric covalent transfer of the O6-alkyl DNA adduct to a cysteine within the active site of MGMT, resulting in the inactivation and subsequent depletion of enzyme activity. MGMT-mediated repair is rapid, with a half-life of 35 hours. MGMT enzyme recovery occurs via de novo synthesis. In malignant glioma patients, MGMT overexpression has been associated with resistance to BCNU and similar alkylating agents and was an independent predictor of poor survival. MGMT is also thought to contribute to temozolomide resistance, which we did not detect in our study. This may be related to the in vitro pharmacokinetic differences between BCNU and temozolomide. | |||
| Disease Class: Malignant glioma [ICD-11: 2A00.2] | [4] | |||
| Resistant Disease | Malignant glioma [ICD-11: 2A00.2] | |||
| Resistant Drug | Temozolomide | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Epithelial mesenchymal transition signaling pathway | Inhibition | hsa01521 | |
| In Vitro Model | U251 cells | Brain | Homo sapiens (Human) | CVCL_0021 |
| LN229 cells | Brain | Homo sapiens (Human) | CVCL_0393 | |
| U373 cells | Brain | Homo sapiens (Human) | CVCL_2219 | |
| U118 cells | Brain | Homo sapiens (Human) | CVCL_0633 | |
| NHA | Brain | Homo sapiens (Human) | N.A. | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay; BrdU incorporation assay | |||
| Mechanism Description | XIST was inversely correlated with miR29c, positively correlated with PS1, positively related with MGMT. XIST can inhibit miR29c expression by directly binding to miR29c and subsequently up-regulate the expression of SP1 and MGMT to promote the chemoresistance of glioma cells to TMZ. | |||
| Disease Class: Glioma [ICD-11: 2A00.1] | [5] | |||
| Resistant Disease | Glioma [ICD-11: 2A00.1] | |||
| Resistant Drug | Temozolomide | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Beta-catenin/MGMT signaling pathway | Regulation | N.A. | |
| In Vitro Model | U251R cells | Brain | Homo sapiens (Human) | N.A. |
| Experiment for Molecule Alteration |
Western blot assay; qRT-PCR | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | In this study, we found that HOTAIR was upregulated in TMZ-resistant GBM cell lines and patients with high HOTAIR expression responded poorly to TMZ therapy. HOTAIR knockdown restored TMZ sensitivity in U251R cells, while HOTAIR overexpression conferred TMZ resistance in U251 cells. Wnt/beta-catenin signaling was enriched in patients with high HOTAIR expression; consistently, HOTAIR positively regulated beta-catenin expression in U251 cells. Moreover, HOTAIR-mediated TMZ resistance was associated with increased MGMT protein level, which resulted from the HOTAIR/miR-214-3p/beta-catenin network. Besides, GBM with high HOTAIR expression exhibited sensitivity to methotrexate. Methotrexate enhanced TMZ sensitivity in U251R cells, accompanied by reduced expression of HOTAIR and beta-catenin. Thus, we conlcude that HOTAIR is a risk factor for TMZ resistance and methotrexate may represent a potential therapeutic drug for patients with high HOTAIR expression level. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Glioblastoma [ICD-11: 2A00.02] | [2], [3] | |||
| Sensitive Disease | Glioblastoma [ICD-11: 2A00.02] | |||
| Sensitive Drug | Temozolomide | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Brain cancer [ICD-11: 2A00] | |||
| The Specified Disease | Brain cancer | |||
| The Studied Tissue | Nervous tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 3.60E-01 Fold-change: -6.55E-03 Z-score: -9.16E-01 |
|||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | U251 cells | Brain | Homo sapiens (Human) | CVCL_0021 |
| LN229 cells | Brain | Homo sapiens (Human) | CVCL_0393 | |
| A172 cells | Brain | Homo sapiens (Human) | CVCL_0131 | |
| T98 cells | Brain | Homo sapiens (Human) | CVCL_B368 | |
| U87 cells | Brain | Homo sapiens (Human) | CVCL_0022 | |
| U118 cells | Brain | Homo sapiens (Human) | CVCL_0633 | |
| U138 cells | Brain | Homo sapiens (Human) | CVCL_0020 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis; Luciferase reporter assay | |||
| Experiment for Drug Resistance |
CCK8 assay; Colony formation assay; TMZ cytotoxicity assay; gamma -H2AX foci formation assay | |||
| Mechanism Description | miR-198 enhances temozolomide sensitivity in glioblastoma by targeting MGMT. | |||
| Disease Class: Glioblastoma [ICD-11: 2A00.02] | [6] | |||
| Sensitive Disease | Glioblastoma [ICD-11: 2A00.02] | |||
| Sensitive Drug | Temozolomide | |||
| Molecule Alteration | Methylation | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell colony | Inhibition | hsa05200 | ||
| Cell viability | Inhibition | hsa05200 | ||
| In Vitro Model | U251 cells | Brain | Homo sapiens (Human) | CVCL_0021 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay; TUNEL assay; Flow cytometry assay | |||
| Mechanism Description | The endogenous protein level of GSk3beta and MGMT was significantly suppressed by combination of MALAT1 knockdown and miR-101 overexpression and the promoter methylation of MGMT was largely promoted by the combination of MALAT1 knockdown and miR-101 overexpression. | |||
| Disease Class: Primary central nervous system lymphoma [ICD-11: 2A8Z.0] | [7] | |||
| Sensitive Disease | Primary central nervous system lymphoma [ICD-11: 2A8Z.0] | |||
| Sensitive Drug | Temozolomide | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Raji cells | Brain | Homo sapiens (Human) | CVCL_0511 |
| Primary central nervous system lymphoma | Spinal cord | Homo sapiens (Human) | N.A. | |
| Experiment for Molecule Alteration |
Western blotting assay | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miR-370 was downregulated in PCNSL tissues, while MGMT was inversely overexpressed.TMZ sensitivity consistently correlated with the overexpression of miR-370 and inhibition of MGMT; miR-370 affected PCNSL cell proliferation and increased apoptosis via MGMT. | |||
| Disease Class: Primary central nervous system lymphoma [ICD-11: 2A8Z.0] | [7] | |||
| Sensitive Disease | Primary central nervous system lymphoma [ICD-11: 2A8Z.0] | |||
| Sensitive Drug | Temozolomide | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Raji Burkitt cells | Bone | Homo sapiens (Human) | N.A. |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Our study described a new miR-370-mediated mechanism of MGMT regulation in PCNSL. We first showed that miR-370 was downregulated in PCNSL tissues, while MGMT was inversely overexpressed. It was also observed that miR-370 suppressed the expression of MGMT. Additionally, upregulation of miR-370 significantly increased TMZ sensitivity dependent of MGMT, thus suppressed Raji cell proliferation and induced apoptosis in vitro. These results suggest that miR-370 is a potential target in PCNSL treatment. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Malignant glioma [ICD-11: 2A00.2] | [1] | |||
| Resistant Disease | Malignant glioma [ICD-11: 2A00.2] | |||
| Resistant Drug | Carmustine | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Malignant gliomas tissue | N.A. | ||
| Experiment for Molecule Alteration |
Immunohistochemistry assay | |||
| Experiment for Drug Resistance |
EDR assay | |||
| Mechanism Description | In vitro drug resistance in malignant gliomas was independent of prior therapy. High-grade glioblastomas showed a lower level of extreme drug resistance than low-grade astrocytomas to cisplatin (11% versus 27%), temozolomide (14% versus 27%), irinotecan (33% versus 53%), and BCNU (29% versus 38%). A substantial percentage of brain tumors overexpressed biomarkers associated with drug resistance, including MGMT (67%), GSTP1 (49%), and mutant p53 (41%). MGMT and GSTP1 overexpression was independently associated with in vitro resistance to BCNU, whereas coexpression of these two markers was associated with the greatest degree of BCNU resistance. | |||
| Disease Class: Anaplastic astrocytoma [ICD-11: 2A00.04] | [1] | |||
| Resistant Disease | Anaplastic astrocytoma [ICD-11: 2A00.04] | |||
| Resistant Drug | Carmustine | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Malignant gliomas tissue | N.A. | ||
| Experiment for Molecule Alteration |
Immunohistochemistry assay | |||
| Experiment for Drug Resistance |
Oncotech EDR assay | |||
| Mechanism Description | For drugs that have evaded cytosolic mechanisms of drug resistance, the nucleus is equipped with the capacity to remove BCNU or temozolomide alkyl groups from the O6-position of guanine via a reaction catalyzed by MGMT. Repair occurs before cross-link formation and involves an irreversible stoichiometric covalent transfer of the O6-alkyl DNA adduct to a cysteine within the active site of MGMT, resulting in the inactivation and subsequent depletion of enzyme activity. MGMT-mediated repair is rapid, with a half-life of 35 hours. MGMT enzyme recovery occurs via de novo synthesis. In malignant glioma patients, MGMT overexpression has been associated with resistance to BCNU and similar alkylating agents and was an independent predictor of poor survival. MGMT is also thought to contribute to temozolomide resistance, which we did not detect in our study. This may be related to the in vitro pharmacokinetic differences between BCNU and temozolomide. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Malignant glioma [ICD-11: 2A00.2] | [1] | |||
| Resistant Disease | Malignant glioma [ICD-11: 2A00.2] | |||
| Resistant Drug | Cisplatin | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Malignant gliomas tissue | N.A. | ||
| Experiment for Molecule Alteration |
Immunohistochemistry assay | |||
| Experiment for Drug Resistance |
EDR assay | |||
| Mechanism Description | In vitro drug resistance in malignant gliomas was independent of prior therapy. High-grade glioblastomas showed a lower level of extreme drug resistance than low-grade astrocytomas to cisplatin (11% versus 27%), temozolomide (14% versus 27%), irinotecan (33% versus 53%), and BCNU (29% versus 38%). A substantial percentage of brain tumors overexpressed biomarkers associated with drug resistance, including MGMT (67%), GSTP1 (49%), and mutant p53 (41%). MGMT and GSTP1 overexpression was independently associated with in vitro resistance to BCNU, whereas coexpression of these two markers was associated with the greatest degree of BCNU resistance. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Malignant glioma [ICD-11: 2A00.2] | [1] | |||
| Resistant Disease | Malignant glioma [ICD-11: 2A00.2] | |||
| Resistant Drug | Dacarbazine | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Malignant gliomas tissue | N.A. | ||
| Experiment for Molecule Alteration |
Immunohistochemistry assay | |||
| Experiment for Drug Resistance |
EDR assay | |||
| Mechanism Description | In vitro drug resistance in malignant gliomas was independent of prior therapy. High-grade glioblastomas showed a lower level of extreme drug resistance than low-grade astrocytomas to cisplatin (11% versus 27%), temozolomide (14% versus 27%), irinotecan (33% versus 53%), and BCNU (29% versus 38%). A substantial percentage of brain tumors overexpressed biomarkers associated with drug resistance, including MGMT (67%), GSTP1 (49%), and mutant p53 (41%). MGMT and GSTP1 overexpression was independently associated with in vitro resistance to BCNU, whereas coexpression of these two markers was associated with the greatest degree of BCNU resistance. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Malignant glioma [ICD-11: 2A00.2] | [1] | |||
| Resistant Disease | Malignant glioma [ICD-11: 2A00.2] | |||
| Resistant Drug | Docetaxel | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Malignant gliomas tissue | N.A. | ||
| Experiment for Molecule Alteration |
Immunohistochemistry assay | |||
| Experiment for Drug Resistance |
EDR assay | |||
| Mechanism Description | In vitro drug resistance in malignant gliomas was independent of prior therapy. High-grade glioblastomas showed a lower level of extreme drug resistance than low-grade astrocytomas to cisplatin (11% versus 27%), temozolomide (14% versus 27%), irinotecan (33% versus 53%), and BCNU (29% versus 38%). A substantial percentage of brain tumors overexpressed biomarkers associated with drug resistance, including MGMT (67%), GSTP1 (49%), and mutant p53 (41%). MGMT and GSTP1 overexpression was independently associated with in vitro resistance to BCNU, whereas coexpression of these two markers was associated with the greatest degree of BCNU resistance. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Malignant glioma [ICD-11: 2A00.2] | [1] | |||
| Resistant Disease | Malignant glioma [ICD-11: 2A00.2] | |||
| Resistant Drug | Irinotecan | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Malignant gliomas tissue | N.A. | ||
| Experiment for Molecule Alteration |
Immunohistochemistry assay | |||
| Experiment for Drug Resistance |
EDR assay | |||
| Mechanism Description | In vitro drug resistance in malignant gliomas was independent of prior therapy. High-grade glioblastomas showed a lower level of extreme drug resistance than low-grade astrocytomas to cisplatin (11% versus 27%), temozolomide (14% versus 27%), irinotecan (33% versus 53%), and BCNU (29% versus 38%). A substantial percentage of brain tumors overexpressed biomarkers associated with drug resistance, including MGMT (67%), GSTP1 (49%), and mutant p53 (41%). MGMT and GSTP1 overexpression was independently associated with in vitro resistance to BCNU, whereas coexpression of these two markers was associated with the greatest degree of BCNU resistance. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Malignant glioma [ICD-11: 2A00.2] | [1] | |||
| Resistant Disease | Malignant glioma [ICD-11: 2A00.2] | |||
| Resistant Drug | Vincristine | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Malignant gliomas tissue | N.A. | ||
| Experiment for Molecule Alteration |
Immunohistochemistry assay | |||
| Experiment for Drug Resistance |
EDR assay | |||
| Mechanism Description | In vitro drug resistance in malignant gliomas was independent of prior therapy. High-grade glioblastomas showed a lower level of extreme drug resistance than low-grade astrocytomas to cisplatin (11% versus 27%), temozolomide (14% versus 27%), irinotecan (33% versus 53%), and BCNU (29% versus 38%). A substantial percentage of brain tumors overexpressed biomarkers associated with drug resistance, including MGMT (67%), GSTP1 (49%), and mutant p53 (41%). MGMT and GSTP1 overexpression was independently associated with in vitro resistance to BCNU, whereas coexpression of these two markers was associated with the greatest degree of BCNU resistance. | |||
Disease- and Tissue-specific Abundances of This Molecule
ICD Disease Classification 02

| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Nervous tissue | |
| The Specified Disease | Brain cancer | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 3.60E-01; Fold-change: -2.72E-02; Z-score: -5.98E-02 | |
|
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |
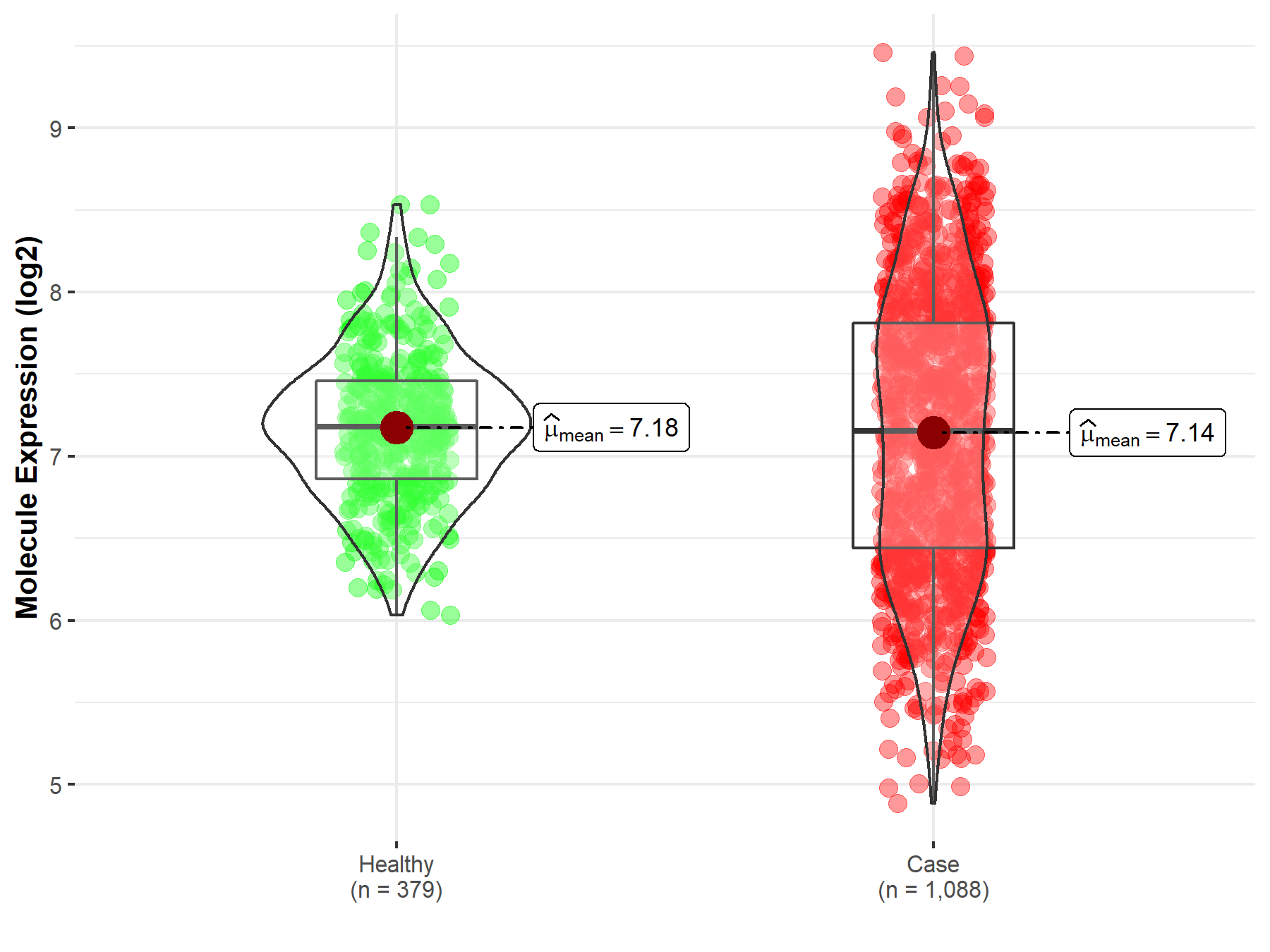
|
Click to View the Clearer Original Diagram |
| The Studied Tissue | Brainstem tissue | |
| The Specified Disease | Glioma | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 4.62E-01; Fold-change: -2.40E-01; Z-score: -6.41E-01 | |
|
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |
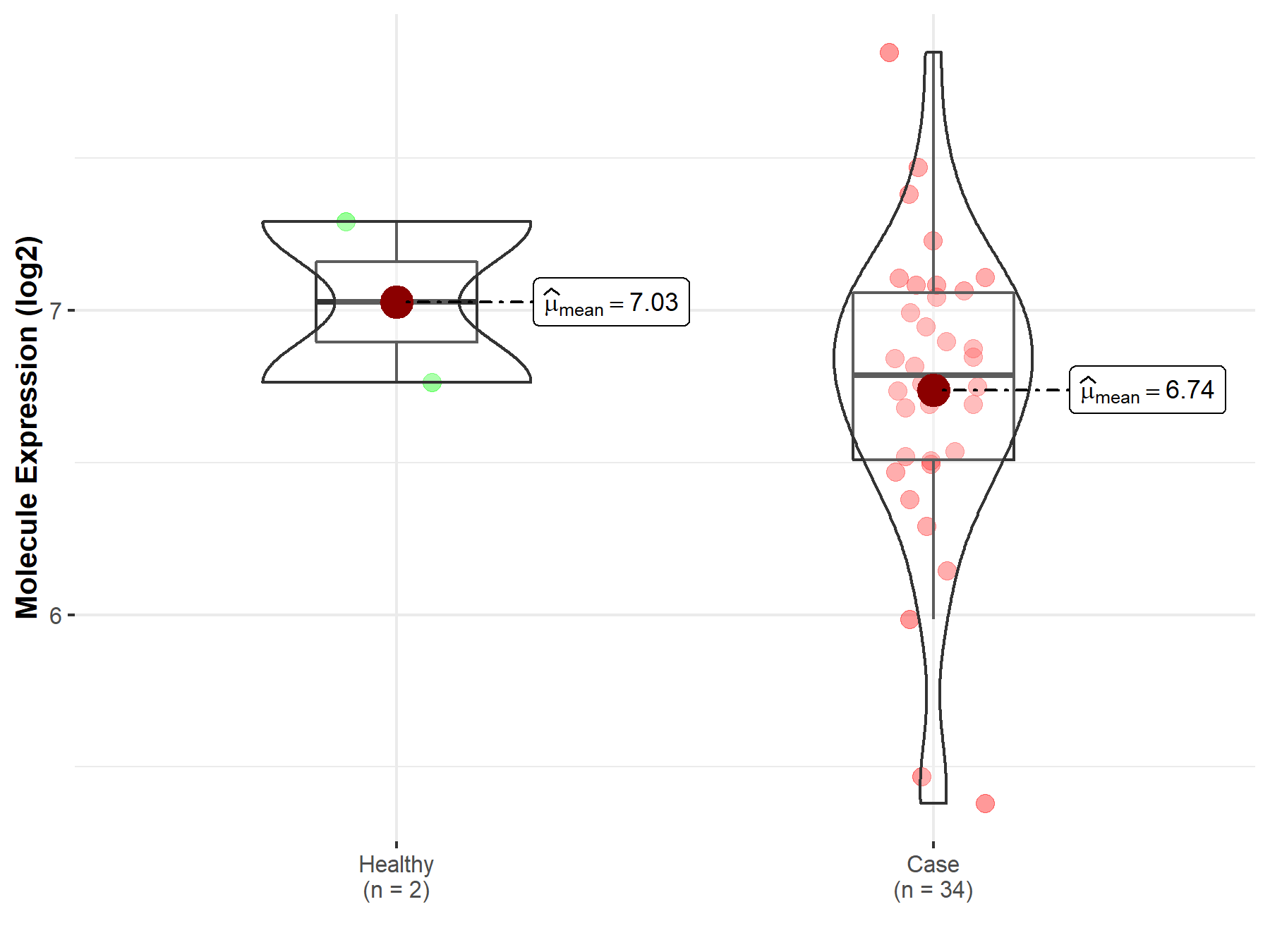
|
Click to View the Clearer Original Diagram |
| The Studied Tissue | White matter | |
| The Specified Disease | Glioma | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 3.65E-01; Fold-change: 2.48E-01; Z-score: 4.03E-01 | |
|
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |
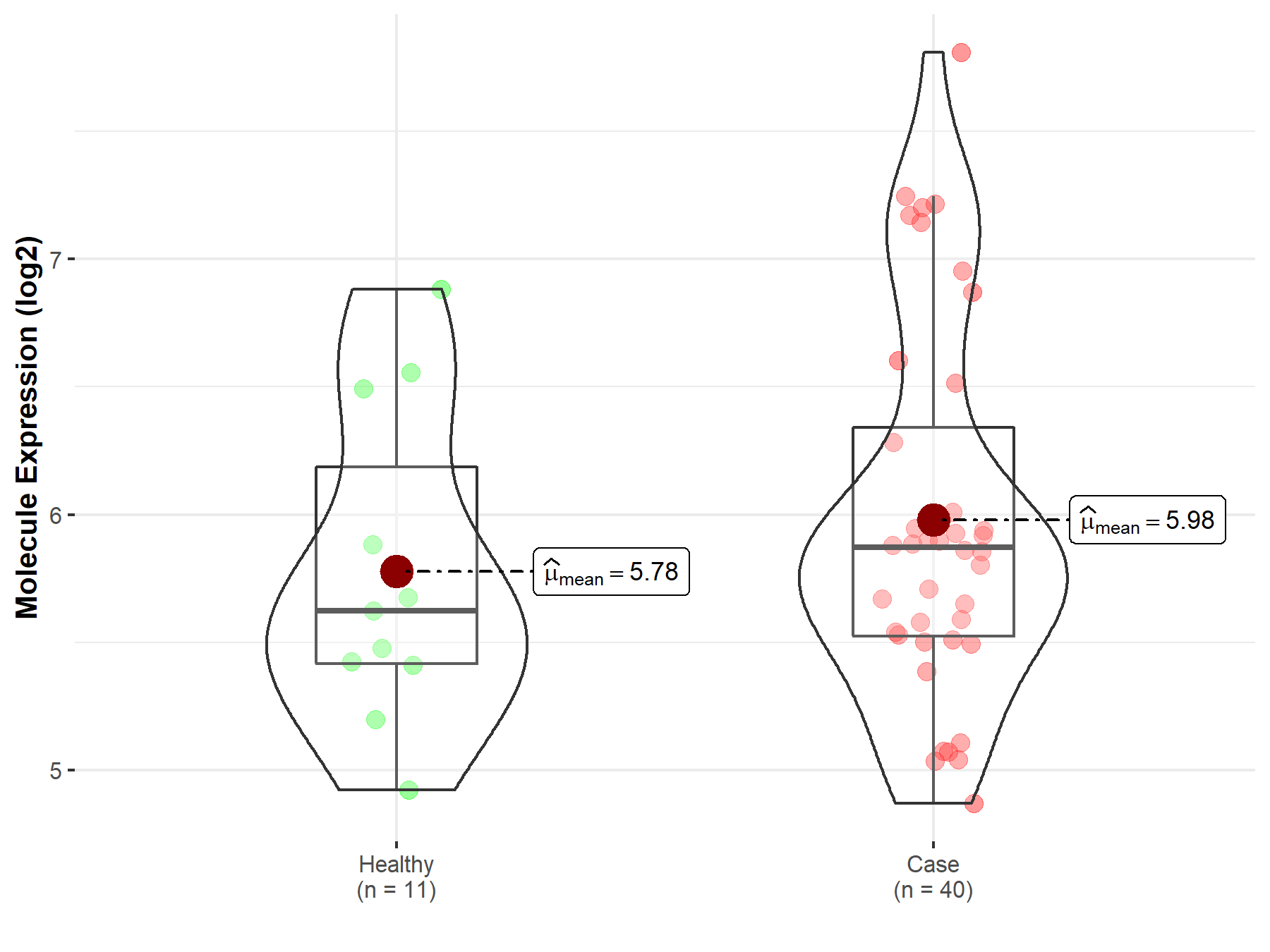
|
Click to View the Clearer Original Diagram |
| The Studied Tissue | Brainstem tissue | |
| The Specified Disease | Neuroectodermal tumor | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 2.61E-03; Fold-change: 1.09E+00; Z-score: 1.61E+00 | |
|
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |
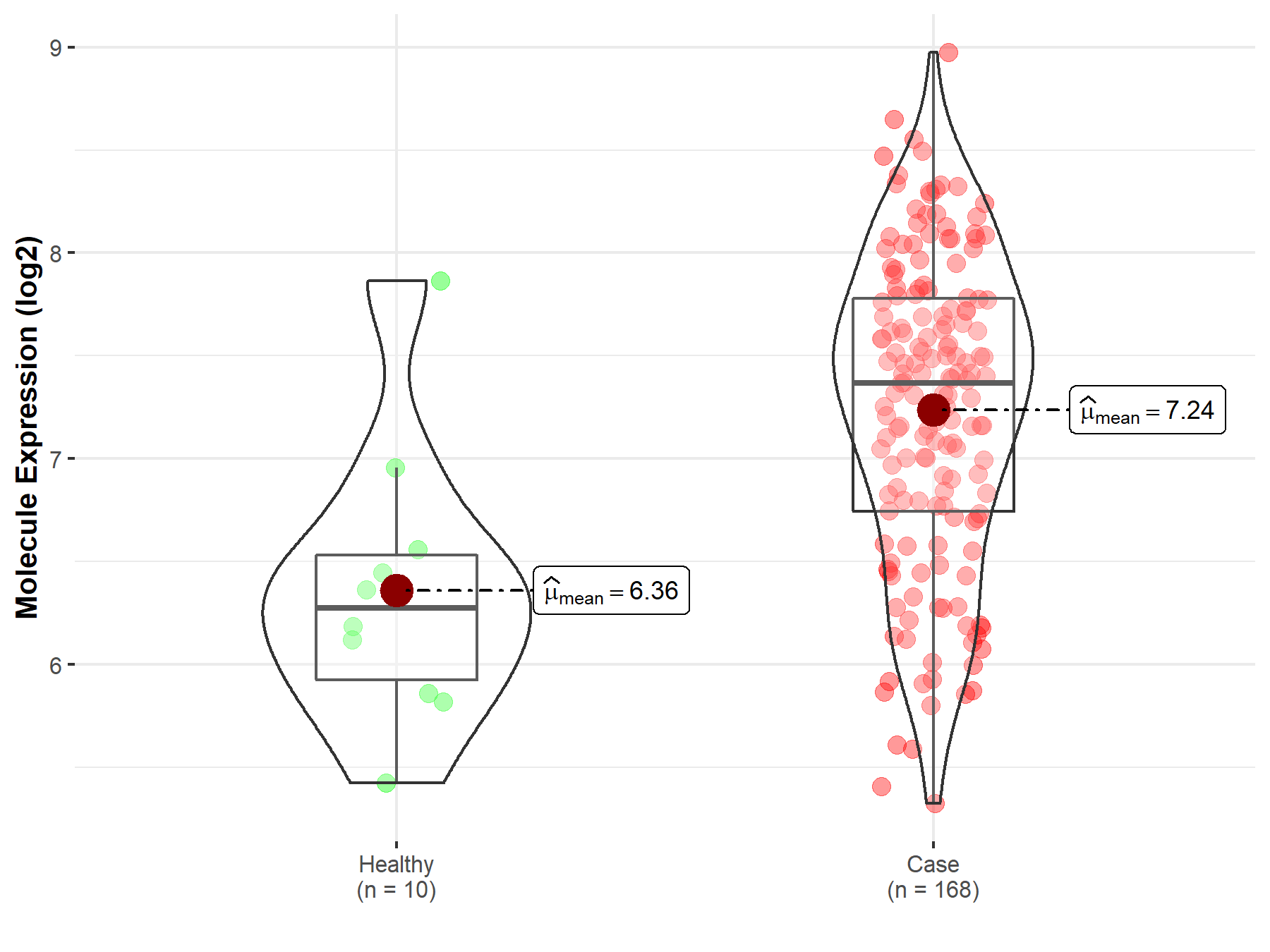
|
Click to View the Clearer Original Diagram |
Tissue-specific Molecule Abundances in Healthy Individuals

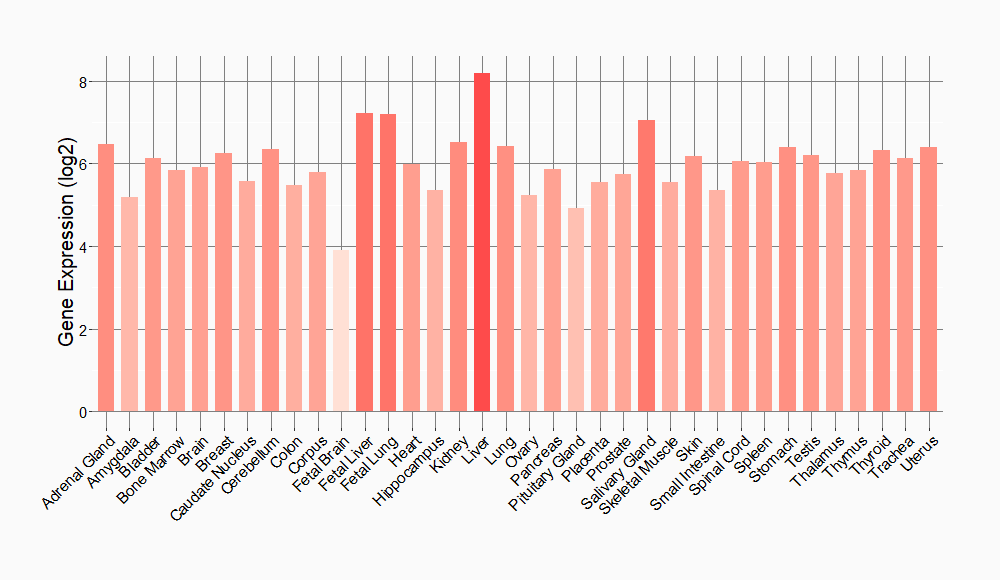
|
||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
