Drug Information
Drug (ID: DG01555) and It's Reported Resistant Information
| Name |
Capivasertib
|
||||
|---|---|---|---|---|---|
| Synonyms |
AZD5363; capivasertib; 1143532-39-1; AZD-5363; AZD 5363; UNII-WFR23M21IE; (S)-4-AMINO-N-(1-(4-CHLOROPHENYL)-3-HYDROXYPROPYL)-1-(7H-PYRROLO[2,3-D]PYRIMIDIN-4-YL)PIPERIDINE-4-CARBOXAMIDE; WFR23M21IE; 4-Amino-N-[(1s)-1-(4-Chlorophenyl)-3-Hydroxypropyl]-1-(7h-Pyrrolo[2,3-D]pyrimidin-4-Yl)piperidine-4-Carboxamide; 4-Piperidinecarboxamide, 4-amino-N-[(1S)-1-(4-chlorophenyl)-3-hydroxypropyl]-1-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-; 4-Amino-N-[(1S)-1-(4-chlorophenyl)-3-hydroxypropyl]-1-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-4-piperidinecarboxamide; C21H25ClN6O2; CHEMBL2325741; cc-638; 4-amino-N-(1-(4-chlorophenyl)-3-hydroxypropyl)-1-(7H-pyrrolo(2,3-d)pyrimidin-4-yl)piperidine-4-carboxamide; Capivasertib (JAN/USAN); Capivasertib (AZD5363); MLS006011179; SCHEMBL390243; GTPL7709; AZC5363; DTXSID40150710; EX-A285; US10654855, Example 9; BDBM443385; 2241AH; MFCD22628785; NSC764039; NSC782347; NSC799347; s8019; ZINC43204023; AKOS027323693; BCP9000365; CCG-268989; CS-1284; DB12218; NSC-764039; NSC-782347; NSC-799347; NCGC00345795-01; NCGC00345795-04; AC-32685; HY-15431; SMR004702948; BCP0726000111; A1387; SW220158-1; D11371; J-514447; Q27074756; 0XZ; ALTERNATIVE ROUTE 1: (S)-4-AMINO-N-(1-(4-CHLOROPHENYL)-3-HYDROXYPROPYL)-1-(7H-PYRROLO[2,3-D]PYRIMIDIN-4-YL)PIPERIDINE-4-CARBOXAMIDE
Click to Show/Hide
|
||||
| Indication |
In total 2 Indication(s)
|
||||
| Structure |
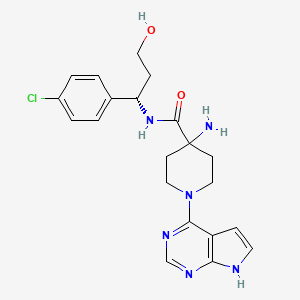
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Resistance Information Discovered by Cell Line Test for This Drug
(1 diseases)
[2]
|
||||
| Target | RAC-gamma serine/threonine-protein kinase (AKT3) | AKT3_HUMAN | [1] | ||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
6
|
||||
| IsoSMILES |
C1CN(CCC1(C(=O)N[C@@H](CCO)C2=CC=C(C=C2)Cl)N)C3=NC=NC4=C3C=CN4
|
||||
| InChI |
InChI=1S/C21H25ClN6O2/c22-15-3-1-14(2-4-15)17(6-12-29)27-20(30)21(23)7-10-28(11-8-21)19-16-5-9-24-18(16)25-13-26-19/h1-5,9,13,17,29H,6-8,10-12,23H2,(H,27,30)(H,24,25,26)/t17-/m0/s1
|
||||
| InChIKey |
JDUBGYFRJFOXQC-KRWDZBQOSA-N
|
||||
| PubChem CID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-02: Benign/in-situ/malignant neoplasm
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | |||||||||||||
|
|
|||||||||||||
| Key Molecule: RAC-alpha serine/threonine-protein kinase (AKT1) | [1] | ||||||||||||
| Sensitive Disease | Brain glioma [ICD-11: 2A00.0] | ||||||||||||
| Molecule Alteration | Missense mutation | p.E17K (c.49G>A) |
|||||||||||
| Wild Type Structure | Method: X-ray diffraction | Resolution: 1.65 Å | |||||||||||
| Mutant Type Structure | Method: X-ray diffraction | Resolution: 1.94 Å | |||||||||||
| Download The Information of Sequence | Download The Structure File | ||||||||||||
-
0
|
-
S
M
M
S
S
D
D
V
V
A
A
I
I
V
V
K
K
E
E
10
|
G
G
W
W
L
L
H
H
K
K
R
R
G
G
E
K
Y
Y
I
I
20
|
K
K
T
T
W
W
R
R
P
P
R
R
Y
Y
F
F
L
L
L
L
30
|
K
K
N
N
D
D
G
G
T
T
F
F
I
I
G
G
Y
Y
K
K
40
|
E
E
R
R
P
P
Q
Q
D
D
V
V
D
D
Q
Q
R
R
E
E
50
|
A
A
P
P
L
L
N
N
N
N
F
F
S
S
V
V
A
A
Q
Q
60
|
C
C
Q
Q
L
L
M
M
K
K
T
T
E
E
R
R
P
P
R
R
70
|
P
P
N
N
T
T
F
F
I
I
I
I
R
R
C
C
L
L
Q
Q
80
|
W
W
T
T
T
T
V
V
I
I
E
E
R
R
T
T
F
F
H
H
90
|
V
V
E
E
T
T
P
P
E
E
E
E
R
R
E
E
E
E
W
W
100
|
T
T
T
T
A
A
I
I
Q
Q
T
T
V
V
A
A
D
D
G
G
110
|
L
L
K
K
K
K
Q
Q
E
E
E
E
E
E
E
E
M
M
D
D
120
|
F
F
R
R
-
S
-
G
|
|||||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| In Vitro Model | Brain | N.A. | |||||||||||
| Mechanism Description | The missense mutation p.E17K (c.49G>A) in gene AKT1 cause the sensitivity of Capivasertib by aberration of the drug's therapeutic target | ||||||||||||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | |||||||||||||
|
|
|||||||||||||
| Key Molecule: RAC-alpha serine/threonine-protein kinase (AKT1) | [3] | ||||||||||||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | ||||||||||||
| Molecule Alteration | Missense mutation | p.E17K (c.49G>A) |
|||||||||||
| Wild Type Structure | Method: X-ray diffraction | Resolution: 1.65 Å | |||||||||||
| Mutant Type Structure | Method: X-ray diffraction | Resolution: 1.94 Å | |||||||||||
| Download The Information of Sequence | Download The Structure File | ||||||||||||
-
0
|
-
S
M
M
S
S
D
D
V
V
A
A
I
I
V
V
K
K
E
E
10
|
G
G
W
W
L
L
H
H
K
K
R
R
G
G
E
K
Y
Y
I
I
20
|
K
K
T
T
W
W
R
R
P
P
R
R
Y
Y
F
F
L
L
L
L
30
|
K
K
N
N
D
D
G
G
T
T
F
F
I
I
G
G
Y
Y
K
K
40
|
E
E
R
R
P
P
Q
Q
D
D
V
V
D
D
Q
Q
R
R
E
E
50
|
A
A
P
P
L
L
N
N
N
N
F
F
S
S
V
V
A
A
Q
Q
60
|
C
C
Q
Q
L
L
M
M
K
K
T
T
E
E
R
R
P
P
R
R
70
|
P
P
N
N
T
T
F
F
I
I
I
I
R
R
C
C
L
L
Q
Q
80
|
W
W
T
T
T
T
V
V
I
I
E
E
R
R
T
T
F
F
H
H
90
|
V
V
E
E
T
T
P
P
E
E
E
E
R
R
E
E
E
E
W
W
100
|
T
T
T
T
A
A
I
I
Q
Q
T
T
V
V
A
A
D
D
G
G
110
|
L
L
K
K
K
K
Q
Q
E
E
E
E
E
E
E
E
M
M
D
D
120
|
F
F
R
R
-
S
-
G
|
|||||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| Mechanism Description | The missense mutation p.E17K (c.49G>A) in gene AKT1 cause the sensitivity of Capivasertib by aberration of the drug's therapeutic target | ||||||||||||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | |||||||||||||
|
|
|||||||||||||
| Key Molecule: RAC-alpha serine/threonine-protein kinase (AKT1) | [4] | ||||||||||||
| Sensitive Disease | Leiomyosarcoma [ICD-11: 2B58.0] | ||||||||||||
| Molecule Alteration | Missense mutation | p.E17K (c.49G>A) |
|||||||||||
| Wild Type Structure | Method: X-ray diffraction | Resolution: 1.65 Å | |||||||||||
| Mutant Type Structure | Method: X-ray diffraction | Resolution: 1.94 Å | |||||||||||
| Download The Information of Sequence | Download The Structure File | ||||||||||||
-
0
|
-
S
M
M
S
S
D
D
V
V
A
A
I
I
V
V
K
K
E
E
10
|
G
G
W
W
L
L
H
H
K
K
R
R
G
G
E
K
Y
Y
I
I
20
|
K
K
T
T
W
W
R
R
P
P
R
R
Y
Y
F
F
L
L
L
L
30
|
K
K
N
N
D
D
G
G
T
T
F
F
I
I
G
G
Y
Y
K
K
40
|
E
E
R
R
P
P
Q
Q
D
D
V
V
D
D
Q
Q
R
R
E
E
50
|
A
A
P
P
L
L
N
N
N
N
F
F
S
S
V
V
A
A
Q
Q
60
|
C
C
Q
Q
L
L
M
M
K
K
T
T
E
E
R
R
P
P
R
R
70
|
P
P
N
N
T
T
F
F
I
I
I
I
R
R
C
C
L
L
Q
Q
80
|
W
W
T
T
T
T
V
V
I
I
E
E
R
R
T
T
F
F
H
H
90
|
V
V
E
E
T
T
P
P
E
E
E
E
R
R
E
E
E
E
W
W
100
|
T
T
T
T
A
A
I
I
Q
Q
T
T
V
V
A
A
D
D
G
G
110
|
L
L
K
K
K
K
Q
Q
E
E
E
E
E
E
E
E
M
M
D
D
120
|
F
F
R
R
-
S
-
G
|
|||||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| In Vitro Model | Breast | N.A. | |||||||||||
| Mechanism Description | The missense mutation p.E17K (c.49G>A) in gene AKT1 cause the sensitivity of Capivasertib by aberration of the drug's therapeutic target | ||||||||||||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | |||||||||||||
|
|
|||||||||||||
| Key Molecule: RAC-alpha serine/threonine-protein kinase (AKT1) | [4] | ||||||||||||
| Sensitive Disease | Parotid gland cancer [ICD-11: 2B67.0] | ||||||||||||
| Molecule Alteration | Missense mutation | p.E17K (c.49G>A) |
|||||||||||
| Wild Type Structure | Method: X-ray diffraction | Resolution: 1.65 Å | |||||||||||
| Mutant Type Structure | Method: X-ray diffraction | Resolution: 1.94 Å | |||||||||||
| Download The Information of Sequence | Download The Structure File | ||||||||||||
-
0
|
-
S
M
M
S
S
D
D
V
V
A
A
I
I
V
V
K
K
E
E
10
|
G
G
W
W
L
L
H
H
K
K
R
R
G
G
E
K
Y
Y
I
I
20
|
K
K
T
T
W
W
R
R
P
P
R
R
Y
Y
F
F
L
L
L
L
30
|
K
K
N
N
D
D
G
G
T
T
F
F
I
I
G
G
Y
Y
K
K
40
|
E
E
R
R
P
P
Q
Q
D
D
V
V
D
D
Q
Q
R
R
E
E
50
|
A
A
P
P
L
L
N
N
N
N
F
F
S
S
V
V
A
A
Q
Q
60
|
C
C
Q
Q
L
L
M
M
K
K
T
T
E
E
R
R
P
P
R
R
70
|
P
P
N
N
T
T
F
F
I
I
I
I
R
R
C
C
L
L
Q
Q
80
|
W
W
T
T
T
T
V
V
I
I
E
E
R
R
T
T
F
F
H
H
90
|
V
V
E
E
T
T
P
P
E
E
E
E
R
R
E
E
E
E
W
W
100
|
T
T
T
T
A
A
I
I
Q
Q
T
T
V
V
A
A
D
D
G
G
110
|
L
L
K
K
K
K
Q
Q
E
E
E
E
E
E
E
E
M
M
D
D
120
|
F
F
R
R
-
S
-
G
|
|||||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| In Vitro Model | Breast | N.A. | |||||||||||
| Mechanism Description | The missense mutation p.E17K (c.49G>A) in gene AKT1 cause the sensitivity of Capivasertib by aberration of the drug's therapeutic target | ||||||||||||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Myeloid cell leukemia 1 (Mcl-1) | [5] | |||
| Sensitive Disease | Oesophagus adenocarcinoma [ICD-11: 2B70.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | KYSE-70-R cells | esophageal | Homo sapiens (Human) | N.A. |
| Experiment for Molecule Alteration |
Western blot assay | |||
| Experiment for Drug Resistance |
Cell viability assay; Combination index assay | |||
| Mechanism Description | We consistently observed an increase in the expression of Mcl-1 in cells exposed to both short and long-term treatment with cisplatin, a drug commonly used in esophageal cancer therapy. Functional analysis showed that Mcl-1 regulates esophageal cancer cell response to cisplatin treatment. Notably, this upregulation of Mcl-1 was not dependent on eukaryotic initiation factor 4E (eIF4E). Instead, it was associated with increased stability due to the activation of Akt. Capivasertib, a potent pan-Akt kinase drug, significantly decreased Mcl-1 level via inhibiting Akt signaling pathway in chemo-resistant cells. In addition, capivasertib not only decreased the viability of chemo-resistant esophageal cancer cells but also synergistically enhanced the effects of cisplatin. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | |||||||||||||
|
|
|||||||||||||
| Key Molecule: PI3-kinase alpha (PIK3CA) | [6] | ||||||||||||
| Sensitive Disease | Gastric adenocarcinoma [ICD-11: 2B72.0] | ||||||||||||
| Molecule Alteration | Missense mutation | p.H1047R (c.3140A>G) |
|||||||||||
| Wild Type Structure | Method: Electron microscopy | Resolution: 2.41 Å | |||||||||||
| Mutant Type Structure | Method: X-ray diffraction | Resolution: 2.61 Å | |||||||||||
| Download The Information of Sequence | Download The Structure File | ||||||||||||
-
M
-
S
-
Y
-
Y
-
H
-
H
-
H
-
-20
|
H
-
H
-
H
-
D
-
Y
-
D
-
I
-
P
-
T
-
T
-
-10
|
E
-
N
-
L
-
Y
-
F
-
Q
-
G
G
A
A
M
M
G
G
0
|
S
S
M
M
P
P
P
P
R
R
P
P
S
S
S
S
G
G
E
E
10
|
L
L
W
W
G
G
I
I
H
H
L
L
M
M
P
P
P
P
R
R
20
|
I
I
L
L
V
V
E
E
C
C
L
L
L
L
P
P
N
N
G
G
30
|
M
M
I
I
V
V
T
T
L
L
E
E
C
C
L
L
R
R
E
E
40
|
A
A
T
T
L
L
I
I
T
T
I
I
K
K
H
H
E
E
L
L
50
|
F
F
K
K
E
E
A
A
R
R
K
K
Y
Y
P
P
L
L
H
H
60
|
Q
Q
L
L
L
L
Q
Q
D
D
E
E
S
S
S
S
Y
Y
I
I
70
|
F
F
V
V
S
S
V
V
T
T
Q
Q
E
E
A
A
E
E
R
R
80
|
E
E
E
E
F
F
F
F
D
D
E
E
T
T
R
R
R
R
L
L
90
|
C
C
D
D
L
L
R
R
L
L
F
F
Q
Q
P
P
F
F
L
L
100
|
K
K
V
V
I
I
E
E
P
P
V
V
G
G
N
N
R
R
E
E
110
|
E
E
K
K
I
I
L
L
N
N
R
R
E
E
I
I
G
G
F
F
120
|
A
A
I
I
G
G
M
M
P
P
V
V
C
C
E
E
F
F
D
D
130
|
M
M
V
V
K
K
D
D
P
P
E
E
V
V
Q
Q
D
D
F
F
140
|
R
R
R
R
N
N
I
I
L
L
N
N
V
V
C
C
K
K
E
E
150
|
A
A
V
V
D
D
L
L
R
R
D
D
L
L
N
N
S
S
P
P
160
|
H
H
S
S
R
R
A
A
M
M
Y
Y
V
V
Y
Y
P
P
P
P
170
|
N
N
V
V
E
E
S
S
S
S
P
P
E
E
L
L
P
P
K
K
180
|
H
H
I
I
Y
Y
N
N
K
K
L
L
D
D
K
K
G
G
Q
Q
190
|
I
I
I
I
V
V
V
V
I
I
W
W
V
V
I
I
V
V
S
S
200
|
P
P
N
N
N
N
D
D
K
K
Q
Q
K
K
Y
Y
T
T
L
L
210
|
K
K
I
I
N
N
H
H
D
D
C
C
V
V
P
P
E
E
Q
Q
220
|
V
V
I
I
A
A
E
E
A
A
I
I
R
R
K
K
K
K
T
T
230
|
R
R
S
S
M
M
L
L
L
L
S
S
S
S
E
E
Q
Q
L
L
240
|
K
K
L
L
C
C
V
V
L
L
E
E
Y
Y
Q
Q
G
G
K
K
250
|
Y
Y
I
I
L
L
K
K
V
V
C
C
G
G
C
C
D
D
E
E
260
|
Y
Y
F
F
L
L
E
E
K
K
Y
Y
P
P
L
L
S
S
Q
Q
270
|
Y
Y
K
K
Y
Y
I
I
R
R
S
S
C
C
I
I
M
M
L
L
280
|
G
G
R
R
M
M
P
P
N
N
L
L
M
M
L
L
M
M
A
A
290
|
K
K
E
E
S
S
L
L
Y
Y
S
S
Q
Q
L
L
P
P
M
M
300
|
D
D
C
C
F
F
T
T
M
M
P
P
S
S
Y
Y
S
S
R
R
310
|
R
R
I
I
S
S
T
T
A
A
T
T
P
P
Y
Y
M
M
N
N
320
|
G
G
E
E
T
T
S
S
T
T
K
K
S
S
L
L
W
W
V
V
330
|
I
I
N
N
S
S
A
A
L
L
R
R
I
I
K
K
I
I
L
L
340
|
C
C
A
A
T
T
Y
Y
V
V
N
N
V
V
N
N
I
I
R
R
350
|
D
D
I
I
D
D
K
K
I
I
Y
Y
V
V
R
R
T
T
G
G
360
|
I
I
Y
Y
H
H
G
G
G
G
E
E
P
P
L
L
C
C
D
D
370
|
N
N
V
V
N
N
T
T
Q
Q
R
R
V
V
P
P
C
C
S
S
380
|
N
N
P
P
R
R
W
W
N
N
E
E
W
W
L
L
N
N
Y
Y
390
|
D
D
I
I
Y
Y
I
I
P
P
D
D
L
L
P
P
R
R
A
A
400
|
A
A
R
R
L
L
C
C
L
L
S
S
I
I
C
C
S
S
V
V
410
|
K
K
G
G
R
R
K
K
G
G
A
A
K
K
E
E
E
E
H
H
420
|
C
C
P
P
L
L
A
A
W
W
G
G
N
N
I
I
N
N
L
L
430
|
F
F
D
D
Y
Y
T
T
D
D
T
T
L
L
V
V
S
S
G
G
440
|
K
K
M
M
A
A
L
L
N
N
L
L
W
W
P
P
V
V
P
P
450
|
H
H
G
G
L
L
E
E
D
D
L
L
L
L
N
N
P
P
I
I
460
|
G
G
V
V
T
T
G
G
S
S
N
N
P
P
N
N
K
K
E
E
470
|
T
T
P
P
C
C
L
L
E
E
L
L
E
E
F
F
D
D
W
W
480
|
F
F
S
S
S
S
V
V
V
V
K
K
F
F
P
P
D
D
M
M
490
|
S
S
V
V
I
I
E
E
E
E
H
H
A
A
N
N
W
W
S
S
500
|
V
V
S
S
R
R
E
E
A
A
G
G
F
F
S
S
Y
Y
S
S
510
|
H
H
A
A
G
G
L
L
S
S
N
N
R
R
L
L
A
A
R
R
520
|
D
D
N
N
E
E
L
L
R
R
E
E
N
N
D
D
K
K
E
E
530
|
Q
Q
L
L
K
K
A
A
I
I
S
S
T
T
R
R
D
D
P
P
540
|
L
L
S
S
E
E
I
I
T
T
E
E
Q
Q
E
E
K
K
D
D
550
|
F
F
L
L
W
W
S
S
H
H
R
R
H
H
Y
Y
C
C
V
V
560
|
T
T
I
I
P
P
E
E
I
I
L
L
P
P
K
K
L
L
L
L
570
|
L
L
S
S
V
V
K
K
W
W
N
N
S
S
R
R
D
D
E
E
580
|
V
V
A
A
Q
Q
M
M
Y
Y
C
C
L
L
V
V
K
K
D
D
590
|
W
W
P
P
P
P
I
I
K
K
P
P
E
E
Q
Q
A
A
M
M
600
|
E
E
L
L
L
L
D
D
C
C
N
N
Y
Y
P
P
D
D
P
P
610
|
M
M
V
V
R
R
G
G
F
F
A
A
V
V
R
R
C
C
L
L
620
|
E
E
K
K
Y
Y
L
L
T
T
D
D
D
D
K
K
L
L
S
S
630
|
Q
Q
Y
Y
L
L
I
I
Q
Q
L
L
V
V
Q
Q
V
V
L
L
640
|
K
K
Y
Y
E
E
Q
Q
Y
Y
L
L
D
D
N
N
L
L
L
L
650
|
V
V
R
R
F
F
L
L
L
L
K
K
K
K
A
A
L
L
T
T
660
|
N
N
Q
Q
R
R
I
I
G
G
H
H
F
F
F
F
F
F
W
W
670
|
H
H
L
L
K
K
S
S
E
E
M
M
H
H
N
N
K
K
T
T
680
|
V
V
S
S
Q
Q
R
R
F
F
G
G
L
L
L
L
L
L
E
E
690
|
S
S
Y
Y
C
C
R
R
A
A
C
C
G
G
M
M
Y
Y
L
L
700
|
K
K
H
H
L
L
N
N
R
R
Q
Q
V
V
E
E
A
A
M
M
710
|
E
E
K
K
L
L
I
I
N
N
L
L
T
T
D
D
I
I
L
L
720
|
K
K
Q
Q
E
E
K
K
K
K
D
D
E
E
T
T
Q
Q
K
K
730
|
V
V
Q
Q
M
M
K
K
F
F
L
L
V
V
E
E
Q
Q
M
M
740
|
R
R
R
R
P
P
D
D
F
F
M
M
D
D
A
A
L
L
Q
Q
750
|
G
G
F
F
L
L
S
S
P
P
L
L
N
N
P
P
A
A
H
H
760
|
Q
Q
L
L
G
G
N
N
L
L
R
R
L
L
E
E
E
E
C
C
770
|
R
R
I
I
M
M
S
S
S
S
A
A
K
K
R
R
P
P
L
L
780
|
W
W
L
L
N
N
W
W
E
E
N
N
P
P
D
D
I
I
M
M
790
|
S
S
E
E
L
L
L
L
F
F
Q
Q
N
N
N
N
E
E
I
I
800
|
I
I
F
F
K
K
N
N
G
G
D
D
D
D
L
L
R
R
Q
Q
810
|
D
D
M
M
L
L
T
T
L
L
Q
Q
I
I
I
I
R
R
I
I
820
|
M
M
E
E
N
N
I
I
W
W
Q
Q
N
N
Q
Q
G
G
L
L
830
|
D
D
L
L
R
R
M
M
L
L
P
P
Y
Y
G
G
C
C
L
L
840
|
S
S
I
I
G
G
D
D
C
C
V
V
G
G
L
L
I
I
E
E
850
|
V
V
V
V
R
R
N
N
S
S
H
H
T
T
I
I
M
M
Q
Q
860
|
I
I
Q
Q
C
C
K
K
G
G
G
G
L
L
K
K
G
G
A
A
870
|
L
L
Q
Q
F
F
N
N
S
S
H
H
T
T
L
L
H
H
Q
Q
880
|
W
W
L
L
K
K
D
D
K
K
N
N
K
K
G
G
E
E
I
I
890
|
Y
Y
D
D
A
A
A
A
I
I
D
D
L
L
F
F
T
T
R
R
900
|
S
S
C
C
A
A
G
G
Y
Y
C
C
V
V
A
A
T
T
F
F
910
|
I
I
L
L
G
G
I
I
G
G
D
D
R
R
H
H
N
N
S
S
920
|
N
N
I
I
M
M
V
V
K
K
D
D
D
D
G
G
Q
Q
L
L
930
|
F
F
H
H
I
I
D
D
F
F
G
G
H
H
F
F
L
L
D
D
940
|
H
H
K
K
K
K
K
K
K
K
F
F
G
G
Y
Y
K
K
R
R
950
|
E
E
R
R
V
V
P
P
F
F
V
V
L
L
T
T
Q
Q
D
D
960
|
F
F
L
L
I
I
V
V
I
I
S
S
K
K
G
G
A
A
Q
Q
970
|
E
E
C
C
T
T
K
K
T
T
R
R
E
E
F
F
E
E
R
R
980
|
F
F
Q
Q
E
E
M
M
C
C
Y
Y
K
K
A
A
Y
Y
L
L
990
|
A
A
I
I
R
R
Q
Q
H
H
A
A
N
N
L
L
F
F
I
I
1000
|
N
N
L
L
F
F
S
S
M
M
M
M
L
L
G
G
S
S
G
G
1010
|
M
M
P
P
E
E
L
L
Q
Q
S
S
F
F
D
D
D
D
I
I
1020
|
A
A
Y
Y
I
I
R
R
K
K
T
T
L
L
A
A
L
L
D
D
1030
|
K
K
T
T
E
E
Q
Q
E
E
A
A
L
L
E
E
Y
Y
F
F
1040
|
M
M
K
K
Q
Q
M
M
N
N
D
D
A
A
H
R
H
H
G
G
1050
|
G
G
W
W
T
T
T
T
K
K
M
M
D
D
W
W
I
I
F
F
1060
|
H
H
T
T
I
I
K
K
Q
Q
H
H
A
A
L
L
N
N
|
|||||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| In Vitro Model | Stomach | N.A. | |||||||||||
| In Vivo Model | GC xenograft (PDGCX) mouse model | Mus musculus | |||||||||||
| Experiment for Molecule Alteration |
Western blot analysis | ||||||||||||
| Experiment for Drug Resistance |
MTS assay | ||||||||||||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: PI3-kinase alpha (PIK3CA) | [6] | |||
| Sensitive Disease | Gastrointestinal system cancer [ICD-11: 2C11.Y] | |||
| Molecule Alteration | Missense mutation | p.E542K (c.1624G>A) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Stomach | N.A. | ||
| In Vivo Model | GC xenograft (PDGCX) mouse model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTS assay | |||
| Key Molecule: PI3-kinase alpha (PIK3CA) | [6] | |||
| Sensitive Disease | Gastrointestinal system cancer [ICD-11: 2C11.Y] | |||
| Molecule Alteration | Missense mutation | p.E545K (c.1633G>A) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Stomach | N.A. | ||
| In Vivo Model | GC xenograft (PDGCX) mouse model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTS assay | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | |||||||||||||
|
|
|||||||||||||
| Key Molecule: RAC-alpha serine/threonine-protein kinase (AKT1) | [7] | ||||||||||||
| Sensitive Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | ||||||||||||
| Molecule Alteration | Missense mutation | p.E17K (c.49G>A) |
|||||||||||
| Wild Type Structure | Method: X-ray diffraction | Resolution: 1.65 Å | |||||||||||
| Mutant Type Structure | Method: X-ray diffraction | Resolution: 1.94 Å | |||||||||||
| Download The Information of Sequence | Download The Structure File | ||||||||||||
-
0
|
-
S
M
M
S
S
D
D
V
V
A
A
I
I
V
V
K
K
E
E
10
|
G
G
W
W
L
L
H
H
K
K
R
R
G
G
E
K
Y
Y
I
I
20
|
K
K
T
T
W
W
R
R
P
P
R
R
Y
Y
F
F
L
L
L
L
30
|
K
K
N
N
D
D
G
G
T
T
F
F
I
I
G
G
Y
Y
K
K
40
|
E
E
R
R
P
P
Q
Q
D
D
V
V
D
D
Q
Q
R
R
E
E
50
|
A
A
P
P
L
L
N
N
N
N
F
F
S
S
V
V
A
A
Q
Q
60
|
C
C
Q
Q
L
L
M
M
K
K
T
T
E
E
R
R
P
P
R
R
70
|
P
P
N
N
T
T
F
F
I
I
I
I
R
R
C
C
L
L
Q
Q
80
|
W
W
T
T
T
T
V
V
I
I
E
E
R
R
T
T
F
F
H
H
90
|
V
V
E
E
T
T
P
P
E
E
E
E
R
R
E
E
E
E
W
W
100
|
T
T
T
T
A
A
I
I
Q
Q
T
T
V
V
A
A
D
D
G
G
110
|
L
L
K
K
K
K
Q
Q
E
E
E
E
E
E
E
E
M
M
D
D
120
|
F
F
R
R
-
S
-
G
|
|||||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| In Vitro Model | Breast | N.A. | |||||||||||
| Mechanism Description | The missense mutation p.E17K (c.49G>A) in gene AKT1 cause the sensitivity of Capivasertib by aberration of the drug's therapeutic target | ||||||||||||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Serine/threonine-protein kinase mTOR (mTOR) | [8] | |||
| Sensitive Disease | Melanoma [ICD-11: 2C30.0] | |||
| Molecule Alteration | Missense mutation | p.H1968Y (c.5902C>T) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | HEK 292T cells | Kidney | Homo sapiens (Human) | N.A. |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK-8 assay | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Multidrug resistance protein 1 (ABCB1) | [2] | |||
| Resistant Disease | Breast adenocarcinoma [ICD-11: 2C60.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | MCF-7/ADR cells | Breast | Homo sapiens (Human) | N.A. |
| Experiment for Drug Resistance |
Annexin V assay | |||
| Mechanism Description | AZD5363 markedly increased apoptosis only in drug-sensitive MCF-7 cells, whereas the same dose of AZD5363 afforded similar levels of apoptosis in resistant MCF-7/ADR | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | |||||||||||||
|
|
|||||||||||||
| Key Molecule: RAC-alpha serine/threonine-protein kinase (AKT1) | [9] | ||||||||||||
| Sensitive Disease | Breast adenocarcinoma [ICD-11: 2C60.1] | ||||||||||||
| Molecule Alteration | Missense mutation | p.E17K (c.49G>A) |
|||||||||||
| Wild Type Structure | Method: X-ray diffraction | Resolution: 1.65 Å | |||||||||||
| Mutant Type Structure | Method: X-ray diffraction | Resolution: 1.94 Å | |||||||||||
| Download The Information of Sequence | Download The Structure File | ||||||||||||
-
0
|
-
S
M
M
S
S
D
D
V
V
A
A
I
I
V
V
K
K
E
E
10
|
G
G
W
W
L
L
H
H
K
K
R
R
G
G
E
K
Y
Y
I
I
20
|
K
K
T
T
W
W
R
R
P
P
R
R
Y
Y
F
F
L
L
L
L
30
|
K
K
N
N
D
D
G
G
T
T
F
F
I
I
G
G
Y
Y
K
K
40
|
E
E
R
R
P
P
Q
Q
D
D
V
V
D
D
Q
Q
R
R
E
E
50
|
A
A
P
P
L
L
N
N
N
N
F
F
S
S
V
V
A
A
Q
Q
60
|
C
C
Q
Q
L
L
M
M
K
K
T
T
E
E
R
R
P
P
R
R
70
|
P
P
N
N
T
T
F
F
I
I
I
I
R
R
C
C
L
L
Q
Q
80
|
W
W
T
T
T
T
V
V
I
I
E
E
R
R
T
T
F
F
H
H
90
|
V
V
E
E
T
T
P
P
E
E
E
E
R
R
E
E
E
E
W
W
100
|
T
T
T
T
A
A
I
I
Q
Q
T
T
V
V
A
A
D
D
G
G
110
|
L
L
K
K
K
K
Q
Q
E
E
E
E
E
E
E
E
M
M
D
D
120
|
F
F
R
R
-
S
-
G
|
|||||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| Mechanism Description | The missense mutation p.E17K (c.49G>A) in gene AKT1 cause the sensitivity of Capivasertib by aberration of the drug's therapeutic target | ||||||||||||
| Key Molecule: RAC-alpha serine/threonine-protein kinase (AKT1) | [4] | ||||||||||||
| Sensitive Disease | HER2 negative breast cancer [ICD-11: 2C60.11] | ||||||||||||
| Molecule Alteration | Missense mutation | p.E17K (c.49G>A) |
|||||||||||
| Wild Type Structure | Method: X-ray diffraction | Resolution: 1.65 Å | |||||||||||
| Mutant Type Structure | Method: X-ray diffraction | Resolution: 1.94 Å | |||||||||||
| Download The Information of Sequence | Download The Structure File | ||||||||||||
-
0
|
-
S
M
M
S
S
D
D
V
V
A
A
I
I
V
V
K
K
E
E
10
|
G
G
W
W
L
L
H
H
K
K
R
R
G
G
E
K
Y
Y
I
I
20
|
K
K
T
T
W
W
R
R
P
P
R
R
Y
Y
F
F
L
L
L
L
30
|
K
K
N
N
D
D
G
G
T
T
F
F
I
I
G
G
Y
Y
K
K
40
|
E
E
R
R
P
P
Q
Q
D
D
V
V
D
D
Q
Q
R
R
E
E
50
|
A
A
P
P
L
L
N
N
N
N
F
F
S
S
V
V
A
A
Q
Q
60
|
C
C
Q
Q
L
L
M
M
K
K
T
T
E
E
R
R
P
P
R
R
70
|
P
P
N
N
T
T
F
F
I
I
I
I
R
R
C
C
L
L
Q
Q
80
|
W
W
T
T
T
T
V
V
I
I
E
E
R
R
T
T
F
F
H
H
90
|
V
V
E
E
T
T
P
P
E
E
E
E
R
R
E
E
E
E
W
W
100
|
T
T
T
T
A
A
I
I
Q
Q
T
T
V
V
A
A
D
D
G
G
110
|
L
L
K
K
K
K
Q
Q
E
E
E
E
E
E
E
E
M
M
D
D
120
|
F
F
R
R
-
S
-
G
|
|||||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| In Vitro Model | Breast | N.A. | |||||||||||
| Mechanism Description | The missense mutation p.E17K (c.49G>A) in gene AKT1 cause the sensitivity of Capivasertib by aberration of the drug's therapeutic target | ||||||||||||
| Key Molecule: RAC-alpha serine/threonine-protein kinase (AKT1) | [4] | ||||||||||||
| Sensitive Disease | HER2 negative breast cancer [ICD-11: 2C60.11] | ||||||||||||
| Molecule Alteration | Missense mutation | p.E17K (c.49G>A) |
|||||||||||
| Wild Type Structure | Method: X-ray diffraction | Resolution: 1.65 Å | |||||||||||
| Mutant Type Structure | Method: X-ray diffraction | Resolution: 1.94 Å | |||||||||||
| Download The Information of Sequence | Download The Structure File | ||||||||||||
-
0
|
-
S
M
M
S
S
D
D
V
V
A
A
I
I
V
V
K
K
E
E
10
|
G
G
W
W
L
L
H
H
K
K
R
R
G
G
E
K
Y
Y
I
I
20
|
K
K
T
T
W
W
R
R
P
P
R
R
Y
Y
F
F
L
L
L
L
30
|
K
K
N
N
D
D
G
G
T
T
F
F
I
I
G
G
Y
Y
K
K
40
|
E
E
R
R
P
P
Q
Q
D
D
V
V
D
D
Q
Q
R
R
E
E
50
|
A
A
P
P
L
L
N
N
N
N
F
F
S
S
V
V
A
A
Q
Q
60
|
C
C
Q
Q
L
L
M
M
K
K
T
T
E
E
R
R
P
P
R
R
70
|
P
P
N
N
T
T
F
F
I
I
I
I
R
R
C
C
L
L
Q
Q
80
|
W
W
T
T
T
T
V
V
I
I
E
E
R
R
T
T
F
F
H
H
90
|
V
V
E
E
T
T
P
P
E
E
E
E
R
R
E
E
E
E
W
W
100
|
T
T
T
T
A
A
I
I
Q
Q
T
T
V
V
A
A
D
D
G
G
110
|
L
L
K
K
K
K
Q
Q
E
E
E
E
E
E
E
E
M
M
D
D
120
|
F
F
R
R
-
S
-
G
|
|||||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| In Vitro Model | Breast | N.A. | |||||||||||
| Mechanism Description | The missense mutation p.E17K (c.49G>A) in gene AKT1 cause the sensitivity of Capivasertib by aberration of the drug's therapeutic target | ||||||||||||
| Key Molecule: RAC-alpha serine/threonine-protein kinase (AKT1) | [7] | ||||||||||||
| Sensitive Disease | ER positive breast cancer [ICD-11: 2C60.6] | ||||||||||||
| Molecule Alteration | Missense mutation | p.E17K (c.49G>A) |
|||||||||||
| Wild Type Structure | Method: X-ray diffraction | Resolution: 1.65 Å | |||||||||||
| Mutant Type Structure | Method: X-ray diffraction | Resolution: 1.94 Å | |||||||||||
| Download The Information of Sequence | Download The Structure File | ||||||||||||
-
0
|
-
S
M
M
S
S
D
D
V
V
A
A
I
I
V
V
K
K
E
E
10
|
G
G
W
W
L
L
H
H
K
K
R
R
G
G
E
K
Y
Y
I
I
20
|
K
K
T
T
W
W
R
R
P
P
R
R
Y
Y
F
F
L
L
L
L
30
|
K
K
N
N
D
D
G
G
T
T
F
F
I
I
G
G
Y
Y
K
K
40
|
E
E
R
R
P
P
Q
Q
D
D
V
V
D
D
Q
Q
R
R
E
E
50
|
A
A
P
P
L
L
N
N
N
N
F
F
S
S
V
V
A
A
Q
Q
60
|
C
C
Q
Q
L
L
M
M
K
K
T
T
E
E
R
R
P
P
R
R
70
|
P
P
N
N
T
T
F
F
I
I
I
I
R
R
C
C
L
L
Q
Q
80
|
W
W
T
T
T
T
V
V
I
I
E
E
R
R
T
T
F
F
H
H
90
|
V
V
E
E
T
T
P
P
E
E
E
E
R
R
E
E
E
E
W
W
100
|
T
T
T
T
A
A
I
I
Q
Q
T
T
V
V
A
A
D
D
G
G
110
|
L
L
K
K
K
K
Q
Q
E
E
E
E
E
E
E
E
M
M
D
D
120
|
F
F
R
R
-
S
-
G
|
|||||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| In Vitro Model | Breast | N.A. | |||||||||||
| Mechanism Description | The missense mutation p.E17K (c.49G>A) in gene AKT1 cause the sensitivity of Capivasertib by aberration of the drug's therapeutic target | ||||||||||||
| Key Molecule: RAC-alpha serine/threonine-protein kinase (AKT1) | [7] | ||||||||||||
| Sensitive Disease | ER negative breast cancer [ICD-11: 2C60.7] | ||||||||||||
| Molecule Alteration | Missense mutation | p.E17K (c.49G>A) |
|||||||||||
| Wild Type Structure | Method: X-ray diffraction | Resolution: 1.65 Å | |||||||||||
| Mutant Type Structure | Method: X-ray diffraction | Resolution: 1.94 Å | |||||||||||
| Download The Information of Sequence | Download The Structure File | ||||||||||||
-
0
|
-
S
M
M
S
S
D
D
V
V
A
A
I
I
V
V
K
K
E
E
10
|
G
G
W
W
L
L
H
H
K
K
R
R
G
G
E
K
Y
Y
I
I
20
|
K
K
T
T
W
W
R
R
P
P
R
R
Y
Y
F
F
L
L
L
L
30
|
K
K
N
N
D
D
G
G
T
T
F
F
I
I
G
G
Y
Y
K
K
40
|
E
E
R
R
P
P
Q
Q
D
D
V
V
D
D
Q
Q
R
R
E
E
50
|
A
A
P
P
L
L
N
N
N
N
F
F
S
S
V
V
A
A
Q
Q
60
|
C
C
Q
Q
L
L
M
M
K
K
T
T
E
E
R
R
P
P
R
R
70
|
P
P
N
N
T
T
F
F
I
I
I
I
R
R
C
C
L
L
Q
Q
80
|
W
W
T
T
T
T
V
V
I
I
E
E
R
R
T
T
F
F
H
H
90
|
V
V
E
E
T
T
P
P
E
E
E
E
R
R
E
E
E
E
W
W
100
|
T
T
T
T
A
A
I
I
Q
Q
T
T
V
V
A
A
D
D
G
G
110
|
L
L
K
K
K
K
Q
Q
E
E
E
E
E
E
E
E
M
M
D
D
120
|
F
F
R
R
-
S
-
G
|
|||||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| In Vitro Model | Breast | N.A. | |||||||||||
| Mechanism Description | The missense mutation p.E17K (c.49G>A) in gene AKT1 cause the sensitivity of Capivasertib by aberration of the drug's therapeutic target | ||||||||||||
| Key Molecule: RAC-alpha serine/threonine-protein kinase (AKT1) | [10] | ||||||||||||
| Sensitive Disease | Breast adenocarcinoma [ICD-11: 2C60.1] | ||||||||||||
| Molecule Alteration | Missense mutation | p.E17K (c.49G>A) |
|||||||||||
| Wild Type Structure | Method: X-ray diffraction | Resolution: 1.65 Å | |||||||||||
| Mutant Type Structure | Method: X-ray diffraction | Resolution: 1.94 Å | |||||||||||
| Download The Information of Sequence | Download The Structure File | ||||||||||||
-
0
|
-
S
M
M
S
S
D
D
V
V
A
A
I
I
V
V
K
K
E
E
10
|
G
G
W
W
L
L
H
H
K
K
R
R
G
G
E
K
Y
Y
I
I
20
|
K
K
T
T
W
W
R
R
P
P
R
R
Y
Y
F
F
L
L
L
L
30
|
K
K
N
N
D
D
G
G
T
T
F
F
I
I
G
G
Y
Y
K
K
40
|
E
E
R
R
P
P
Q
Q
D
D
V
V
D
D
Q
Q
R
R
E
E
50
|
A
A
P
P
L
L
N
N
N
N
F
F
S
S
V
V
A
A
Q
Q
60
|
C
C
Q
Q
L
L
M
M
K
K
T
T
E
E
R
R
P
P
R
R
70
|
P
P
N
N
T
T
F
F
I
I
I
I
R
R
C
C
L
L
Q
Q
80
|
W
W
T
T
T
T
V
V
I
I
E
E
R
R
T
T
F
F
H
H
90
|
V
V
E
E
T
T
P
P
E
E
E
E
R
R
E
E
E
E
W
W
100
|
T
T
T
T
A
A
I
I
Q
Q
T
T
V
V
A
A
D
D
G
G
110
|
L
L
K
K
K
K
Q
Q
E
E
E
E
E
E
E
E
M
M
D
D
120
|
F
F
R
R
-
S
-
G
|
|||||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| In Vitro Model | MCF10A cells | Breast | Homo sapiens (Human) | CVCL_0598 | |||||||||
| In Vivo Model | Male nude mouse (nu/nu:Alpk) xenograft model | Mus musculus | |||||||||||
| Mechanism Description | The missense mutation p.E17K (c.49G>A) in gene AKT1 cause the sensitivity of Capivasertib by aberration of the drug's therapeutic target | ||||||||||||
| Key Molecule: RAC-alpha serine/threonine-protein kinase (AKT1) | [11] | ||||||||||||
| Sensitive Disease | Breast adenocarcinoma [ICD-11: 2C60.1] | ||||||||||||
| Molecule Alteration | Duplication | p.P68_C77 (c.202_231) |
|||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| Mechanism Description | The duplication p.P68_C77 (c.202_231) in gene AKT1 cause the sensitivity of Capivasertib by aberration of the drug's therapeutic target. | ||||||||||||
|
|
|||||||||||||
| Key Molecule: Multidrug resistance protein 1 (ABCB1) | [2] | ||||||||||||
| Sensitive Disease | Breast adenocarcinoma [ICD-11: 2C60.1] | ||||||||||||
| Molecule Alteration | Expression | S2702T |
|||||||||||
| Experimental Note | Revealed Based on the Cell Line Data | ||||||||||||
| In Vitro Model | MCF7 cells | Breast | Homo sapiens (Human) | CVCL_0031 | |||||||||
| Experiment for Drug Resistance |
Annexin V assay | ||||||||||||
| Mechanism Description | AZD5363 markedly increased apoptosis only in drug-sensitive MCF-7 cells, whereas the same dose of AZD5363 afforded similar levels of apoptosis in resistant MCF-7/ADR | ||||||||||||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | |||||||||||||
|
|
|||||||||||||
| Key Molecule: RAC-alpha serine/threonine-protein kinase (AKT1) | [9] | ||||||||||||
| Sensitive Disease | Ovarian cancer [ICD-11: 2C73.0] | ||||||||||||
| Molecule Alteration | Missense mutation | p.E17K (c.49G>A) |
|||||||||||
| Wild Type Structure | Method: X-ray diffraction | Resolution: 1.65 Å | |||||||||||
| Mutant Type Structure | Method: X-ray diffraction | Resolution: 1.94 Å | |||||||||||
| Download The Information of Sequence | Download The Structure File | ||||||||||||
-
0
|
-
S
M
M
S
S
D
D
V
V
A
A
I
I
V
V
K
K
E
E
10
|
G
G
W
W
L
L
H
H
K
K
R
R
G
G
E
K
Y
Y
I
I
20
|
K
K
T
T
W
W
R
R
P
P
R
R
Y
Y
F
F
L
L
L
L
30
|
K
K
N
N
D
D
G
G
T
T
F
F
I
I
G
G
Y
Y
K
K
40
|
E
E
R
R
P
P
Q
Q
D
D
V
V
D
D
Q
Q
R
R
E
E
50
|
A
A
P
P
L
L
N
N
N
N
F
F
S
S
V
V
A
A
Q
Q
60
|
C
C
Q
Q
L
L
M
M
K
K
T
T
E
E
R
R
P
P
R
R
70
|
P
P
N
N
T
T
F
F
I
I
I
I
R
R
C
C
L
L
Q
Q
80
|
W
W
T
T
T
T
V
V
I
I
E
E
R
R
T
T
F
F
H
H
90
|
V
V
E
E
T
T
P
P
E
E
E
E
R
R
E
E
E
E
W
W
100
|
T
T
T
T
A
A
I
I
Q
Q
T
T
V
V
A
A
D
D
G
G
110
|
L
L
K
K
K
K
Q
Q
E
E
E
E
E
E
E
E
M
M
D
D
120
|
F
F
R
R
-
S
-
G
|
|||||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| Experiment for Molecule Alteration |
Whole-exome sequencing | ||||||||||||
| Key Molecule: RAC-alpha serine/threonine-protein kinase (AKT1) | [7] | ||||||||||||
| Sensitive Disease | Ovarian cancer [ICD-11: 2C73.0] | ||||||||||||
| Molecule Alteration | Missense mutation | p.Q79K (c.235C>A) |
|||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| In Vitro Model | Breast | N.A. | |||||||||||
| Mechanism Description | The missense mutation p.Q79K (c.235C>A) in gene AKT1 cause the sensitivity of Capivasertib by aberration of the drug's therapeutic target | ||||||||||||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | |||||||||||||
|
|
|||||||||||||
| Key Molecule: RAC-alpha serine/threonine-protein kinase (AKT1) | [9] | ||||||||||||
| Sensitive Disease | Endometrial adenocarcinoma [ICD-11: 2C76.0] | ||||||||||||
| Molecule Alteration | Missense mutation | p.E17K (c.49G>A) |
|||||||||||
| Wild Type Structure | Method: X-ray diffraction | Resolution: 1.65 Å | |||||||||||
| Mutant Type Structure | Method: X-ray diffraction | Resolution: 1.94 Å | |||||||||||
| Download The Information of Sequence | Download The Structure File | ||||||||||||
-
0
|
-
S
M
M
S
S
D
D
V
V
A
A
I
I
V
V
K
K
E
E
10
|
G
G
W
W
L
L
H
H
K
K
R
R
G
G
E
K
Y
Y
I
I
20
|
K
K
T
T
W
W
R
R
P
P
R
R
Y
Y
F
F
L
L
L
L
30
|
K
K
N
N
D
D
G
G
T
T
F
F
I
I
G
G
Y
Y
K
K
40
|
E
E
R
R
P
P
Q
Q
D
D
V
V
D
D
Q
Q
R
R
E
E
50
|
A
A
P
P
L
L
N
N
N
N
F
F
S
S
V
V
A
A
Q
Q
60
|
C
C
Q
Q
L
L
M
M
K
K
T
T
E
E
R
R
P
P
R
R
70
|
P
P
N
N
T
T
F
F
I
I
I
I
R
R
C
C
L
L
Q
Q
80
|
W
W
T
T
T
T
V
V
I
I
E
E
R
R
T
T
F
F
H
H
90
|
V
V
E
E
T
T
P
P
E
E
E
E
R
R
E
E
E
E
W
W
100
|
T
T
T
T
A
A
I
I
Q
Q
T
T
V
V
A
A
D
D
G
G
110
|
L
L
K
K
K
K
Q
Q
E
E
E
E
E
E
E
E
M
M
D
D
120
|
F
F
R
R
-
S
-
G
|
|||||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| Mechanism Description | The missense mutation p.E17K (c.49G>A) in gene AKT1 cause the sensitivity of Capivasertib by aberration of the drug's therapeutic target | ||||||||||||
| Key Molecule: RAC-alpha serine/threonine-protein kinase (AKT1) | [4] | ||||||||||||
| Sensitive Disease | Endometrial adenocarcinoma [ICD-11: 2C76.0] | ||||||||||||
| Molecule Alteration | Missense mutation | p.E17K (c.49G>A) |
|||||||||||
| Wild Type Structure | Method: X-ray diffraction | Resolution: 1.65 Å | |||||||||||
| Mutant Type Structure | Method: X-ray diffraction | Resolution: 1.94 Å | |||||||||||
| Download The Information of Sequence | Download The Structure File | ||||||||||||
-
0
|
-
S
M
M
S
S
D
D
V
V
A
A
I
I
V
V
K
K
E
E
10
|
G
G
W
W
L
L
H
H
K
K
R
R
G
G
E
K
Y
Y
I
I
20
|
K
K
T
T
W
W
R
R
P
P
R
R
Y
Y
F
F
L
L
L
L
30
|
K
K
N
N
D
D
G
G
T
T
F
F
I
I
G
G
Y
Y
K
K
40
|
E
E
R
R
P
P
Q
Q
D
D
V
V
D
D
Q
Q
R
R
E
E
50
|
A
A
P
P
L
L
N
N
N
N
F
F
S
S
V
V
A
A
Q
Q
60
|
C
C
Q
Q
L
L
M
M
K
K
T
T
E
E
R
R
P
P
R
R
70
|
P
P
N
N
T
T
F
F
I
I
I
I
R
R
C
C
L
L
Q
Q
80
|
W
W
T
T
T
T
V
V
I
I
E
E
R
R
T
T
F
F
H
H
90
|
V
V
E
E
T
T
P
P
E
E
E
E
R
R
E
E
E
E
W
W
100
|
T
T
T
T
A
A
I
I
Q
Q
T
T
V
V
A
A
D
D
G
G
110
|
L
L
K
K
K
K
Q
Q
E
E
E
E
E
E
E
E
M
M
D
D
120
|
F
F
R
R
-
S
-
G
|
|||||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| In Vitro Model | Breast | N.A. | |||||||||||
| Mechanism Description | The missense mutation p.E17K (c.49G>A) in gene AKT1 cause the sensitivity of Capivasertib by aberration of the drug's therapeutic target | ||||||||||||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | |||||||||||||
|
|
|||||||||||||
| Key Molecule: RAC-alpha serine/threonine-protein kinase (AKT1) | [7] | ||||||||||||
| Sensitive Disease | Cervical cancer [ICD-11: 2C77.0] | ||||||||||||
| Molecule Alteration | Missense mutation | p.E17K (c.49G>A) |
|||||||||||
| Wild Type Structure | Method: X-ray diffraction | Resolution: 1.65 Å | |||||||||||
| Mutant Type Structure | Method: X-ray diffraction | Resolution: 1.94 Å | |||||||||||
| Download The Information of Sequence | Download The Structure File | ||||||||||||
-
0
|
-
S
M
M
S
S
D
D
V
V
A
A
I
I
V
V
K
K
E
E
10
|
G
G
W
W
L
L
H
H
K
K
R
R
G
G
E
K
Y
Y
I
I
20
|
K
K
T
T
W
W
R
R
P
P
R
R
Y
Y
F
F
L
L
L
L
30
|
K
K
N
N
D
D
G
G
T
T
F
F
I
I
G
G
Y
Y
K
K
40
|
E
E
R
R
P
P
Q
Q
D
D
V
V
D
D
Q
Q
R
R
E
E
50
|
A
A
P
P
L
L
N
N
N
N
F
F
S
S
V
V
A
A
Q
Q
60
|
C
C
Q
Q
L
L
M
M
K
K
T
T
E
E
R
R
P
P
R
R
70
|
P
P
N
N
T
T
F
F
I
I
I
I
R
R
C
C
L
L
Q
Q
80
|
W
W
T
T
T
T
V
V
I
I
E
E
R
R
T
T
F
F
H
H
90
|
V
V
E
E
T
T
P
P
E
E
E
E
R
R
E
E
E
E
W
W
100
|
T
T
T
T
A
A
I
I
Q
Q
T
T
V
V
A
A
D
D
G
G
110
|
L
L
K
K
K
K
Q
Q
E
E
E
E
E
E
E
E
M
M
D
D
120
|
F
F
R
R
-
S
-
G
|
|||||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| In Vitro Model | Breast | N.A. | |||||||||||
| Mechanism Description | The missense mutation p.E17K (c.49G>A) in gene AKT1 cause the sensitivity of Capivasertib by aberration of the drug's therapeutic target | ||||||||||||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Androgen receptor (AR) | [12] | |||
| Sensitive Disease | Prostate cancer [ICD-11: 2C82.0] | |||
| Molecule Alteration | Function | Activation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | PI3K/AKT signaling pathway | Inhibition | hsa04151 | |
| In Vitro Model | LNCaP clone FGC cells | N.A. | Homo sapiens (Human) | CVCL_1379 |
| 22Rv-1 cells | Prostate | Homo sapiens (Human) | CVCL_1045 | |
| Experiment for Molecule Alteration |
Cell protein extraction assay; Western blot assay; qRT-PCR; Luciferase reporter assay | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | This study revealed the role of p35-CDK5 in between PI3K/Akt and AR by utilizing LNCaP and 22Rv1 cells. Through the TCGA database analysis, we observed a positive correlation between PTEN and p35 expression, implying a potential negative correlation between PI3K/Akt activation and p35-CDK5. Inhibiting PI3K/Akt with LY294002, Capivasertib (AZD5363), or using an inactive Akt mutant significantly increased p35 expression and subsequently enhanced AR stability and activation in PCa cells. On the other hand, CDK5-knockdown reversed these effects. The involvement of the beta-catenin/Egr1-axis was observed in regulating PI3K/Akt inhibition and p35-CDK5 activation, implying a possible mechanistic connection. Importantly, CDK5 knockdown further reduced PI3K/Akt-inhibition-induced AR and cell viability maintenance, suggesting a compensatory role for CDK5-AR in maintaining cell viability under Akt inhibition. In conclusion, PI3K/Akt inhibition could trigger p35-CDK5-dependent AR activation and cell viability, highlighting p35-CDK5 as a critical link connecting PI3K/Akt inhibition to AR activation and pivotal in PCa cell resistance to PI3K/Akt blockade. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | |||||||||||||
|
|
|||||||||||||
| Key Molecule: RAC-alpha serine/threonine-protein kinase (AKT1) | [7] | ||||||||||||
| Sensitive Disease | Granulosa cell tumor [ICD-11: 2F76.0] | ||||||||||||
| Molecule Alteration | Missense mutation | p.E17K (c.49G>A) |
|||||||||||
| Wild Type Structure | Method: X-ray diffraction | Resolution: 1.65 Å | |||||||||||
| Mutant Type Structure | Method: X-ray diffraction | Resolution: 1.94 Å | |||||||||||
| Download The Information of Sequence | Download The Structure File | ||||||||||||
-
0
|
-
S
M
M
S
S
D
D
V
V
A
A
I
I
V
V
K
K
E
E
10
|
G
G
W
W
L
L
H
H
K
K
R
R
G
G
E
K
Y
Y
I
I
20
|
K
K
T
T
W
W
R
R
P
P
R
R
Y
Y
F
F
L
L
L
L
30
|
K
K
N
N
D
D
G
G
T
T
F
F
I
I
G
G
Y
Y
K
K
40
|
E
E
R
R
P
P
Q
Q
D
D
V
V
D
D
Q
Q
R
R
E
E
50
|
A
A
P
P
L
L
N
N
N
N
F
F
S
S
V
V
A
A
Q
Q
60
|
C
C
Q
Q
L
L
M
M
K
K
T
T
E
E
R
R
P
P
R
R
70
|
P
P
N
N
T
T
F
F
I
I
I
I
R
R
C
C
L
L
Q
Q
80
|
W
W
T
T
T
T
V
V
I
I
E
E
R
R
T
T
F
F
H
H
90
|
V
V
E
E
T
T
P
P
E
E
E
E
R
R
E
E
E
E
W
W
100
|
T
T
T
T
A
A
I
I
Q
Q
T
T
V
V
A
A
D
D
G
G
110
|
L
L
K
K
K
K
Q
Q
E
E
E
E
E
E
E
E
M
M
D
D
120
|
F
F
R
R
-
S
-
G
|
|||||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| In Vitro Model | Breast | N.A. | |||||||||||
| Mechanism Description | The missense mutation p.E17K (c.49G>A) in gene AKT1 cause the sensitivity of Capivasertib by aberration of the drug's therapeutic target | ||||||||||||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
