Drug Information
Drug (ID: DG01464) and It's Reported Resistant Information
| Name |
Flutamide
|
||||
|---|---|---|---|---|---|
| Synonyms |
Flutamide; 13311-84-7; Eulexin; Niftolide; Niftholide; 2-Methyl-N-[4-nitro-3-(trifluoromethyl)phenyl]propanamide; Sch 13521; NFBA; niftolid; Drogenil; Flutamin; Cebatrol, veterinary; Flutamida; Flutamidum; 4'-Nitro-3'-trifluoromethylisobutyranilide; Sch-13521; 2-Methyl-N-(4-nitro-3-[trifluoromethyl]phenyl)propanamide; N-(4-NITRO-3-(TRIFLUOROMETHYL)PHENYL)ISOBUTYRAMIDE; SCH13521; MFCD00072009; NSC 215876; Flutamide (Eulexin); alpha,alpha,alpha-Trifluoro-2-methyl-4'-nitro-m-propionotoluidide; UNII-76W6J0943E; CHEMBL806; Propanamide, 2-methyl-N-[4-nitro-3-(trifluoromethyl)phenyl]-; 4-Nitro-3-(trifluoromethyl)isobutyranilide; CHEBI:5132; Propanamide, 2-methyl-N-(4-nitro-3-(trifluoromethyl)phenyl)-; 76W6J0943E; NSC-215876; NCGC00015452-09; Eulexine; Chimax; 4'-Nitro-3'-trifluoromethylisobutyramilide; CAS-13311-84-7; Ham's F-12 medium; Flutamidum [INN-Latin]; DSSTox_CID_11121; DSSTox_RID_78899; DSSTox_GSID_32004; Flutamida [INN-Spanish]; Flutamide USP25; N-[4-Nitro-3-(trifluoromethyl)phenyl]isobutyramide; Prostandril; Odyne; SMR000058187; Eulexin (TN); CCRIS 7246; m-Propionotoluidide,.alpha.,.alpha.-trifluoro-; SR-01000075888; EINECS 236-341-9; BRN 2157663; .alpha.,.alpha.-Trifluoro-2-methyl-4'-nitro-m-propionotoluidide; m-Propionotoluidide,.alpha.,.alpha.-trifluoro-2-methyl-4'-nitro-; 4'-Nitro-3'-(trifluoromethyl)isobutyranilide; Flutamide [USAN:USP:INN:BAN]; Flutamide,(S); Prestwick_228; NK-601; Spectrum_001210; 2-Methyl-N-[4-nitro-3-(trifluoromethyl)phenyl]propionamide; CPD000058187; Prestwick0_000180; Prestwick1_000180; Prestwick2_000180; Prestwick3_000180; Spectrum2_001201; Spectrum3_001421; Spectrum4_000829; Spectrum5_001450; Lopac-F-9397; F0663; Flutamide (pubertal study); F 9397; SCHEMBL3934; Lopac0_000557; BSPBio_000079; BSPBio_003122; KBioGR_001377; KBioSS_001690; MLS000069634; MLS001065596; MLS002548892; DivK1c_000459; SPECTRUM1500995; SPBio_000982; SPBio_002000; Flutamide (JP17/USP/INN); BPBio1_000087; GTPL6943; CHEMBL4759307; DTXSID7032004; SCHEMBL12932289; HMS501G21; KBio1_000459; KBio2_001690; KBio2_004258; KBio2_006826; KBio3_002342; NINDS_000459; HMS1568D21; HMS1921O16; HMS2090I18; HMS2092O14; HMS2095D21; HMS2230P19; HMS3259I03; HMS3261P15; HMS3373C12; HMS3655G22; HMS3712D21; Pharmakon1600-01500995; AMY32524; BCP23006; HY-B0022; ZINC3812944; Tox21_110154; Tox21_202169; Tox21_300536; Tox21_500557; BDBM50131270; CCG-39105; m-Propionotoluidide, 2-methyl-4'-nitro-alpha,alpha,alpha-triflouro-; NSC147834; NSC215876; NSC757817; s1908; AKOS001025465; AKOS025243203; m-Propionotoluidide, .alpha.,.alpha.,.alpha.-trifluoro-2-methyl-4'-nitro-; Tox21_110154_1; AB02835; DB00499; KS-5091; LP00557; MCULE-7498839065; NC00451; NSC-147834; NSC-757817; SDCCGSBI-0050540.P004; IDI1_000459; NCGC00015452-01; NCGC00015452-02; NCGC00015452-03; NCGC00015452-04; NCGC00015452-05; NCGC00015452-06; NCGC00015452-07; NCGC00015452-08; NCGC00015452-10; NCGC00015452-11; NCGC00015452-12; NCGC00015452-13; NCGC00015452-14; NCGC00015452-15; NCGC00015452-16; NCGC00015452-19; NCGC00015452-20; NCGC00015452-32; NCGC00091460-01; NCGC00091460-02; NCGC00091460-03; NCGC00091460-04; NCGC00091460-05; NCGC00091460-06; NCGC00091460-07; NCGC00091460-08; NCGC00091460-09; NCGC00254495-01; NCGC00259718-01; NCGC00261242-01; AC-24192; BF166239; SY036411; SBI-0050540.P003; DB-042163; 3'-Trifluoromethyl-4'-Nitro-Isobutyranilide; AB00052188; EU-0100557; FT-0626493; FT-0668764; SW196536-4; 4''-nitro-3''-trifluoromethylisobutyranilide; C07653; D00586; J10037; AB00052188-09; AB00052188_10; AB00052188_11; 311F847; A806562; Q418669; Q-201131; SR-01000075888-1; SR-01000075888-6; SR-01000075888-7; SR-01000075888-9; BRD-K28307902-001-05-0; Flutamide, certified reference material, TraceCERT(R); Z56755651; a,a,a-Trifluoro-2-methyl-4'-nitro- m-propionotoluidide; Flutamide, European Pharmacopoeia (EP) Reference Standard; 2-Methyl-N-[4-nitro-3-(trifluoromethyl)phenyl]propanamide #; Flutamide, United States Pharmacopeia (USP) Reference Standard; 2-methyl-N-[4-nitro-3-(trifluoromethyl)phenyl]propanamide;Flutamide; alpha,alpha,alpha-trifluoro-2-methyl-4''-nitro-m-propionotoluidide; .alpha.,.alpha.,.alpha.-Trifluoro-2-methyl-4'-nitro-m-propionotoluidide; m-Propionotoluidide, 2-methyl-4'-nitro-.alpha.,.alpha.,.alpha.-trifluoro-; m-Propionotoluidide, alpha,alpha,alpha-trifluoro-2-methyl-4'-nitro- (8CI); Flutamide for system suitability, European Pharmacopoeia (EP) Reference Standard; 37209-54-4
Click to Show/Hide
|
||||
| Indication |
In total 4 Indication(s)
|
||||
| Structure |
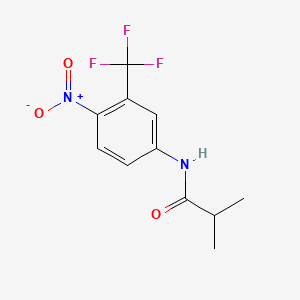
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Clinically Reported Resistance for This Drug
(1 diseases)
[1]
Disease(s) with Resistance Information Discovered by Cell Line Test for This Drug
(1 diseases)
[2]
|
||||
| Target | Candida Thymidylate synthase (Candi TMP1) | TYSY_CANAL | [1] | ||
| Dihydrothymine dehydrogenase (DPYD) | DPYD_HUMAN | [1] | |||
| TERT messenger RNA (TERT mRNA) | TERT_HUMAN | [1] | |||
| Thymidylate synthase messenger RNA (TYMS mRNA) | TYSY_HUMAN | [1] | |||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
2
|
||||
| IsoSMILES |
CC(C)C(=O)NC1=CC(=C(C=C1)[N+](=O)[O-])C(F)(F)F
|
||||
| InChI |
InChI=1S/C11H11F3N2O3/c1-6(2)10(17)15-7-3-4-9(16(18)19)8(5-7)11(12,13)14/h3-6H,1-2H3,(H,15,17)
|
||||
| InChIKey |
MKXKFYHWDHIYRV-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
| VARIDT ID | |||||
| INTEDE ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-02: Benign/in-situ/malignant neoplasm
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | |||||||||||||
|
|
|||||||||||||
| Key Molecule: Androgen receptor (AR) | [1] | ||||||||||||
| Resistant Disease | Prostate cancer [ICD-11: 2C82.0] | ||||||||||||
| Molecule Alteration | Missense mutation | p.T878A (c.2632A>G) |
|||||||||||
| Wild Type Structure | Method: X-ray diffraction | Resolution: 1.44 Å | |||||||||||
| Mutant Type Structure | Method: X-ray diffraction | Resolution: 1.20 Å | |||||||||||
| Download The Information of Sequence | Download The Structure File | ||||||||||||
-
-
M
-
G
-
S
-
S
-
H
-
H
650
|
-
H
-
H
-
H
-
H
-
S
-
S
-
G
-
L
-
V
-
P
660
|
-
R
-
G
-
S
-
H
-
M
-
I
-
E
-
G
-
Y
-
E
670
|
-
C
-
Q
P
P
I
I
F
F
L
L
N
N
V
V
L
L
E
E
680
|
A
A
I
I
E
E
P
P
G
G
V
V
V
V
C
C
A
A
G
G
690
|
H
H
D
D
N
N
N
N
Q
Q
P
P
D
D
S
S
F
F
A
A
700
|
A
A
L
L
L
L
S
S
S
S
L
L
N
N
E
E
L
L
G
G
710
|
E
E
R
R
Q
Q
L
L
V
V
H
H
V
V
V
V
K
K
W
W
720
|
A
A
K
K
A
A
L
L
P
P
G
G
F
F
R
R
N
N
L
L
730
|
H
H
V
V
D
D
D
D
Q
Q
M
M
A
A
V
V
I
I
Q
Q
740
|
Y
Y
S
S
W
W
M
M
G
G
L
L
M
M
V
V
F
F
A
A
750
|
M
M
G
G
W
W
R
R
S
S
F
F
T
T
N
N
V
V
N
N
760
|
S
S
R
R
M
M
L
L
Y
Y
F
F
A
A
P
P
D
D
L
L
770
|
V
V
F
F
N
N
E
E
Y
Y
R
R
M
M
H
H
K
K
S
S
780
|
R
R
M
M
Y
Y
S
S
Q
Q
C
C
V
V
R
R
M
M
R
R
790
|
H
H
L
L
S
S
Q
Q
E
E
F
F
G
G
W
W
L
L
Q
Q
800
|
I
I
T
T
P
P
Q
Q
E
E
F
F
L
L
C
C
M
M
K
K
810
|
A
A
L
L
L
L
L
L
F
F
S
S
I
I
I
I
P
P
V
V
820
|
D
D
G
G
L
L
K
K
N
N
Q
Q
K
K
F
F
F
F
D
D
830
|
E
E
L
L
R
R
M
M
N
N
Y
Y
I
I
K
K
E
E
L
L
840
|
D
D
R
R
I
I
I
I
A
A
C
C
A
K
R
R
K
K
N
N
850
|
P
P
T
T
S
S
C
C
S
S
R
R
R
R
F
F
Y
Y
Q
Q
860
|
L
L
T
T
K
K
L
L
L
L
D
D
S
S
V
V
Q
Q
P
P
870
|
I
I
A
A
R
R
E
E
L
L
H
H
Q
Q
F
F
T
A
F
F
880
|
D
D
L
L
L
L
I
I
K
K
S
S
H
H
M
M
V
V
S
S
890
|
V
V
D
D
F
F
P
P
E
E
M
M
M
M
A
A
E
E
I
I
900
|
I
I
S
S
V
V
Q
Q
V
V
P
P
K
K
I
I
L
L
S
S
910
|
G
G
K
K
V
V
K
K
P
P
I
I
Y
Y
F
F
H
H
T
T
920
|
Q
Q
|
|||||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| Key Molecule: Androgen receptor (AR) | [1] | ||||||||||||
| Resistant Disease | Prostate cancer [ICD-11: 2C82.0] | ||||||||||||
| Molecule Alteration | Missense mutation | p.T877A |
|||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| Key Molecule: Androgen receptor (AR) | [2] | ||||||||||||
| Resistant Disease | Prostate cancer [ICD-11: 2C82.0] | ||||||||||||
| Molecule Alteration | Expression | Up-regulation |
|||||||||||
| Experimental Note | Revealed Based on the Cell Line Data | ||||||||||||
| In Vitro Model | LN-FLU cells | Prostate | Homo sapiens (Human) | N.A. | |||||||||
| Experiment for Molecule Alteration |
Western blot assay | ||||||||||||
| Experiment for Drug Resistance |
MTT assay | ||||||||||||
| Mechanism Description | To obtain molecular evidence of the acquired resistance of the LN-FLU cells, we checked the expressions of significant proteins involved in prostate cell growth. As shown in, the LN-FLU cells showed less expression of the androgen receptor (AR) compared with the parental LNCaP cells, further confirming the androgen refractory state of the cells. | ||||||||||||
|
|
|||||||||||||
| Key Molecule: P-glycoprotein 1A (ABCB1A) | [2] | ||||||||||||
| Resistant Disease | Prostate cancer [ICD-11: 2C82.0] | ||||||||||||
| Molecule Alteration | Expression | Down-regulation |
|||||||||||
| Experimental Note | Revealed Based on the Cell Line Data | ||||||||||||
| In Vitro Model | LN-FLU cells | Prostate | Homo sapiens (Human) | N.A. | |||||||||
| Experiment for Molecule Alteration |
Western blot assay | ||||||||||||
| Experiment for Drug Resistance |
MTT assay | ||||||||||||
| Mechanism Description | ABCB1A, a well-known multidrug-resistant protein that expels drugs outside the cell, thereby promoting drug resistance, was increased in the LN-FLU cells | ||||||||||||
|
|
|||||||||||||
| Key Molecule: Serine/threonine-protein kinase mTOR (mTOR) | [2] | ||||||||||||
| Resistant Disease | Prostate cancer [ICD-11: 2C82.0] | ||||||||||||
| Molecule Alteration | Phosphorylation | Up-regulation |
|||||||||||
| Experimental Note | Revealed Based on the Cell Line Data | ||||||||||||
| Cell Pathway Regulation | PI3K/AKT/mTOR signaling pathway | Inhibition | hsa04151 | ||||||||||
| In Vitro Model | LN-FLU cells | Prostate | Homo sapiens (Human) | N.A. | |||||||||
| Experiment for Molecule Alteration |
Western blot assay | ||||||||||||
| Experiment for Drug Resistance |
MTT assay | ||||||||||||
| Mechanism Description | The PI3K/Akt/mTOR pathway is involved in the regulation of cancer cell survival, proliferation, growth, and metabolism. In most prostate cancer cell lines, the PIP3 phosphatase PTEN, which antagonizes this pathway, is mutated and therefore the PI3K/Akt/mTOR pathway is activated. To examine the functioning of this pathway, we determined the expressions and phosphorylation levels of Akt and mTOR via Western blot. As shown in, in the drug-resistant LN-FLU cells, both the phosphorylation levels and overall expressions of Akt and mTOR were decreased when compared to the LNCaP cells. The results in the LN-FLU cells were similar to those observed in the androgen-resistant PC3 cells, which were used as a positive control. The decrease in Akt and mTOR signaling suggests a proliferative arrest of the drug-resistant LN-FLU cells. | ||||||||||||
| Key Molecule: AKT serine/threonine kinase (AKT) | [2] | ||||||||||||
| Resistant Disease | Prostate cancer [ICD-11: 2C82.0] | ||||||||||||
| Molecule Alteration | Phosphorylation | Up-regulation |
|||||||||||
| Experimental Note | Revealed Based on the Cell Line Data | ||||||||||||
| Cell Pathway Regulation | PI3K/AKT/mTOR signaling pathway | Inhibition | hsa04151 | ||||||||||
| In Vitro Model | LN-FLU cells | Prostate | Homo sapiens (Human) | N.A. | |||||||||
| Experiment for Molecule Alteration |
Western blot assay | ||||||||||||
| Experiment for Drug Resistance |
MTT assay | ||||||||||||
| Mechanism Description | The PI3K/Akt/mTOR pathway is involved in the regulation of cancer cell survival, proliferation, growth, and metabolism. In most prostate cancer cell lines, the PIP3 phosphatase PTEN, which antagonizes this pathway, is mutated and therefore the PI3K/Akt/mTOR pathway is activated. To examine the functioning of this pathway, we determined the expressions and phosphorylation levels of Akt and mTOR via Western blot. As shown in, in the drug-resistant LN-FLU cells, both the phosphorylation levels and overall expressions of Akt and mTOR were decreased when compared to the LNCaP cells. The results in the LN-FLU cells were similar to those observed in the androgen-resistant PC3 cells, which were used as a positive control. The decrease in Akt and mTOR signaling suggests a proliferative arrest of the drug-resistant LN-FLU cells. | ||||||||||||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
