Drug Information
Drug (ID: DG01311) and It's Reported Resistant Information
| Name |
Olanzapine
|
||||
|---|---|---|---|---|---|
| Synonyms |
Olanzapine; 132539-06-1; Zyprexa; Olansek; Zyprexa Zydis; Zalasta; Zyprexa Velotab; Zyprexa Intramuscular; Zolafren; Zypadhera; LY-170053; 2-Methyl-4-(4-methylpiperazin-1-yl)-10H-benzo[b]thieno[2,3-e][1,4]diazepine; 2-methyl-4-(4-methylpiperazin-1-yl)-10H-thieno[2,3-b][1,5]benzodiazepine; Olzapin; Lanzac; Oferta; Olanzapine Mylan; LY 170053; 2-Methyl-4-(4-methyl-1-piperazinyl)-10H-thieno[2,3-b][1,5]benzodiazepine; C17H20N4S; UNII-N7U69T4SZR; 2-methyl-4-(4-methylpiperazin-1-yl)-5H-thieno[3,2-c][1,5]benzodiazepine; LY170053; 10H-thieno[2,3-b][1,5]benzodiazepine, 2-methyl-4-(4-methyl-1-piperazinyl)-; CHEMBL715; N7U69T4SZR; 2-Methyl-4-(4-methyl-1-piperazinyl)-10H-thieno(2,3-b)(1,5)benzodiazepine; CHEBI:7735; MFCD00866702; 5-methyl-8-(4-methylpiperazin-1-yl)-4-thia-2,9-diazatricyclo[8.4.0.0^{3,7}]tetradeca-1(10),3(7),5,8,11,13-hexaene; olanzapina; NCGC00096077-03; DSSTox_CID_3388; 2-Methyl-4-(4-methyl-1-piperazinyl)- 10H-thieno[2,3-b][1,5]benzodiazepine; DSSTox_RID_77010; DSSTox_GSID_23388; olanzapinum; Olanzapin; Midax; Olazax; Olanzapine Teva; Olazax Disperzi; 2-methyl-4-(4-methyl-1-piperazinyl)-5H-thieno[3,2-c][1,5]benzodiazepine; Olanzapine Teva; 10H-Thieno(2,3-b)(1,5)benzodiazepine, 2-methyl-4-(4-methyl-1-piperazinyl)-; Olanzapine Glenmark; SMR000466345; Olanzapine Neopharma; CAS-132539-06-1; SR-01000759343; Olanzapine cipla; Olanzapine apotex; ALKS-7921; HSDB 8155; Olanzapine [USAN:USP:INN:BAN]; Olanzapine solution; Olanzapine- Bio-X; Olanzapine (Zyprexa); KS-1090; GTPL47; BIDD:PXR0138; SCHEMBL28763; Olanzapine (JAN/USP/INN); US8802672, Olanzapine; MLS000759457; MLS001165781; MLS001195646; MLS001424057; BIDD:GT0332; SCHEMBL117695; SPECTRUM1505024; Olanzapine (LY-170053); DTXSID9023388; Olanzapine, >=98% (HPLC); BDBM35254; BDBM82479; CHEBI:94534; AMY7709; HMS2051H05; HMS2089M04; HMS2093I04; HMS2233F24; HMS3374L02; HMS3393H05; HMS3657I15; HMS3714J03; HMS3743A09; HMS3884J21; ACT03231; ALBB-027265; BCP04917; NSC_4585; ZINC3873830; Tox21_111556; AC-665; NSC754829; NSC801187; s2493; STK634338; STL388024; ZINC52957434; 2-methyl-4-(4-methylpiperazin-1-yl)-5H-thieno[2,3-b][1,5]benzodiazepine; AKOS000282888; AKOS005566122; Olanzapine 1.0 mg/ml in Acetonitrile; Tox21_111556_1; BCP9001021; CCG-100922; CS-1114; DB00334; MCULE-1620364835; NC00172; NSC-754829; NSC-801187; NCGC00096077-01; NCGC00096077-04; NCGC00096077-05; NCGC00096077-06; NCGC00096077-18; NCGC00389791-02; BO164166; HY-14541; I960; SBI-0206786.P001; CAS_132539-06-1; FT-0673219; O0393; SW220248-1; C07322; D00454; J10363; AB00639907-06; AB00639907-07; AB00639907_08; AB00639907_09; 539O061; A806453; L000455; L005958; Q201872; J-006186; SR-01000759343-4; SR-01000759343-6; Z1868057799; Olanzapine, European Pharmacopoeia (EP) Reference Standard; Olanzapine, United States Pharmacopeia (USP) Reference Standard; Olanzapine, Pharmaceutical Secondary Standard; Certified Reference Material; (E)-2-methyl-4-(4-methylpiperazin-1-yl)-10H-benzo[b]thieno[2,3-e][1,4]diazepine; 2-Methyl-10-(4-methyl-piperazin-1-yl)-4H-3-thia-4,9-diaza-benzo[f]azulene; 2-methyl-4-(4-methyl-1-piperazinyl) -10h-thieno[2,3-b][1,5]benzodiazepine; 2-methyl-4-(4-methyl-1-piperazinyl)-10H-thieno-[2,3-b][1,5]benzodiazepine; 2-Methyl-4-(4-methyl-1-piperazinyl)-10H-thieno[2,3-b][1,5]benzodiazepine #; 2-Methyl-4-(4-methylpiperazin-1-yl)-10H- thieno[2,3-b][1,5]benzodiazepin; Olanzapine for system suitability, European Pharmacopoeia (EP) Reference Standard; Olanzapine-d8, 100 mug/mL in acetonitrile, ampule of 1 mL, certified reference material; Olanzapine solution, 1.0 mg/mL in acetonitrile, ampule of 1 mL, certified reference material
Click to Show/Hide
|
||||
| Indication |
In total 1 Indication(s)
|
||||
| Structure |
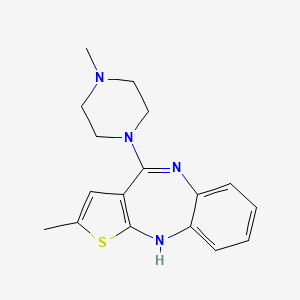
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Resistance Information Validated by in-vivo Model for This Drug
(1 diseases)
[1]
|
||||
| Target | Dopamine D2 receptor (D2R) | DRD2_HUMAN | [1] | ||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
C17H20N4S
|
||||
| IsoSMILES |
CC1=CC2=C(S1)NC3=CC=CC=C3N=C2N4CCN(CC4)C
|
||||
| InChI |
1S/C17H20N4S/c1-12-11-13-16(21-9-7-20(2)8-10-21)18-14-5-3-4-6-15(14)19-17(13)22-12/h3-6,11,19H,7-10H2,1-2H3
|
||||
| InChIKey |
KVWDHTXUZHCGIO-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
| VARIDT ID | |||||
| INTEDE ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-06: Mental/behavioural/neurodevelopmental disorders
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Mitogen-activated protein kinase 8 (MAPK8) | [1] | |||
| Resistant Disease | Schizophrenia [ICD-11: 6A20.0] | |||
| Molecule Alteration | Function | Activation |
||
| Experimental Note | Discovered Using In-vivo Testing Model | |||
| In Vivo Model | Female C57BL/6J mouse model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis; RT-qPCR | |||
| Experiment for Drug Resistance |
Flow cytometry | |||
| Mechanism Description | JNK downregulation improves olanzapine-induced insulin resistance by suppressing IRS1Ser307 phosphorylation and reducing inflammation. | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
