Drug Information
Drug (ID: DG01060) and It's Reported Resistant Information
| Name |
Cefsulodin
|
||||
|---|---|---|---|---|---|
| Synonyms |
Cefsulodin; Cefsulodine; Cefsulodinum; Cefsulodino; 62587-73-9; UNII-OV42LHE42B; OV42LHE42B; (6R,7R)-3-[(4-carbamoylpyridin-1-ium-1-yl)methyl]-8-oxo-7-[[(2R)-2-phenyl-2-sulfoacetyl]amino]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate; CHEBI:3507; (6R,7R)-3-[(4-carbamoylpyridinium-1-yl)methyl]-8-oxo-7-{[(2R)-2-phenyl-2-sulfoacetyl]amino}-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate; 7beta-{[(2R)-2-phenyl-2-sulfoacetyl]amino}-3-(4-carbamoylpyridinium-1-yl)methyl-3,4-didehydrocepham-4-carboxylate; Cefsulodin [INN:BAN]; Cefsulodine [INN-French]; Cefsulodinum [INN-Latin]; Cefsulodino [INN-Spanish]; Cefonomil; Cefsulodin (INN); Epitope ID:120379; SCHEMBL65525; CGP7174/E; GTPL10783; CGP-7174-E; DB13499; (6R,7R)-3-((4-carbamoylpyridinio)methyl)-8-oxo-7-((R)-2-phenyl-2-sulfoacetamido)-5-thia-1-azabicyclo(4.2.0)oct-2-en-2-carboxylat; 4-Carbamoyl-1-(((6R,7R)-2-carboxy-8-oxo-7-((2R)-2-phenyl-2-sulfoacetaido)-5-thia-1-azabicyclo(4.2.0)oct-2-en-3-yl)methyl)pyridinium hydroxide, inner salt; D07653; Pyridinium, 4-(aminocarbonyl)-1-((2-carboxy-8-oxo-7-((phenylsulfoacetyl)amino)-5-thia-1-azabicyclo(4.2.0)oct-2-en-3-yl)methyl)-, inner salt, (6R-(6alpha,7beta(R*)))-
Click to Show/Hide
|
||||
| Indication |
In total 1 Indication(s)
|
||||
| Structure |
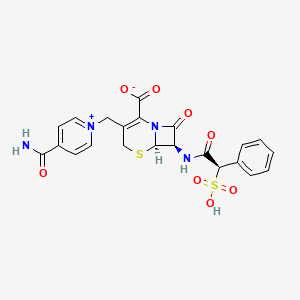
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Clinically Reported Resistance for This Drug
(1 diseases)
[1]
Disease(s) with Resistance Information Discovered by Cell Line Test for This Drug
(1 diseases)
[2]
|
||||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
C22H20N4O8S2
|
||||
| IsoSMILES |
C1C(=C(N2[C@H](S1)[C@@H](C2=O)NC(=O)[C@@H](C3=CC=CC=C3)S(=O)(=O)O)C(=O)[O-])C[N+]4=CC=C(C=C4)C(=O)N
|
||||
| InChI |
1S/C22H20N4O8S2/c23-18(27)13-6-8-25(9-7-13)10-14-11-35-21-15(20(29)26(21)16(14)22(30)31)24-19(28)17(36(32,33)34)12-4-2-1-3-5-12/h1-9,15,17,21H,10-11H2,(H4-,23,24,27,28,30,31,32,33,34)/t15-,17-,21-/m1/s1
|
||||
| InChIKey |
SYLKGLMBLAAGSC-QLVMHMETSA-N
|
||||
| PubChem CID | |||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-01: Infectious/parasitic diseases
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: 16S rRNA adenine dimethyltransferase (KsgA) | [2] | |||
| Resistant Disease | Lactobacillus casei infection [ICD-11: 1A00-1C4Z] | |||
| Molecule Alteration | Missense mutation | A1518/1519 |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Pseudomonas aeruginosa | 1763 | ||
| Experiment for Molecule Alteration |
PCR; Southern blot assay | |||
| Experiment for Drug Resistance |
MIC assay | |||
| Mechanism Description | SOD enzymatic activity and SodM protein levels are reduced in the ksgA mutant strain;The absence of ksgA contributes to an altered antibiotic response | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
