Drug Information
Drug (ID: DG00998) and It's Reported Resistant Information
| Name |
Tazobactam
|
||||
|---|---|---|---|---|---|
| Synonyms |
Tazobactam; 89786-04-9; Tazobactam acid; Tazobactamum; YTR-830H; YTR 830H; UNII-SE10G96M8W; CL-298741; CHEMBL404; YTR 830; CHEBI:9421; SE10G96M8W; (2S,3S,5R)-3-((1H-1,2,3-triazol-1-yl)methyl)-3-methyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid 4,4-dioxide; (2S,3S,5R)-3-methyl-7-oxo-3-(1H-1,2,3-triazol-1-ylmethyl)-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid 4,4-dioxide; CL 298,741; YTR 830 H; 89786-04-9 (free acid); (2S,3S,5R)-3-METHYL-4,4,7-TRIOXO-3-(1H-1,2,3-TRIAZOL-1-YLMETHYL)-4-THIA-1-AZABICYCLO[3.2.0]HEPTANE-2-CARBOXYLIC ACID; (2S,3S,5R)-3-methyl-4,4,7-trioxo-3-(triazol-1-ylmethyl)-4lambda6-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid; (2S,3S,5R)-3-Methyl-7-oxo-3-(1H-1,2,3-triazol-1-ylmethyl)-4-thia-1-azabicyclo(3.2.0)heptane-2-carboxylic acid 4,4-dioxide; SR-01000872598; 4-Thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid, 3-methyl-7-oxo-3-(1H-1,2,3-triazol-1-ylmethyl)-, 4,4-dioxide, (2S,3S,5R)-; 89785-84-2; Tazobactamum [INN-Latin]; YTR830H; CCRIS 2203; YTR830; NSC-759887; Tazobactam,(S); Tazobactam [USAN:USP:INN:BAN]; (2S,3S,5R)-3-methyl-4,4,7-trioxo-3-(triazol-1-ylmethyl)-4 ^{6}-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid; Cl 298741; BRN 4787943; CL298741; Tazobactam;Tazobactan acid; EC 618-303-7; BIDD:GT0212; SCHEMBL122302; CXA201; Tazobactam (JP17/USP/INN); DTXSID8023634; GTPL10789; HMS3714G17; (2S,3S,5S)-3-methyl-7-oxo-3-(1H-1,2,3-triazol-1-ylmethyl)-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid 4,4-dioxide; BCP09757; EX-A1377; HY-B1418; ZINC3787060; BDBM50053173; MFCD00867002; s3077; AKOS015960801; AC-7620; CCG-207890; CS-4914; DB01606; DS-8301; NSC 759887; (2S,3S,5R)-3-methyl-4,4,7-trioxo-3-(triazol-1-ylmethyl)-4$l^{6}-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid; (2S,3S,5R)-3-Methyl-7-oxo-3-(1H-1,2,3-triazol-1-ylmethyl)-4-thia-1-azabicyclo(3.2.0)heptane-2-carboxylic acid, 4,4-dioxide; T3732; C07771; D00660; D78146; T-1445; 786T049; A843310; Q423376; Tazobactam, Antibiotic for Culture Media Use Only; SR-01000872598-1; SR-01000872598-2; 89786-04-9, 89785-84-2 (sodium salt); BRD-K14312455-001-01-6; (2S,3S,5R)-3-methyl-4,4,7-trioxo-3-(1-triazolylmethyl)-4$l^{6}-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid; (2S,3S,5R)-3-methyl-4,4,7-trioxo-3-(1H-1,2,3-triazol-1-ylmethyl)-4$l^{6}-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid; (2S,3S,5R)-3-methyl-4,4,7-trioxo-3-[(1H-1,2,3-triazol-1-yl)methyl]-4lambda(6)-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid; (2S,3S,5R)-3-Methyl-4,4,7-trioxo-3-[1,2,3]triazol-1-ylmethyl-4-thia-1-aza-bicyclo[3.2.0]heptane-2-carboxylic acid; (2S,3S,5R)-3-Methyl-4,4,7-trioxo-3-[1,2,3]triazol-1-ylmethyl-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid; (2S,3S,5R)-3-Methyl-4,4,7-trioxo-3-[1,2,3]triazol-1-ylmethyl-4lambda 6-thia-1-aza-bicyclo[3.2.0]heptane-2-carboxylic acid; (2S,3S,5R)-3-methyl-4,4,7-trioxo-3-[1,2,3]triazol-1-ylmethyl-4lambda6-thia-1-aza-bicyclo[3.2.0]heptane-2-carboxylic acid; (2S,3S,5R)-3-methyl-4,4,7-tris(oxidanylidene)-3-(1,2,3-triazol-1-ylmethyl)-4$l^{6}-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid; 3-Methyl-4,4,7-trioxo-3-[1,2,3]triazol-1-ylmethyl-4lambda*6*-thia-1-aza-bicyclo[3.2.0]heptane-2-carboxylic acid; 4-Thia-1-azabicyclo(3.2.0)heptane-2-carboxylic acid, 3-methyl-7-oxo-3-(1H-1,2,3-triazol-1-ylmethyl)-, 4,4-dioxide, (2S-(2alpha,3beta,5alpha))-; 4-Thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid, 3-methyl-7- oxo-3-(1H-1,2,3-triazol-1-ylmethyl)-, 4,4-dioxide, [2S- (2alpha,3beta,5alpha)]-
Click to Show/Hide
|
||||
| Indication |
In total 1 Indication(s)
|
||||
| Structure |
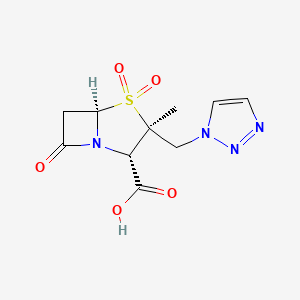
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Resistance Information Validated by in-vivo Model for This Drug
(1 diseases)
[1]
|
||||
| Target | Staphylococcus Beta-lactamase (Stap-coc blaZ) | BLAC_STAAU | [1] | ||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
C10H12N4O5S
|
||||
| IsoSMILES |
C[C@@]1([C@@H](N2[C@H](S1(=O)=O)CC2=O)C(=O)O)CN3C=CN=N3
|
||||
| InChI |
1S/C10H12N4O5S/c1-10(5-13-3-2-11-12-13)8(9(16)17)14-6(15)4-7(14)20(10,18)19/h2-3,7-8H,4-5H2,1H3,(H,16,17)/t7-,8+,10+/m1/s1
|
||||
| InChIKey |
LPQZKKCYTLCDGQ-WEDXCCLWSA-N
|
||||
| PubChem CID | |||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
| INTEDE ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-01: Infectious/parasitic diseases
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Beta-lactamase (Q6QJ55) | [1] | |||
| Resistant Disease | Carbapenem-nonsusceptible Pseudomonas aeruginosa infection [ICD-11: 1A00-1C4Z] | |||
| Molecule Alteration | Function | Inhibition |
||
| Experimental Note | Discovered Using In-vivo Testing Model | |||
| In Vitro Model | Carbapenem-nonsusceptible Pseudomonas aeruginosa strain | 287 | ||
| Experiment for Molecule Alteration |
Whole-genome sequencing assay | |||
| Mechanism Description | Ceftolozane/tazobactam (C/T), a novel beta-lactam/beta-lactamase inhibitor combination, addresses an unmet medical need in patients with these multidrug-resistant P. aeruginosa infections. | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
