Drug Information
Drug (ID: DG00658) and It's Reported Resistant Information
| Name |
Pregabalin
|
||||
|---|---|---|---|---|---|
| Synonyms |
Pregabalin; 148553-50-8; Lyrica; (S)-3-(Aminomethyl)-5-methylhexanoic acid; 3-isobutyl GABA; (3S)-3-(aminomethyl)-5-methylhexanoic acid; CI-1008; (S)-Pregabalin; Hexanoic acid, 3-(aminomethyl)-5-methyl-, (3S)-; CI 1008; PD 144723; UNII-55JG375S6M; PD-144723; (S)-3-Isobutyl GABA; CHEMBL1059; CHEBI:64356; (3S)-3-(aminomethyl)-5-methyl-hexanoic acid; 55JG375S6M; MFCD00917044; Hexanoic acid, 3-(aminomethyl)-5-ethyl-, (3S)-; (R-)-3-isobutyl GABA; Hexanoic acid, 3-(aminomethyl)-5-methyl-, (S)-; Pregabalina; Pregabaline; Vronogabic; Nervalin; Pregablin; Pregabalin mylan; Pregabalin sandoz; HSDB 7530; Pregabalin zentiva; NSC-759256; Pregabalin [USAN:INN:BAN:JAN]; NCGC00095186-01; Pregabalin- Bio-X; Lyrica (TN); Pregabalin sandoz gmbh; (S)-3-(Aminomethyl)-5-methylhexanoicacid; SCHEMBL8227; DSSTox_CID_25950; DSSTox_RID_81246; DSSTox_GSID_45950; Pregabalin (JAN/USAN/INN); (S)-(+)-4-amino-3-(2-methylpropyl)butanoic acid; GTPL5484; ZINC5152; DEA No. 2782; Pregabalin, >=97% (NMR); DTXSID1045950; HMS3715J16; Pregabalin 1.0 mg/ml in Methanol; YNP-1807; Tox21_111475; BDBM50164279; AKOS001476611; AKOS005145504; Lyrica;CI-1008;PD-144723; AC-1158; CCG-221247; CM14412; CS-1247; DB00230; KS-5378; NSC 759256; (S)-3-aminomethyl-5-methylhexanoic acid; NCGC00346738-01; 121GE001; BP163672; HY-17414; (S)-3-Aminomethyl-5-methyl-hexanoic acid; AM20080369; CAS-148553-50-8; EN300-92104; (3S)-3-(aminomethyl)-5-methyl hexanoic acid; (S)-(+)-3-Aminomethyl-5-Methylhexanoic Acid; D02716; AB01563007_01; 553P508; A808784; Q412174; SR-01000942257; SR-01000942257-2; Pregabalin, EuropePharmacopoeia (EP) Reference Standard; Z2757554242; 1414928-41-8
Click to Show/Hide
|
||||
| Structure |
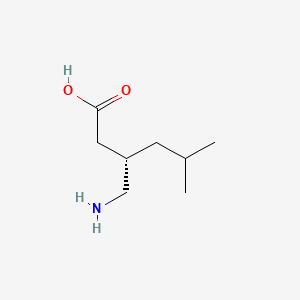
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Resistance Information Validated by in-vivo Model for This Drug
(1 diseases)
[1]
Disease(s) with Clinically Reported Resistance for This Drug
(1 diseases)
[2]
|
||||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
C8H17NO2
|
||||
| IsoSMILES |
CC(C)C[C@@H](CC(=O)O)CN
|
||||
| InChI |
1S/C8H17NO2/c1-6(2)3-7(5-9)4-8(10)11/h6-7H,3-5,9H2,1-2H3,(H,10,11)/t7-/m0/s1
|
||||
| InChIKey |
AYXYPKUFHZROOJ-ZETCQYMHSA-N
|
||||
| PubChem CID | |||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
| VARIDT ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-08: Nervous system diseases
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Glutathione synthetase (GSH) | [1] | |||
| Resistant Disease | mitochondrial refractory epilepsy [ICD-11: 8A60.A] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Discovered Using In-vivo Testing Model | |||
| In Vivo Model | Swiss albino mice model | Mus musculus | ||
| Experiment for Molecule Alteration |
Ellman assay | |||
| Experiment for Drug Resistance |
Pre-treatment resistance testing; Post-treatment resistance testing | |||
| Mechanism Description | The involvement of complex I in drug resistance is well established in epilepsy; therefore, the model chosen for this study was rotenone corneal kindled model of drug resistance using rotenone as a selective irreversible inhibitor of complex I, which have shown resistance to drugs such as valproate, levetiracetam, lamotrigine, pregabalin, carbamazepine, zonisamide, topiramate, gabapentin and their combinations | |||
| Key Molecule: Quinone reductase 1 (NQO1) | [1] | |||
| Resistant Disease | mitochondrial refractory epilepsy [ICD-11: 8A60.A] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Discovered Using In-vivo Testing Model | |||
| Cell Pathway Regulation | Nrf2 signaling pathway | Inhibition | hsa05208 | |
| In Vivo Model | Swiss albino mice model | Mus musculus | ||
| Experiment for Molecule Alteration |
ELISA assay | |||
| Experiment for Drug Resistance |
Pre-treatment resistance testing; Post-treatment resistance testing | |||
| Mechanism Description | The involvement of complex I in drug resistance is well established in epilepsy; therefore, the model chosen for this study was rotenone corneal kindled model of drug resistance using rotenone as a selective irreversible inhibitor of complex I, which have shown resistance to drugs such as valproate, levetiracetam, lamotrigine, pregabalin, carbamazepine, zonisamide, topiramate, gabapentin and their combinations | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
