Drug Information
Drug (ID: DG00284) and It's Reported Resistant Information
| Name |
Alpelisib
|
||||
|---|---|---|---|---|---|
| Synonyms |
Alpelisib; 1217486-61-7; BYL-719; BYL719; UNII-08W5N2C97Q; BYL 719; Alpelisib (BYL719); (S)-N1-(4-Methyl-5-(2-(1,1,1-trifluoro-2-methylpropan-2-yl)pyridin-4-yl)thiazol-2-yl)pyrrolidine-1,2-dicarboxamide; NVP-BYL719; (2S)-N1-[4-Methyl-5-[2-(2,2,2-trifluoro-1,1-dimethylethyl)-4-pyridinyl]-2-thiazolyl]-1,2-pyrrolidinedicarboxamide; CHEMBL2396661; 08W5N2C97Q; AK146107; C19H22F3N5O2S; (S)-N1-(4-Methyl-5-(2-(1,1,1-trifluoro-2-methylpropan-2-yl)-pyridin-4-yl)thiazol-2-yl)pyrrolidine-1,2-dicarboxamide
Click to Show/Hide
|
||||
| Indication |
In total 2 Indication(s)
|
||||
| Structure |
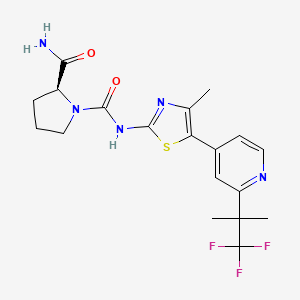
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Clinically Reported Resistance for This Drug
(1 diseases)
Disease(s) with Resistance Information Discovered by Cell Line Test for This Drug
(2 diseases)
[3]
[4]
|
||||
| Target | PI3-kinase alpha (PIK3CA) | PK3CA_HUMAN | [2] | ||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
C19H22F3N5O2S
|
||||
| IsoSMILES |
CC1=C(SC(=N1)NC(=O)N2CCC[C@H]2C(=O)N)C3=CC(=NC=C3)C(C)(C)C(F)(F)F
|
||||
| InChI |
1S/C19H22F3N5O2S/c1-10-14(11-6-7-24-13(9-11)18(2,3)19(20,21)22)30-16(25-10)26-17(29)27-8-4-5-12(27)15(23)28/h6-7,9,12H,4-5,8H2,1-3H3,(H2,23,28)(H,25,26,29)/t12-/m0/s1
|
||||
| InChIKey |
STUWGJZDJHPWGZ-LBPRGKRZSA-N
|
||||
| PubChem CID | |||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
| INTEDE ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-02: Benign/in-situ/malignant neoplasm
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (PIK3CA) | [3] | |||
| Resistant Disease | Breast adenocarcinoma [ICD-11: 2C60.1] | |||
| Molecule Alteration | Mutation | E17K |
||
| Experiment for Molecule Alteration |
Cell-Free DNA extraction assay; ddPCR | |||
| Mechanism Description | Although secondary?PIK3CA?mutations were previously reported to increase sensitivity to PI3Kalpha inhibitors, we identified emergent secondary resistance mutations in?PIK3CA?that alter the inhibitor binding pocket. Some mutations had differential effects on PI3Kalpha-selective versus pan-PI3K inhibitors, but resistance induced by all mutations could be overcome by the novel allosteric pan-mutant-selective PI3Kalpha-inhibitor RLY-2608. Together, these findings provide insights to guide strategies to overcome resistance in?PIK3CA-mutated cancers. | |||
| Key Molecule: Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (PIK3CA) | [3] | |||
| Resistant Disease | Breast adenocarcinoma [ICD-11: 2C60.1] | |||
| Molecule Alteration | Mutation | Q24K/L28M/R30Q/A92K RASs |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Experiment for Molecule Alteration |
Cell-Free DNA extraction assay; ddPCR | |||
| Mechanism Description | Although secondary?PIK3CA?mutations were previously reported to increase sensitivity to PI3Kalpha inhibitors, we identified emergent secondary resistance mutations in?PIK3CA?that alter the inhibitor binding pocket. Some mutations had differential effects on PI3Kalpha-selective versus pan-PI3K inhibitors, but resistance induced by all mutations could be overcome by the novel allosteric pan-mutant-selective PI3Kalpha-inhibitor RLY-2608. Together, these findings provide insights to guide strategies to overcome resistance in?PIK3CA-mutated cancers. | |||
|
|
||||
| Key Molecule: Phosphatase and tensin homolog (PTEN) | [1], [2] | |||
| Resistant Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Molecule Alteration | Structural variation | Copy number loss |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | PI3K signaling pathway | Activation | hsa04151 | |
| Experiment for Molecule Alteration |
Whole genome sequencing assay; Whole exome sequencing assay | |||
| Experiment for Drug Resistance |
Tetrazolium-based MTT assay | |||
| Mechanism Description | We conclude that parallel genetic evolution of separate metastatic sites with different PTEN genomic alterations leads to a convergent PTEN-null phenotype resistant to PI(3)kalpha inhibition. | |||
| Key Molecule: AKT serine/threonine kinase 1 (AKT1) | [3] | |||
| Resistant Disease | Breast adenocarcinoma [ICD-11: 2C60.1] | |||
| Molecule Alteration | Mutation | Q24K/L28M/R30Q/A92K RASs |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | T-47D cells | N.A. | Homo sapiens (Human) | CVCL_0553 |
| Experiment for Molecule Alteration |
Cell-Free DNA extraction assay; ddPCR | |||
| Mechanism Description | Although secondary?PIK3CA?mutations were previously reported to increase sensitivity to PI3Kalpha inhibitors, we identified emergent secondary resistance mutations in?PIK3CA?that alter the inhibitor binding pocket. Some mutations had differential effects on PI3Kalpha-selective versus pan-PI3K inhibitors, but resistance induced by all mutations could be overcome by the novel allosteric pan-mutant-selective PI3Kalpha-inhibitor RLY-2608. Together, these findings provide insights to guide strategies to overcome resistance in?PIK3CA-mutated cancers. | |||
| Key Molecule: AKT serine/threonine kinase 1 (AKT1) | [3] | |||
| Resistant Disease | Breast adenocarcinoma [ICD-11: 2C60.1] | |||
| Molecule Alteration | Mutation | W780R |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | T-47D cells | N.A. | Homo sapiens (Human) | CVCL_0553 |
| Experiment for Molecule Alteration |
Cell-Free DNA extraction assay; ddPCR | |||
| Mechanism Description | Although secondary?PIK3CA?mutations were previously reported to increase sensitivity to PI3Kalpha inhibitors, we identified emergent secondary resistance mutations in?PIK3CA?that alter the inhibitor binding pocket. Some mutations had differential effects on PI3Kalpha-selective versus pan-PI3K inhibitors, but resistance induced by all mutations could be overcome by the novel allosteric pan-mutant-selective PI3Kalpha-inhibitor RLY-2608. Together, these findings provide insights to guide strategies to overcome resistance in?PIK3CA-mutated cancers. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Growth factor receptor-bound protein 2 (GRB2) | [4] | |||
| Resistant Disease | Head and neck cancer [ICD-11: 2D42.0] | |||
| Molecule Alteration | Interaction | K143R? |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | MAPK signaling pathway | Activation | hsa04010 | |
| In Vitro Model | Cal-33 cells | Tongue | Homo sapiens (Human) | CVCL_1108 |
| FaDu cells | Pharynx | Homo sapiens (Human) | CVCL_1218 | |
| HSC-4 cells | Cervical lymph node | Homo sapiens (Human) | CVCL_1289 | |
| SAS cells | Oral | Homo sapiens (Human) | CVCL_1675 | |
| UT-SCC-5 cells | Head and Neck | Homo sapiens (Human) | CVCL_7858 | |
| UT-SCC-8 cells | Head and Neck | Homo sapiens (Human) | CVCL_7869 | |
| UT-SCC-14 cells | Head and Neck | Homo sapiens (Human) | CVCL_7810 | |
| UT-SCC-15 cells | Head and Neck | Homo sapiens (Human) | CVCL_7811 | |
| Experiment for Molecule Alteration |
Whole exome sequencing assay; Western blot assay; Akt activity assay; 3D foci assay; Phosphorylation pathway analysis; Gene enrichment assay; Network analysis; Functional enrichment analysis | |||
| Experiment for Drug Resistance |
3D colony formation assay | |||
| Mechanism Description | In terms of PI3K/Akt pathway activity, Alpelisib treatment reduced phosphorylation of Akt (Ser473), GSK3beta (Ser9) and 4E-BP1 (Ser65) to a similar extent in responder and non-responder cell models (Fig. 2A, B and S2). Likewise, Akt activity was not significantly modified upon Alpelisib exposure (Fig. 2C). Taken together, our data show that inhibition of PI3Kalpha kinase causes varying degrees of radiochemosensitization in different HNSCC models and without an obvious mutational biomarker to predict drug effect. | |||
| Key Molecule: Integrin beta-1 (ITGB1) | [4] | |||
| Resistant Disease | Head and neck cancer [ICD-11: 2D42.0] | |||
| Molecule Alteration | Function | Activation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | PI3Kalpha and beta1 integrin signaling pathway | Regulation | N.A. | |
| In Vitro Model | Cal-33 cells | Tongue | Homo sapiens (Human) | CVCL_1108 |
| FaDu cells | Pharynx | Homo sapiens (Human) | CVCL_1218 | |
| HSC-4 cells | Cervical lymph node | Homo sapiens (Human) | CVCL_1289 | |
| SAS cells | Oral | Homo sapiens (Human) | CVCL_1675 | |
| UT-SCC-5 cells | Head and Neck | Homo sapiens (Human) | CVCL_7858 | |
| UT-SCC-8 cells | Head and Neck | Homo sapiens (Human) | CVCL_7869 | |
| UT-SCC-14 cells | Head and Neck | Homo sapiens (Human) | CVCL_7810 | |
| UT-SCC-15 cells | Head and Neck | Homo sapiens (Human) | CVCL_7811 | |
| Experiment for Molecule Alteration |
Whole exome sequencing assay; Western blot assay; Akt activity assay; 3D foci assay; Phosphorylation pathway analysis; Gene enrichment assay; Network analysis; Functional enrichment analysis | |||
| Experiment for Drug Resistance |
3D colony formation assay | |||
| Mechanism Description | In terms of PI3K/Akt pathway activity, Alpelisib treatment reduced phosphorylation of Akt (Ser473), GSK3beta (Ser9) and 4E-BP1 (Ser65) to a similar extent in responder and non-responder cell models (Fig. 2A, B and S2). Likewise, Akt activity was not significantly modified upon Alpelisib exposure (Fig. 2C). Taken together, our data show that inhibition of PI3Kalpha kinase causes varying degrees of radiochemosensitization in different HNSCC models and without an obvious mutational biomarker to predict drug effect. | |||
| Key Molecule: Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (PIK3CA) | [4] | |||
| Resistant Disease | Head and neck cancer [ICD-11: 2D42.0] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | MAPK signaling pathway | Activation | hsa04010 | |
| In Vitro Model | Cal-33 cells | Tongue | Homo sapiens (Human) | CVCL_1108 |
| FaDu cells | Pharynx | Homo sapiens (Human) | CVCL_1218 | |
| HSC-4 cells | Cervical lymph node | Homo sapiens (Human) | CVCL_1289 | |
| SAS cells | Oral | Homo sapiens (Human) | CVCL_1675 | |
| UT-SCC-5 cells | Head and Neck | Homo sapiens (Human) | CVCL_7858 | |
| UT-SCC-8 cells | Head and Neck | Homo sapiens (Human) | CVCL_7869 | |
| UT-SCC-14 cells | Head and Neck | Homo sapiens (Human) | CVCL_7810 | |
| UT-SCC-15 cells | Head and Neck | Homo sapiens (Human) | CVCL_7811 | |
| Experiment for Molecule Alteration |
Whole exome sequencing assay; Western blot assay; Akt activity assay; 3D foci assay; Phosphorylation pathway analysis; Gene enrichment assay; Network analysis; Functional enrichment analysis | |||
| Experiment for Drug Resistance |
3D colony formation assay | |||
| Mechanism Description | We demonstrate that Alpelisib, Copanlisib and AZD8186 but not Idelalisib enhance radio- and radiochemosensitivity in the majority of HNSCC cell models (= responders) in a manner independent of PIK3CA mutation status. However, Alpelisib promotes MAPK signaling in non-responders compared to responders without profound impact on Akt, NFkappaB, TGFbeta, JAK/STAT signaling and DNA repair. Bioinformatic analyses identified unique gene mutations associated with extracellular matrix to be more frequent in non-responder cell models than in responders. Finally, we demonstrate that targeting of the cell adhesion molecule beta1 integrin on top of Alpelisib sensitizes non-responders to radiochemotherapy. Taken together, our study demonstrates the sensitizing potential of Alpelisib and other PI3K inhibitors in HNSCC models and uncovers a novel beta1 integrin-dependent mechanism that may prove useful in overcoming resistance to PI3K inhibitors. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: RAC-alpha serine/threonine-protein kinase (AKT1) | [4] | |||
| Sensitive Disease | Head and neck cancer [ICD-11: 2D42.0] | |||
| Molecule Alteration | Phosphorylation | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | PI3K/AKT signaling pathway | Inhibition | hsa04151 | |
| In Vitro Model | Cal-33 cells | Tongue | Homo sapiens (Human) | CVCL_1108 |
| FaDu cells | Pharynx | Homo sapiens (Human) | CVCL_1218 | |
| HSC-4 cells | Cervical lymph node | Homo sapiens (Human) | CVCL_1289 | |
| SAS cells | Oral | Homo sapiens (Human) | CVCL_1675 | |
| UT-SCC-5 cells | Head and Neck | Homo sapiens (Human) | CVCL_7858 | |
| UT-SCC-8 cells | Head and Neck | Homo sapiens (Human) | CVCL_7869 | |
| UT-SCC-14 cells | Head and Neck | Homo sapiens (Human) | CVCL_7810 | |
| UT-SCC-15 cells | Head and Neck | Homo sapiens (Human) | CVCL_7811 | |
| Experiment for Molecule Alteration |
Whole exome sequencing assay; Western blot assay; Akt activity assay; 3D foci assay; Phosphorylation pathway analysis; Gene enrichment assay; Network analysis; Functional enrichment analysis | |||
| Experiment for Drug Resistance |
3D colony formation assay | |||
| Mechanism Description | In terms of PI3K/Akt pathway activity, Alpelisib treatment reduced phosphorylation of Akt (Ser473), GSK3beta (Ser9) and 4E-BP1 (Ser65) to a similar extent in responder and non-responder cell models (Fig. 2A, B and S2). Likewise, Akt activity was not significantly modified upon Alpelisib exposure (Fig. 2C). Taken together, our data show that inhibition of PI3Kalpha kinase causes varying degrees of radiochemosensitization in different HNSCC models and without an obvious mutational biomarker to predict drug effect. | |||
| Key Molecule: Eukaryotic translation initiation factor 4E-binding protein 1 (EIF4EBP1) | [4] | |||
| Sensitive Disease | Head and neck cancer [ICD-11: 2D42.0] | |||
| Molecule Alteration | Phosphorylation | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | PI3K/AKT signaling pathway | Inhibition | hsa04151 | |
| In Vitro Model | Cal-33 cells | Tongue | Homo sapiens (Human) | CVCL_1108 |
| FaDu cells | Pharynx | Homo sapiens (Human) | CVCL_1218 | |
| HSC-4 cells | Cervical lymph node | Homo sapiens (Human) | CVCL_1289 | |
| SAS cells | Oral | Homo sapiens (Human) | CVCL_1675 | |
| UT-SCC-5 cells | Head and Neck | Homo sapiens (Human) | CVCL_7858 | |
| UT-SCC-8 cells | Head and Neck | Homo sapiens (Human) | CVCL_7869 | |
| UT-SCC-14 cells | Head and Neck | Homo sapiens (Human) | CVCL_7810 | |
| UT-SCC-15 cells | Head and Neck | Homo sapiens (Human) | CVCL_7811 | |
| Experiment for Molecule Alteration |
Whole exome sequencing assay; Western blot assay; Akt activity assay; 3D foci assay; Phosphorylation pathway analysis; Gene enrichment assay; Network analysis; Functional enrichment analysis | |||
| Experiment for Drug Resistance |
3D colony formation assay | |||
| Mechanism Description | In terms of PI3K/Akt pathway activity, Alpelisib treatment reduced phosphorylation of Akt (Ser473), GSK3beta (Ser9) and 4E-BP1 (Ser65) to a similar extent in responder and non-responder cell models (Fig. 2A, B and S2). Likewise, Akt activity was not significantly modified upon Alpelisib exposure (Fig. 2C). Taken together, our data show that inhibition of PI3Kalpha kinase causes varying degrees of radiochemosensitization in different HNSCC models and without an obvious mutational biomarker to predict drug effect. | |||
| Key Molecule: GSK3B-interacting protein (GSKIP) | [4] | |||
| Sensitive Disease | Head and neck cancer [ICD-11: 2D42.0] | |||
| Molecule Alteration | Phosphorylation | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | PI3K/AKT signaling pathway | Inhibition | hsa04151 | |
| In Vitro Model | Cal-33 cells | Tongue | Homo sapiens (Human) | CVCL_1108 |
| FaDu cells | Pharynx | Homo sapiens (Human) | CVCL_1218 | |
| HSC-4 cells | Cervical lymph node | Homo sapiens (Human) | CVCL_1289 | |
| SAS cells | Oral | Homo sapiens (Human) | CVCL_1675 | |
| UT-SCC-5 cells | Head and Neck | Homo sapiens (Human) | CVCL_7858 | |
| UT-SCC-8 cells | Head and Neck | Homo sapiens (Human) | CVCL_7869 | |
| UT-SCC-14 cells | Head and Neck | Homo sapiens (Human) | CVCL_7810 | |
| UT-SCC-15 cells | Head and Neck | Homo sapiens (Human) | CVCL_7811 | |
| Experiment for Molecule Alteration |
Whole exome sequencing assay; Western blot assay; Akt activity assay; 3D foci assay; Phosphorylation pathway analysis; Gene enrichment assay; Network analysis; Functional enrichment analysis | |||
| Experiment for Drug Resistance |
3D colony formation assay | |||
| Mechanism Description | In terms of PI3K/Akt pathway activity, Alpelisib treatment reduced phosphorylation of Akt (Ser473), GSK3beta (Ser9) and 4E-BP1 (Ser65) to a similar extent in responder and non-responder cell models (Fig. 2A, B and S2). Likewise, Akt activity was not significantly modified upon Alpelisib exposure (Fig. 2C). Taken together, our data show that inhibition of PI3Kalpha kinase causes varying degrees of radiochemosensitization in different HNSCC models and without an obvious mutational biomarker to predict drug effect. | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
