Drug Information
Drug (ID: DG00229) and It's Reported Resistant Information
| Name |
Osimertinib
|
||||
|---|---|---|---|---|---|
| Synonyms |
Tagrisso
Click to Show/Hide
|
||||
| Indication |
In total 2 Indication(s)
|
||||
| Structure |
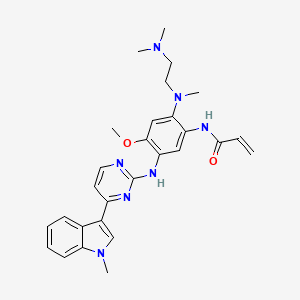
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Clinically Reported Resistance for This Drug
(1 diseases)
[1]
Disease(s) with Resistance Information Discovered by Cell Line Test for This Drug
(2 diseases)
[3]
[4]
|
||||
| Target | Epidermal growth factor receptor (EGFR) | EGFR_HUMAN | [1] | ||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
C28H33N7O2
|
||||
| IsoSMILES |
CN1C=C(C2=CC=CC=C21)C3=NC(=NC=C3)NC4=C(C=C(C(=C4)NC(=O)C=C)N(C)CCN(C)C)OC
|
||||
| InChI |
1S/C28H33N7O2/c1-7-27(36)30-22-16-23(26(37-6)17-25(22)34(4)15-14-33(2)3)32-28-29-13-12-21(31-28)20-18-35(5)24-11-9-8-10-19(20)24/h7-13,16-18H,1,14-15H2,2-6H3,(H,30,36)(H,29,31,32)
|
||||
| InChIKey |
DUYJMQONPNNFPI-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
| VARIDT ID | |||||
| INTEDE ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-02: Benign/in-situ/malignant neoplasm
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | |||||||||||||
|
|
|||||||||||||
| Key Molecule: Insulin-like growth factor 2 mRNA-binding protein 3 (IGF2BP3) | [5] | ||||||||||||
| Metabolic Type | Mitochondrial metabolism | ||||||||||||
| Resistant Disease | Non-small cell lung carcinoma [ICD-11: 2C25.Y] | ||||||||||||
| Molecule Alteration | Expression | Up-regulation |
|||||||||||
| Differential expression of the molecule in resistant disease | |||||||||||||
| Classification of Disease | Lung cancer [ICD-11: 2C25] | ||||||||||||
| The Specified Disease | Non-small cell lung carcinoma | ||||||||||||
| The Studied Tissue | Lung tissue | ||||||||||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.38E-05 Fold-change: 6.29E-01 Z-score: 4.51E+00 |
||||||||||||
| Experimental Note | Revealed Based on the Cell Line Data | ||||||||||||
| In Vivo Model | Nude mice , with PC-9/GR cell lines | Mice | |||||||||||
| Experiment for Molecule Alteration |
qRT-PCR | ||||||||||||
| Experiment for Drug Resistance |
Tumor volume assay | ||||||||||||
| Mechanism Description | Furthermore, we revealed that targeting IGF2BP3 can markedly enhance the sensitivity of TKIs in NSCLC and this effect was strongly dependent on the coordinated induction of COX6B2, a key downstream target of IGF2BP3 in mitochondrial OXPHOS energy production. Overall, our study revealed a novel mechanism of TKI resistance involved in IGF2BP3-dependent cross-talk between epigenetic modifications and metabolic reprogramming through the IGF2BP3-COX6B5 axis in NSCLC. | ||||||||||||
| Key Molecule: Insulin-like growth factor 2 mRNA-binding protein 3 (IGF2BP3) | [5] | ||||||||||||
| Metabolic Type | Mitochondrial metabolism | ||||||||||||
| Resistant Disease | Non-small cell lung carcinoma [ICD-11: 2C25.Y] | ||||||||||||
| Molecule Alteration | Expression | Up-regulation |
|||||||||||
| Differential expression of the molecule in resistant disease | |||||||||||||
| Classification of Disease | Lung cancer [ICD-11: 2C25] | ||||||||||||
| The Specified Disease | Non-small cell lung carcinoma | ||||||||||||
| The Studied Tissue | Lung tissue | ||||||||||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.38E-05 Fold-change: 6.29E-01 Z-score: 4.51E+00 |
||||||||||||
| Experimental Note | Revealed Based on the Cell Line Data | ||||||||||||
| In Vivo Model | Nude mice , with fresh tissue from patient | Mice | |||||||||||
| Experiment for Molecule Alteration |
qRT-PCR | ||||||||||||
| Experiment for Drug Resistance |
Tumor volume assay | ||||||||||||
| Mechanism Description | Furthermore, we revealed that targeting IGF2BP3 can markedly enhance the sensitivity of TKIs in NSCLC and this effect was strongly dependent on the coordinated induction of COX6B2, a key downstream target of IGF2BP3 in mitochondrial OXPHOS energy production. Overall, our study revealed a novel mechanism of TKI resistance involved in IGF2BP3-dependent cross-talk between epigenetic modifications and metabolic reprogramming through the IGF2BP3-COX6B4 axis in NSCLC. | ||||||||||||
| Key Molecule: Nuclear receptor coactivator 4 (NCOA4) | [6] | ||||||||||||
| Metabolic Type | Mitochondrial metabolism | ||||||||||||
| Resistant Disease | Non-small cell lung carcinoma [ICD-11: 2C25.Y] | ||||||||||||
| Molecule Alteration | Expression | Up-regulation |
|||||||||||
| Differential expression of the molecule in resistant disease | |||||||||||||
| Classification of Disease | Lung cancer [ICD-11: 2C25] | ||||||||||||
| The Specified Disease | Non-small cell lung carcinoma | ||||||||||||
| The Studied Tissue | Lung tissue | ||||||||||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 9.00E-02 Fold-change: 6.78E-02 Z-score: 1.70E+00 |
||||||||||||
| Experimental Note | Revealed Based on the Cell Line Data | ||||||||||||
| In Vitro Model | H1975 cells | Lung | Homo sapiens (Human) | CVCL_B0JT | |||||||||
| Experiment for Molecule Alteration |
Western blot analysis | ||||||||||||
| Experiment for Drug Resistance |
PI/Annexin V apoptosis assay | ||||||||||||
| Mechanism Description | Mechanically, Osi treatment induces an elevation of NCOA4, a key protein of ferritinophagy, which maintains the synthesis of iron-sulfur cluster (ISC) proteins of electron transport chain and OXPHOS. Additionally, active ISC protein synthesis in adaptive-resistant cells significantly increases the sensitivity to copper ions. Combining Osi with elesclomol, a copper ion ionophore, significantly increases the efficacy of Osi, with no additional toxicity. Altogether, this study reveals the mechanisms of NCOA4-mediated ferritinophagy in Osi adaptive resistance and introduces a promising new therapy of combining copper ionophores to improve its initial efficacy. | ||||||||||||
| Key Molecule: Family with sequence similarity 83 member B (FAM83B) | [6] | ||||||||||||
| Metabolic Type | Mitochondrial metabolism | ||||||||||||
| Resistant Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | ||||||||||||
| Molecule Alteration | Expression | Up-regulation |
|||||||||||
| Differential expression of the molecule in resistant disease | |||||||||||||
| Classification of Disease | Lung cancer [ICD-11: 2C25] | ||||||||||||
| The Specified Disease | Lung adenocarcinoma | ||||||||||||
| The Studied Tissue | Lung tissue | ||||||||||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 3.89E-39 Fold-change: 1.68E+00 Z-score: 1.75E+01 |
||||||||||||
| Experimental Note | Revealed Based on the Cell Line Data | ||||||||||||
| In Vitro Model | A431 cells | Skin | Homo sapiens (Human) | CVCL_0037 | |||||||||
| Experiment for Molecule Alteration |
Western blot analysis | ||||||||||||
| Experiment for Drug Resistance |
PI/Annexin V apoptosis assay | ||||||||||||
| Mechanism Description | Mechanically, Osi treatment induces an elevation of NCOA4, a key protein of ferritinophagy, which maintains the synthesis of iron-sulfur cluster (ISC) proteins of electron transport chain and OXPHOS. Additionally, active ISC protein synthesis in adaptive-resistant cells significantly increases the sensitivity to copper ions. Combining Osi with elesclomol, a copper ion ionophore, significantly increases the efficacy of Osi, with no additional toxicity. Altogether, this study reveals the mechanisms of NCOA9-mediated ferritinophagy in Osi adaptive resistance and introduces a promising new therapy of combining copper ionophores to improve its initial efficacy. | ||||||||||||
| Key Molecule: Family with sequence similarity 83 member B (FAM83B) | [6] | ||||||||||||
| Metabolic Type | Mitochondrial metabolism | ||||||||||||
| Resistant Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | ||||||||||||
| Molecule Alteration | Expression | Up-regulation |
|||||||||||
| Differential expression of the molecule in resistant disease | |||||||||||||
| Classification of Disease | Lung cancer [ICD-11: 2C25] | ||||||||||||
| The Specified Disease | Lung adenocarcinoma | ||||||||||||
| The Studied Tissue | Lung tissue | ||||||||||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 3.89E-39 Fold-change: 1.68E+00 Z-score: 1.75E+01 |
||||||||||||
| Experimental Note | Revealed Based on the Cell Line Data | ||||||||||||
| In Vitro Model | H1650 cells | Pleural effusion | Homo sapiens (Human) | CVCL_4V01 | |||||||||
| Experiment for Molecule Alteration |
Western blot analysis | ||||||||||||
| Experiment for Drug Resistance |
PI/Annexin V apoptosis assay | ||||||||||||
| Mechanism Description | Mechanically, Osi treatment induces an elevation of NCOA4, a key protein of ferritinophagy, which maintains the synthesis of iron-sulfur cluster (ISC) proteins of electron transport chain and OXPHOS. Additionally, active ISC protein synthesis in adaptive-resistant cells significantly increases the sensitivity to copper ions. Combining Osi with elesclomol, a copper ion ionophore, significantly increases the efficacy of Osi, with no additional toxicity. Altogether, this study reveals the mechanisms of NCOA8-mediated ferritinophagy in Osi adaptive resistance and introduces a promising new therapy of combining copper ionophores to improve its initial efficacy. | ||||||||||||
| Key Molecule: Family with sequence similarity 83 member B (FAM83B) | [6] | ||||||||||||
| Metabolic Type | Mitochondrial metabolism | ||||||||||||
| Resistant Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | ||||||||||||
| Molecule Alteration | Expression | Up-regulation |
|||||||||||
| Differential expression of the molecule in resistant disease | |||||||||||||
| Classification of Disease | Lung cancer [ICD-11: 2C25] | ||||||||||||
| The Specified Disease | Lung adenocarcinoma | ||||||||||||
| The Studied Tissue | Lung tissue | ||||||||||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 3.89E-39 Fold-change: 1.68E+00 Z-score: 1.75E+01 |
||||||||||||
| Experimental Note | Revealed Based on the Cell Line Data | ||||||||||||
| In Vitro Model | HCC4006 cells | Lung | Homo sapiens (Human) | CVCL_1269 | |||||||||
| Experiment for Molecule Alteration |
Western blot analysis | ||||||||||||
| Experiment for Drug Resistance |
PI/Annexin V apoptosis assay | ||||||||||||
| Mechanism Description | Mechanically, Osi treatment induces an elevation of NCOA4, a key protein of ferritinophagy, which maintains the synthesis of iron-sulfur cluster (ISC) proteins of electron transport chain and OXPHOS. Additionally, active ISC protein synthesis in adaptive-resistant cells significantly increases the sensitivity to copper ions. Combining Osi with elesclomol, a copper ion ionophore, significantly increases the efficacy of Osi, with no additional toxicity. Altogether, this study reveals the mechanisms of NCOA7-mediated ferritinophagy in Osi adaptive resistance and introduces a promising new therapy of combining copper ionophores to improve its initial efficacy. | ||||||||||||
| Key Molecule: Family with sequence similarity 83 member B (FAM83B) | [6] | ||||||||||||
| Metabolic Type | Mitochondrial metabolism | ||||||||||||
| Resistant Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | ||||||||||||
| Molecule Alteration | Expression | Up-regulation |
|||||||||||
| Differential expression of the molecule in resistant disease | |||||||||||||
| Classification of Disease | Lung cancer [ICD-11: 2C25] | ||||||||||||
| The Specified Disease | Lung adenocarcinoma | ||||||||||||
| The Studied Tissue | Lung tissue | ||||||||||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 3.89E-39 Fold-change: 1.68E+00 Z-score: 1.75E+01 |
||||||||||||
| Experimental Note | Revealed Based on the Cell Line Data | ||||||||||||
| In Vitro Model | PC-9 cells | Lung | Homo sapiens (Human) | CVCL_B260 | |||||||||
| Experiment for Molecule Alteration |
Western blot analysis | ||||||||||||
| Experiment for Drug Resistance |
PI/Annexin V apoptosis assay | ||||||||||||
| Mechanism Description | Mechanically, Osi treatment induces an elevation of NCOA4, a key protein of ferritinophagy, which maintains the synthesis of iron-sulfur cluster (ISC) proteins of electron transport chain and OXPHOS. Additionally, active ISC protein synthesis in adaptive-resistant cells significantly increases the sensitivity to copper ions. Combining Osi with elesclomol, a copper ion ionophore, significantly increases the efficacy of Osi, with no additional toxicity. Altogether, this study reveals the mechanisms of NCOA6-mediated ferritinophagy in Osi adaptive resistance and introduces a promising new therapy of combining copper ionophores to improve its initial efficacy. | ||||||||||||
| Key Molecule: Family with sequence similarity 83 member B (FAM83B) | [6] | ||||||||||||
| Metabolic Type | Mitochondrial metabolism | ||||||||||||
| Resistant Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | ||||||||||||
| Molecule Alteration | Expression | Up-regulation |
|||||||||||
| Differential expression of the molecule in resistant disease | |||||||||||||
| Classification of Disease | Lung cancer [ICD-11: 2C25] | ||||||||||||
| The Specified Disease | Lung adenocarcinoma | ||||||||||||
| The Studied Tissue | Lung tissue | ||||||||||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 3.89E-39 Fold-change: 1.68E+00 Z-score: 1.75E+01 |
||||||||||||
| Experimental Note | Revealed Based on the Cell Line Data | ||||||||||||
| In Vitro Model | HCC827 cells | Lung | Homo sapiens (Human) | CVCL_2063 | |||||||||
| Experiment for Molecule Alteration |
Western blot analysis | ||||||||||||
| Experiment for Drug Resistance |
PI/Annexin V apoptosis assay | ||||||||||||
| Mechanism Description | Mechanically, Osi treatment induces an elevation of NCOA4, a key protein of ferritinophagy, which maintains the synthesis of iron-sulfur cluster (ISC) proteins of electron transport chain and OXPHOS. Additionally, active ISC protein synthesis in adaptive-resistant cells significantly increases the sensitivity to copper ions. Combining Osi with elesclomol, a copper ion ionophore, significantly increases the efficacy of Osi, with no additional toxicity. Altogether, this study reveals the mechanisms of NCOA5-mediated ferritinophagy in Osi adaptive resistance and introduces a promising new therapy of combining copper ionophores to improve its initial efficacy. | ||||||||||||
| Key Molecule: Solute carrier family 1 member 3 (SLC1A3) | [7] | ||||||||||||
| Metabolic Type | Glutamine metabolism | ||||||||||||
| Resistant Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | ||||||||||||
| Molecule Alteration | Expression | Up-regulation |
|||||||||||
| Differential expression of the molecule in resistant disease | |||||||||||||
| Classification of Disease | Lung cancer [ICD-11: 2C25] | ||||||||||||
| The Specified Disease | Lung adenocarcinoma | ||||||||||||
| The Studied Tissue | Lung tissue | ||||||||||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.31E-19 Fold-change: 1.29E+00 Z-score: 1.00E+01 |
||||||||||||
| Experimental Note | Revealed Based on the Cell Line Data | ||||||||||||
| In Vitro Model | PC-9 cells | Lung | Homo sapiens (Human) | CVCL_B260 | |||||||||
| Experiment for Molecule Alteration |
qRT-PCR; Western blot analysis | ||||||||||||
| Experiment for Drug Resistance |
Cell viability assay | ||||||||||||
| Mechanism Description | SLC1A3 had the highest mRNA expression level in PC-9/OsmR2 compared to PC-9 cells by microarray analysis and SLC1A3 was increased by flow cytometry. In PC-9/OsmR2 cells, osimertinib sensitivity was significantly increased in combination with siSLC1A3. Because SLC1A3 functions in glutamic acid transport, osimertinib with a glutaminase inhibitor (CB-839) or an SLC1A3 inhibitor (TFB-TBOA) increased the sensitivity. | ||||||||||||
| Key Molecule: Cytochrome c oxidase subunit 6B2 (COX6B2) | [5] | ||||||||||||
| Metabolic Type | Mitochondrial metabolism | ||||||||||||
| Resistant Disease | Non-small cell lung carcinoma [ICD-11: 2C25.Y] | ||||||||||||
| Molecule Alteration | Expression | Up-regulation |
|||||||||||
| Experimental Note | Revealed Based on the Cell Line Data | ||||||||||||
| In Vivo Model | Nude mice , with fresh tissue from patient | Mice | |||||||||||
| Experiment for Molecule Alteration |
qRT-PCR | ||||||||||||
| Experiment for Drug Resistance |
Tumor volume assay | ||||||||||||
| Mechanism Description | Furthermore, we revealed that targeting IGF2BP3 can markedly enhance the sensitivity of TKIs in NSCLC and this effect was strongly dependent on the coordinated induction of COX6B2, a key downstream target of IGF2BP3 in mitochondrial OXPHOS energy production. Overall, our study revealed a novel mechanism of TKI resistance involved in IGF2BP3-dependent cross-talk between epigenetic modifications and metabolic reprogramming through the IGF2BP3-COX6B4 axis in NSCLC. | ||||||||||||
| Key Molecule: Cytochrome c oxidase subunit 6B2 (COX6B2) | [5] | ||||||||||||
| Metabolic Type | Mitochondrial metabolism | ||||||||||||
| Resistant Disease | Non-small cell lung carcinoma [ICD-11: 2C25.Y] | ||||||||||||
| Molecule Alteration | Expression | Up-regulation |
|||||||||||
| Experimental Note | Revealed Based on the Cell Line Data | ||||||||||||
| In Vivo Model | Nude mice , with PC-9/GR cell lines | Mice | |||||||||||
| Experiment for Molecule Alteration |
qRT-PCR | ||||||||||||
| Experiment for Drug Resistance |
Tumor volume assay | ||||||||||||
| Mechanism Description | Furthermore, we revealed that targeting IGF2BP3 can markedly enhance the sensitivity of TKIs in NSCLC and this effect was strongly dependent on the coordinated induction of COX6B2, a key downstream target of IGF2BP3 in mitochondrial OXPHOS energy production. Overall, our study revealed a novel mechanism of TKI resistance involved in IGF2BP3-dependent cross-talk between epigenetic modifications and metabolic reprogramming through the IGF2BP3-COX6B5 axis in NSCLC. | ||||||||||||
| Key Molecule: . | [3] | ||||||||||||
| Metabolic Type | Glucose metabolism | ||||||||||||
| Resistant Disease | Non-small cell lung carcinoma [ICD-11: 2C25.Y] | ||||||||||||
| Molecule Alteration | . | . |
|||||||||||
| Experimental Note | Revealed Based on the Cell Line Data | ||||||||||||
| Cell Pathway Regulation | MAPK signaling pathway | Activation | hsa04010 | ||||||||||
| Insulin signaling pathway | Activation | hsa04910 | |||||||||||
| mTOR signaling pathway | Activation | hsa04150 | |||||||||||
| In Vitro Model | HEK 293 cells | Kidney | Homo sapiens (Human) | CVCL_0045 | |||||||||
| Fibroblast cells | Lung | Homo sapiens (Human) | N.A. | ||||||||||
| Gefitinib-resistant NSCLC cells | Lung | Homo sapiens (Human) | N.A. | ||||||||||
| H1975 parental cells | Lung | Homo sapiens (Human) | CVCL_1511 | ||||||||||
| Experiment for Drug Resistance |
MTT assay | ||||||||||||
| Mechanism Description | We found that the combined use of EGFR-TKIs and EGCG significantly reversed the Warburg effect by suppressing glycolysis while boosting mitochondrial respiration, which was accompanied by increased cellular ROS and decreased lactate secretion. The combination effectively activated the AMPK pathway while inhibited both ERK/MAPK and AKT/mTOR pathways, leading to cell cycle arrest and apoptosis, particularly in drug-resistant NSCLC cells. | ||||||||||||
| Key Molecule: . | [3] | ||||||||||||
| Metabolic Type | Glucose metabolism | ||||||||||||
| Resistant Disease | Non-small cell lung carcinoma [ICD-11: 2C25.Y] | ||||||||||||
| Molecule Alteration | . | . |
|||||||||||
| Experimental Note | Revealed Based on the Cell Line Data | ||||||||||||
| Cell Pathway Regulation | MAPK signaling pathway | Activation | hsa04010 | ||||||||||
| Insulin signaling pathway | Activation | hsa04910 | |||||||||||
| mTOR signaling pathway | Activation | hsa04150 | |||||||||||
| In Vivo Model | NCI-H1975 xenograft-bearing mice; nude mice bearing AR cell subcutaneous xenografts | Mice | |||||||||||
| Experiment for Drug Resistance |
Tumor volume assay | ||||||||||||
| Mechanism Description | We found that the combined use of EGFR-TKIs and EGCG significantly reversed the Warburg effect by suppressing glycolysis while boosting mitochondrial respiration, which was accompanied by increased cellular ROS and decreased lactate secretion. The combination effectively activated the AMPK pathway while inhibited both ERK/MAPK and AKT/mTOR pathways, leading to cell cycle arrest and apoptosis, particularly in drug-resistant NSCLC cells. | ||||||||||||
|
|
|||||||||||||
| Key Molecule: Epidermal growth factor receptor (EGFR) | [1] | ||||||||||||
| Resistant Disease | Non-small cell lung cancer [ICD-11: 2C25.Y] | ||||||||||||
| Molecule Alteration | Missense mutation | p.C797S |
|||||||||||
| Wild Type Structure | Method: X-ray diffraction | Resolution: 2.64 Å | |||||||||||
| Mutant Type Structure | Method: X-ray diffraction | Resolution: 2.20 Å | |||||||||||
| Download The Information of Sequence | Download The Structure File | ||||||||||||
-
-
G
G
A
S
M
M
G
G
G
E
E
A
A
P
P
700
|
N
N
Q
Q
A
A
L
L
L
L
R
R
I
I
L
L
K
K
E
E
710
|
T
T
E
E
F
F
K
K
K
K
I
I
K
K
V
V
L
L
G
G
720
|
S
S
G
G
A
A
F
F
G
G
T
T
V
V
Y
Y
K
K
G
G
730
|
L
L
W
W
I
I
P
P
E
E
G
G
E
E
K
K
V
V
K
K
740
|
I
I
P
P
V
V
A
A
I
I
K
K
E
E
L
L
R
R
E
E
750
|
A
A
T
T
S
S
P
P
K
K
A
A
N
N
K
K
E
E
I
I
760
|
L
L
D
D
E
E
A
A
Y
Y
V
V
M
M
A
A
S
S
V
V
770
|
D
D
N
N
P
P
H
H
V
V
C
C
R
R
L
L
L
L
G
G
780
|
I
I
C
C
L
L
T
T
S
S
T
T
V
V
Q
Q
L
L
I
I
790
|
T
M
Q
Q
L
L
M
M
P
P
F
F
G
G
C
S
L
L
L
L
800
|
D
D
Y
Y
V
V
R
R
E
E
H
H
K
K
D
D
N
N
I
I
810
|
G
G
S
S
Q
Q
Y
Y
L
L
L
L
N
N
W
W
C
C
V
V
820
|
Q
Q
I
I
A
A
K
K
G
G
M
M
N
N
Y
Y
L
L
E
E
830
|
D
D
R
R
R
R
L
L
V
V
H
H
R
R
D
D
L
L
A
A
840
|
A
A
R
R
N
N
V
V
L
L
V
V
K
K
T
T
P
P
Q
Q
850
|
H
H
V
V
K
K
I
I
T
T
D
D
F
F
G
G
L
L
A
A
860
|
K
K
L
L
L
L
G
G
A
A
E
E
E
E
K
K
E
E
Y
Y
870
|
H
H
A
A
E
E
G
G
G
G
K
K
V
V
P
P
I
I
K
K
880
|
W
W
M
M
A
A
L
L
E
E
S
S
I
I
L
L
H
H
R
R
890
|
I
I
Y
Y
T
T
H
H
Q
Q
S
S
D
D
V
V
W
W
S
S
900
|
Y
Y
G
G
V
V
T
T
V
V
W
W
E
E
L
L
M
M
T
T
910
|
F
F
G
G
S
S
K
K
P
P
Y
Y
D
D
G
G
I
I
P
P
920
|
A
A
S
S
E
E
I
I
S
S
S
S
I
I
L
L
E
E
K
K
930
|
G
G
E
E
R
R
L
L
P
P
Q
Q
P
P
P
P
I
I
C
C
940
|
T
T
I
I
D
D
V
V
Y
Y
M
M
I
I
M
M
V
V
K
K
950
|
C
C
W
W
M
M
I
I
D
D
A
A
D
D
S
S
R
R
P
P
960
|
K
K
F
F
R
R
E
E
L
L
I
I
I
I
E
E
F
F
S
S
970
|
K
K
M
M
A
A
R
R
D
D
P
P
Q
Q
R
R
Y
Y
L
L
980
|
V
V
I
I
Q
Q
G
G
D
D
E
E
R
R
M
M
H
H
L
L
990
|
P
P
S
S
P
P
T
T
D
D
S
S
N
N
F
F
Y
Y
R
R
1000
|
A
A
L
L
M
M
D
D
E
E
E
E
D
D
M
M
D
D
D
D
1010
|
V
V
V
V
D
D
A
A
D
D
E
E
Y
Y
L
L
I
I
P
P
1020
|
Q
Q
-
Q
-
G
|
|||||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| Experiment for Molecule Alteration |
Cell-free plasma DNA assay; Next generation assay; Droplet digital PCR assay | ||||||||||||
| Experiment for Drug Resistance |
Progression-free survival assay | ||||||||||||
| Mechanism Description | Acquired EGFR C797S mediates resistance to AZD9291 in advanced non-small cell lung cancer harboring EGFR T790M. | ||||||||||||
| Key Molecule: Epidermal growth factor receptor (EGFR) | [8] | ||||||||||||
| Resistant Disease | Non-small cell lung cancer [ICD-11: 2C25.Y] | ||||||||||||
| Molecule Alteration | Missense mutation | p.T790M |
|||||||||||
| Wild Type Structure | Method: X-ray diffraction | Resolution: 3.10 Å | |||||||||||
| Mutant Type Structure | Method: X-ray diffraction | Resolution: 3.05 Å | |||||||||||
| Download The Information of Sequence | Download The Structure File | ||||||||||||
-
-
S
G
G
E
E
A
A
P
P
700
|
N
N
Q
Q
A
A
L
L
L
L
R
R
I
I
L
L
K
K
E
E
710
|
T
T
E
E
F
F
K
K
K
K
I
I
K
K
V
V
L
L
G
G
720
|
S
S
G
G
A
A
F
F
G
G
T
T
V
V
Y
Y
K
K
G
G
730
|
L
L
W
W
I
I
P
P
E
E
G
G
E
E
K
K
V
V
K
K
740
|
I
I
P
P
V
V
A
A
I
I
K
K
E
E
L
L
R
R
E
E
750
|
A
A
T
T
S
S
P
P
K
K
A
A
N
N
K
K
E
E
I
I
760
|
L
L
D
D
E
E
A
A
Y
Y
V
V
M
M
A
A
S
S
V
V
770
|
D
D
N
N
P
P
H
H
V
V
C
C
R
R
L
L
L
L
G
G
780
|
I
I
C
C
L
L
T
T
S
S
T
T
V
V
Q
Q
L
L
I
I
790
|
T
M
Q
Q
L
L
M
M
P
P
F
F
G
G
C
C
L
L
L
L
800
|
D
D
Y
Y
V
V
R
R
E
E
H
H
K
K
D
D
N
N
I
I
810
|
G
G
S
S
Q
Q
Y
Y
L
L
L
L
N
N
W
W
C
C
V
V
820
|
Q
Q
I
I
A
A
K
K
G
G
M
M
N
N
Y
Y
L
L
E
E
830
|
D
D
R
R
R
R
L
L
V
V
H
H
R
R
D
D
L
L
A
A
840
|
A
A
R
R
N
N
V
V
L
L
V
V
K
K
T
T
P
P
Q
Q
850
|
H
H
V
V
K
K
I
I
T
T
D
D
F
F
G
G
L
L
A
A
860
|
K
K
L
L
L
L
G
G
A
A
E
E
E
E
K
K
E
E
Y
Y
870
|
H
H
A
A
E
E
G
G
G
G
K
K
V
V
P
P
I
I
K
K
880
|
W
W
M
M
A
A
L
L
E
E
S
S
I
I
L
L
H
H
R
R
890
|
I
I
Y
Y
T
T
H
H
Q
Q
S
S
D
D
V
V
W
W
S
S
900
|
Y
Y
G
G
V
V
T
T
V
V
W
W
E
E
L
L
M
M
T
T
910
|
F
F
G
G
S
S
K
K
P
P
Y
Y
D
D
G
G
I
I
P
P
920
|
A
A
S
S
E
E
I
I
S
S
S
S
I
I
L
L
E
E
K
K
930
|
G
G
E
E
R
R
L
L
P
P
Q
Q
P
P
P
P
I
I
C
C
940
|
T
T
I
I
D
D
V
V
Y
Y
M
M
I
I
M
M
V
V
K
K
950
|
C
C
W
W
M
M
I
I
D
D
A
A
D
D
S
S
R
R
P
P
960
|
K
K
F
F
R
R
E
E
L
L
I
I
I
I
E
E
F
F
S
S
970
|
K
K
M
M
A
A
R
R
D
D
P
P
Q
Q
R
R
Y
Y
L
L
980
|
V
V
I
I
Q
Q
G
G
D
D
E
E
R
R
M
M
H
H
L
L
990
|
P
P
S
S
P
P
T
T
D
D
S
S
N
N
F
F
Y
Y
R
R
1000
|
A
A
L
L
M
M
D
D
E
E
E
E
D
D
M
M
D
D
D
D
1010
|
V
V
V
V
D
D
A
A
D
D
E
E
Y
Y
L
L
I
I
P
P
1020
|
Q
Q
Q
Q
G
G
|
|||||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| Experiment for Molecule Alteration |
Sanger sequencing assay; Fluorescence in situ hybridization assay; Real-time polymerase chain reaction assay; Targeted exome sequencing assay | ||||||||||||
| Experiment for Drug Resistance |
Computed tomography assay | ||||||||||||
| Mechanism Description | Acquired resistance mechanisms of AZD9291 in patients with EGFRT790M-mutant NSCLC who failed treatment with first-generation EGFR TkIs include the loss of EGFRT790M-mutant clones plus alternative pathway activation or histologic transformation and EGFR ligand-dependent activation. | ||||||||||||
| Key Molecule: Oncogenic epidermal growth factor receptor (EGFR) | [9] | ||||||||||||
| Resistant Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | ||||||||||||
| Molecule Alteration | Missense mutation | Exon 20 insertion mutations |
|||||||||||
| Experimental Note | Revealed Based on the Cell Line Data | ||||||||||||
| Cell Pathway Regulation | PI3K-Akt signaling pathway | Inhibition | hsa04151 | ||||||||||
| In Vitro Model | Ba/F3 murine cells | Bone marrow | Homo sapiens (Human) | N.A. | |||||||||
| Bosc23 cells | Fetal kidney | Homo sapiens (Human) | CVCL_4401 | ||||||||||
| Experiment for Molecule Alteration |
GeneSeq assay | ||||||||||||
| Experiment for Drug Resistance |
Cell proliferation assay; Immunoblotting assay | ||||||||||||
| Mechanism Description | Mechanisms of acquired EGFR TKI resistance of this mutant remained underreported. | ||||||||||||
| Key Molecule: Epidermal growth factor receptor (EGFR) | [2] | ||||||||||||
| Resistant Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | ||||||||||||
| Molecule Alteration | Mutation | . |
|||||||||||
| Experiment for Drug Resistance |
Statistical analysis | ||||||||||||
| Mechanism Description | EGFR-TKI Rechallenge With Another TKI may be a useful treatment option after first-line osimertinib. | ||||||||||||
|
|
|||||||||||||
| Key Molecule: Serine/threonine-protein kinase PLK1 (PLK1) | [10] | ||||||||||||
| Resistant Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | ||||||||||||
| Molecule Alteration | Expression | Down-regulation |
|||||||||||
| Experimental Note | Revealed Based on the Cell Line Data | ||||||||||||
| Cell Pathway Regulation | PLK1 regulatory signalling pathway | Regulation | N.A. | ||||||||||
| Cell cycle | Activation | hsa04110 | |||||||||||
| In Vitro Model | NCI-H1975 cells | Lung | Homo sapiens (Human) | CVCL_1511 | |||||||||
| Experiment for Molecule Alteration |
qRT-PCR | ||||||||||||
| Mechanism Description | The PLK1 inhibitors GSK 461364 and BI 2536 had synergistic effect with osimertinib. Compared with osimertinib-sensitive cells, PLK1 regulatory pathway and cell cycle pathway were significantly activated in osimertinib-resistant cells. In NSCLC patients with epidermal growth factor receptor mutations treated with osimertinib,?PLK1?mRNA levels were negatively correlated with progression free survival of patients (R= -0.62,?P<0.05), indicating that excessive activation of PLK1 in NSCLC cells may cause cell resistant to osimertinib. Further?in vitro?experiments showed that IC50?of PLK1 inhibitors BI 6727 and GSK 461364 in osimertinib-resistant cells were lower than those in sensitive ones. Compared with the mono treatment of osimertinib, PLK1 inhibitors combined with osimertinib behaved significantly stronger effect on the proliferation of osimertinib-resistant cells. | ||||||||||||
| Key Molecule: Serine/threonine-protein phosphatase 2B catalytic subunit beta isoform (PPP3CB) | [11] | ||||||||||||
| Resistant Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | ||||||||||||
| Molecule Alteration | Expression | Up-regulation |
|||||||||||
| Experimental Note | Revealed Based on the Cell Line Data | ||||||||||||
| Cell Pathway Regulation | Ca2+/calcineurin/MEK/ERK1/2 signaling pathway | Regulation | N.A. | ||||||||||
| In Vivo Model | Patient-derived EGFR-mutant lung adenocarcinoma model | Homo sapiens | |||||||||||
| Experiment for Molecule Alteration |
Western blot assay | ||||||||||||
| Mechanism Description | Here, we show that a?PPP3CB?transcript that encodes full-length catalytic subunit 2B of calcineurin accumulates in EGFR-mutant NSCLC cells with acquired resistance against different EGFR TKIs and in post-progression biopsies of NSCLC patients treated with EGFR TKIs. Neutralization of?PPP3CB?by siRNA or inactivation of calcineurin by cyclosporin A induces apoptosis in resistant cells treated with EGFR TKIs. Mechanistically, EGFR TKIs increase the cytosolic level of calcium and trigger activation of a calcineurin/MEK/ERK pathway that prevents apoptosis. Combining EGFR, calcineurin, and MEK inhibitors overcomes resistance to EGFR TKI in both in vitro and in vivo models. Our results identify PPP3CB overexpression as a new mechanism of acquired resistance to EGFR TKIs, and provide a promising therapeutic approach for NSCLC patients that progress under TKI treatment. | ||||||||||||
| Key Molecule: AKT serine/threonine kinase (AKT) | [12] | ||||||||||||
| Resistant Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | ||||||||||||
| Molecule Alteration | Phosphorylation | Up-regulation |
|||||||||||
| Experimental Note | Revealed Based on the Cell Line Data | ||||||||||||
| Cell Pathway Regulation | PI3K+Akt signaling pathway | Activation | hsa04151 | ||||||||||
| In Vitro Model | PC-9 cells | Lung | Homo sapiens (Human) | CVCL_B260 | |||||||||
| HEK293 FT cells | Kidney | Homo sapiens (Human) | CVCL_6911 | ||||||||||
| NCI-H1975 cells | Lung | Homo sapiens (Human) | CVCL_1511 | ||||||||||
| HCC827 cells | Lung | Homo sapiens (Human) | CVCL_2063 | ||||||||||
| In Vivo Model | BALB/c female nude mice model | Mus musculus | |||||||||||
| Experiment for Molecule Alteration |
Western blot assay; Immunofluorescence staining assay; Immunohistochemistry; RNA sequencing assay | ||||||||||||
| Experiment for Drug Resistance |
Cell viability assay; Colony formation assay; EdU incorporation assay; Cell apoptosis assay | ||||||||||||
| Mechanism Description | Osimertinib, a third-generation epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI), has demonstrated significant clinical benefits in the treatment of EGFR-mutated non-small cell lung cancer (NSCLC). However, inevitable acquired resistance to osimertinib limits its clinical utility, and there is a lack of effective countermeasures. Here, we established osimertinib-resistant cell lines and performed drug library screening. This screening identified ivacaftor, a cystic fibrosis transmembrane conductance regulator (CFTR) potentiator, as a synergistic enhancer of osimertinib-induced anti-tumor activity both in vitro and in vivo. Mechanistically, ivacaftor facilitated the colocalization of CFTR and PTEN on the plasma membrane to promote the function of PTEN, subsequently inhibiting the PI3K/AKT signaling pathway and suppressing tumor growth. In summary, our study suggests that activating CFTR enhances osimertinib-induced anti-tumor activity by regulating the PTEN-AKT axis. Furthermore, ivacaftor and osimertinib constitute a potential combination strategy for treating osimertinib-resistant EGFR-mutated NSCLC patients. | ||||||||||||
| Key Molecule: Phosphatase and tensin homolog (PTEN) | [12] | ||||||||||||
| Resistant Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | ||||||||||||
| Molecule Alteration | Expression | Down-regulation |
|||||||||||
| Experimental Note | Revealed Based on the Cell Line Data | ||||||||||||
| Cell Pathway Regulation | PI3K+Akt signaling pathway | Activation | hsa04151 | ||||||||||
| In Vitro Model | PC-9 cells | Lung | Homo sapiens (Human) | CVCL_B260 | |||||||||
| HEK293 FT cells | Kidney | Homo sapiens (Human) | CVCL_6911 | ||||||||||
| NCI-H1975 cells | Lung | Homo sapiens (Human) | CVCL_1511 | ||||||||||
| HCC827 cells | Lung | Homo sapiens (Human) | CVCL_2063 | ||||||||||
| In Vivo Model | BALB/c female nude mice model | Mus musculus | |||||||||||
| Experiment for Molecule Alteration |
Western blot assay; Immunofluorescence staining assay; Immunohistochemistry; RNA sequencing assay | ||||||||||||
| Experiment for Drug Resistance |
Cell viability assay; Colony formation assay; EdU incorporation assay; Cell apoptosis assay | ||||||||||||
| Mechanism Description | Osimertinib, a third-generation epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI), has demonstrated significant clinical benefits in the treatment of EGFR-mutated non-small cell lung cancer (NSCLC). However, inevitable acquired resistance to osimertinib limits its clinical utility, and there is a lack of effective countermeasures. Here, we established osimertinib-resistant cell lines and performed drug library screening. This screening identified ivacaftor, a cystic fibrosis transmembrane conductance regulator (CFTR) potentiator, as a synergistic enhancer of osimertinib-induced anti-tumor activity both in vitro and in vivo. Mechanistically, ivacaftor facilitated the colocalization of CFTR and PTEN on the plasma membrane to promote the function of PTEN, subsequently inhibiting the PI3K/AKT signaling pathway and suppressing tumor growth. In summary, our study suggests that activating CFTR enhances osimertinib-induced anti-tumor activity by regulating the PTEN-AKT axis. Furthermore, ivacaftor and osimertinib constitute a potential combination strategy for treating osimertinib-resistant EGFR-mutated NSCLC patients. | ||||||||||||
| Key Molecule: Cystic fibrosis transmembrane conductance regulator (CFTR) | [12] | ||||||||||||
| Resistant Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | ||||||||||||
| Molecule Alteration | Expression | Down-regulation |
|||||||||||
| Experimental Note | Revealed Based on the Cell Line Data | ||||||||||||
| Cell Pathway Regulation | PI3K+Akt signaling pathway | Activation | hsa04151 | ||||||||||
| In Vitro Model | PC-9 cells | Lung | Homo sapiens (Human) | CVCL_B260 | |||||||||
| HEK293 FT cells | Kidney | Homo sapiens (Human) | CVCL_6911 | ||||||||||
| NCI-H1975 cells | Lung | Homo sapiens (Human) | CVCL_1511 | ||||||||||
| HCC827 cells | Lung | Homo sapiens (Human) | CVCL_2063 | ||||||||||
| In Vivo Model | BALB/c female nude mice model | Mus musculus | |||||||||||
| Experiment for Molecule Alteration |
Western blot assay; Immunofluorescence staining assay; Immunohistochemistry; RNA sequencing assay | ||||||||||||
| Experiment for Drug Resistance |
Cell viability assay; Colony formation assay; EdU incorporation assay; Cell apoptosis assay | ||||||||||||
| Mechanism Description | Osimertinib, a third-generation epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI), has demonstrated significant clinical benefits in the treatment of EGFR-mutated non-small cell lung cancer (NSCLC). However, inevitable acquired resistance to osimertinib limits its clinical utility, and there is a lack of effective countermeasures. Here, we established osimertinib-resistant cell lines and performed drug library screening. This screening identified ivacaftor, a cystic fibrosis transmembrane conductance regulator (CFTR) potentiator, as a synergistic enhancer of osimertinib-induced anti-tumor activity both in vitro and in vivo. Mechanistically, ivacaftor facilitated the colocalization of CFTR and PTEN on the plasma membrane to promote the function of PTEN, subsequently inhibiting the PI3K/AKT signaling pathway and suppressing tumor growth. In summary, our study suggests that activating CFTR enhances osimertinib-induced anti-tumor activity by regulating the PTEN-AKT axis. Furthermore, ivacaftor and osimertinib constitute a potential combination strategy for treating osimertinib-resistant EGFR-mutated NSCLC patients. | ||||||||||||
| Key Molecule: Serine/threonine-protein phosphatase 2B catalytic subunit beta isoform (PPP3CB) | [11] | ||||||||||||
| Resistant Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | ||||||||||||
| Molecule Alteration | Expression | Down-regulation |
|||||||||||
| Experimental Note | Revealed Based on the Cell Line Data | ||||||||||||
| Cell Pathway Regulation | Ca2+/calcineurin/MEK/ERK1/2 signaling pathway | Regulation | N.A. | ||||||||||
| In Vitro Model | PC9/DR cells | N.A. | Homo sapiens (Human) | N.A. | |||||||||
| PC9/GR cells | N.A. | Homo sapiens (Human) | N.A. | ||||||||||
| PC9/OR cells | N.A. | Homo sapiens (Human) | N.A. | ||||||||||
| Experiment for Molecule Alteration |
Western blot assay | ||||||||||||
| Experiment for Drug Resistance |
MTS assay; Flow cytometric assay; Colony formation assay | ||||||||||||
| Mechanism Description | Here, we show that a?PPP3CB?transcript that encodes full-length catalytic subunit 2B of calcineurin accumulates in EGFR-mutant NSCLC cells with acquired resistance against different EGFR TKIs and in post-progression biopsies of NSCLC patients treated with EGFR TKIs. Neutralization of?PPP3CB?by siRNA or inactivation of calcineurin by cyclosporin A induces apoptosis in resistant cells treated with EGFR TKIs. Mechanistically, EGFR TKIs increase the cytosolic level of calcium and trigger activation of a calcineurin/MEK/ERK pathway that prevents apoptosis. Combining EGFR, calcineurin, and MEK inhibitors overcomes resistance to EGFR TKI in both in vitro and in vivo models. Our results identify PPP3CB overexpression as a new mechanism of acquired resistance to EGFR TKIs, and provide a promising therapeutic approach for NSCLC patients that progress under TKI treatment. | ||||||||||||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Oncogenic epidermal growth factor receptor (EGFR) | [9] | |||
| Sensitive Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | |||
| Molecule Alteration | Missense mutation | Exon 20 insertion mutations |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | PI3K-Akt signaling pathway | Inhibition | hsa04151 | |
| In Vitro Model | Ba/F3 murine cells | Bone marrow | Homo sapiens (Human) | N.A. |
| Bosc23 cells | Fetal kidney | Homo sapiens (Human) | CVCL_4401 | |
| Experiment for Molecule Alteration |
GeneSeq assay | |||
| Experiment for Drug Resistance |
Cell proliferation assay; Immunoblotting assay | |||
| Mechanism Description | Mechanisms of acquired EGFR TKI resistance of this mutant remained underreported. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Receptor tyrosine-protein kinase erbB-2 (ERBB2) | [4] | |||
| Resistant Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Molecule Alteration | Duplication | p.Y772_A775 (c.2314_2325)/p.A775_G776insYVMA |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 |
| Experiment for Molecule Alteration |
Sanger cDNA sequencing assay | |||
| Experiment for Drug Resistance |
CCK-8 assay | |||
| Key Molecule: Receptor tyrosine-protein kinase erbB-2 (ERBB2) | [4] | |||
| Resistant Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Molecule Alteration | Duplication | p.G778_P780 (c.2332_2340)/p.780_Y781insGSP |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 |
| Experiment for Molecule Alteration |
Sanger cDNA sequencing assay | |||
| Experiment for Drug Resistance |
CCK-8 assay | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Receptor tyrosine-protein kinase erbB-2 (ERBB2) | [4] | |||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Molecule Alteration | Complex-indel | p.G776_776delinsVC (c.2326_2328delinsGTATGT) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 |
| Experiment for Molecule Alteration |
Sanger cDNA sequencing assay | |||
| Experiment for Drug Resistance |
CCK-8 assay | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
