Drug Information
Drug (ID: DG00096) and It's Reported Resistant Information
| Name |
Quizartinib
|
||||
|---|---|---|---|---|---|
| Synonyms |
Quizartinib
Click to Show/Hide
|
||||
| Indication |
In total 2 Indication(s)
|
||||
| Structure |
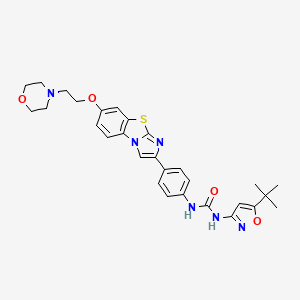
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Clinically Reported Resistance for This Drug
(1 diseases)
Disease(s) with Resistance Information Discovered by Cell Line Test for This Drug
(1 diseases)
[4]
|
||||
| Target | Fms-like tyrosine kinase 3 (FLT-3) | FLT3_HUMAN | [1] | ||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
C29H32N6O4S
|
||||
| IsoSMILES |
CC(C)(C)C1=CC(=NO1)NC(=O)NC2=CC=C(C=C2)C3=CN4C5=C(C=C(C=C5)OCCN6CCOCC6)SC4=N3
|
||||
| InChI |
1S/C29H32N6O4S/c1-29(2,3)25-17-26(33-39-25)32-27(36)30-20-6-4-19(5-7-20)22-18-35-23-9-8-21(16-24(23)40-28(35)31-22)38-15-12-34-10-13-37-14-11-34/h4-9,16-18H,10-15H2,1-3H3,(H2,30,32,33,36)
|
||||
| InChIKey |
CVWXJKQAOSCOAB-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-02: Benign/in-situ/malignant neoplasm
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Receptor-type tyrosine-protein kinase FLT3 (FLT3) | [1], [2] | |||
| Resistant Disease | Acute myeloid leukemia [ICD-11: 2A60.0] | |||
| Molecule Alteration | Missense mutation | p.F691L |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vivo Model | A retrospective survey in conducting clinical studies | Homo sapiens | ||
| Experiment for Molecule Alteration |
Subsequent bidirectional sequencing assay | |||
| Experiment for Drug Resistance |
Vi-cell XR automated cell viability analysis | |||
| Mechanism Description | Overall, these data support a primarily structural mechanism for AC220 resistance mediated by mutations at F691, D835 and Y842, although further studies are necessary for definitive confirmation. We speculate that the ability to retain inhibitory activity against activation loop substitutions at D835 and Y842 will require a type I FLT3 kinase inhibitor capable of effectively binding to the active, DFG-in conformation of the kinase. | |||
| Key Molecule: Receptor-type tyrosine-protein kinase FLT3 (FLT3) | [1] | |||
| Resistant Disease | Acute myeloid leukemia [ICD-11: 2A60.0] | |||
| Molecule Alteration | Missense mutation | p.D835V |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vivo Model | A retrospective survey in conducting clinical studies | Homo sapiens | ||
| Experiment for Molecule Alteration |
Subsequent bidirectional sequencing assay | |||
| Experiment for Drug Resistance |
Vi-cell XR automated cell viability analysis | |||
| Mechanism Description | Overall, these data support a primarily structural mechanism for AC220 resistance mediated by mutations at F691, D835 and Y842, although further studies are necessary for definitive confirmation. We speculate that the ability to retain inhibitory activity against activation loop substitutions at D835 and Y842 will require a type I FLT3 kinase inhibitor capable of effectively binding to the active, DFG-in conformation of the kinase. | |||
| Key Molecule: Receptor-type tyrosine-protein kinase FLT3 (FLT3) | [1] | |||
| Resistant Disease | Acute myeloid leukemia [ICD-11: 2A60.0] | |||
| Molecule Alteration | Missense mutation | p.D835F |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vivo Model | A retrospective survey in conducting clinical studies | Homo sapiens | ||
| Experiment for Molecule Alteration |
Subsequent bidirectional sequencing assay | |||
| Experiment for Drug Resistance |
Vi-cell XR automated cell viability analysis | |||
| Mechanism Description | Overall, these data support a primarily structural mechanism for AC220 resistance mediated by mutations at F691, D835 and Y842, although further studies are necessary for definitive confirmation. We speculate that the ability to retain inhibitory activity against activation loop substitutions at D835 and Y842 will require a type I FLT3 kinase inhibitor capable of effectively binding to the active, DFG-in conformation of the kinase. | |||
| Key Molecule: Receptor-type tyrosine-protein kinase FLT3 (FLT3) | [1], [3] | |||
| Resistant Disease | Acute myeloid leukemia [ICD-11: 2A60.0] | |||
| Molecule Alteration | Missense mutation | p.D835Y |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vivo Model | A retrospective survey in conducting clinical studies | Homo sapiens | ||
| Experiment for Molecule Alteration |
Subsequent bidirectional sequencing assay | |||
| Experiment for Drug Resistance |
Vi-cell XR automated cell viability analysis | |||
| Mechanism Description | Overall, these data support a primarily structural mechanism for AC220 resistance mediated by mutations at F691, D835 and Y842, although further studies are necessary for definitive confirmation. We speculate that the ability to retain inhibitory activity against activation loop substitutions at D835 and Y842 will require a type I FLT3 kinase inhibitor capable of effectively binding to the active, DFG-in conformation of the kinase. | |||
| Key Molecule: Receptor-type tyrosine-protein kinase FLT3 (FLT3) | [5] | |||
| Resistant Disease | Acute myeloid leukemia [ICD-11: 2A60.0] | |||
| Molecule Alteration | Missense mutation | p.F691 |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Experiment for Molecule Alteration |
FISH assay; Comparative genomic hybridization array assay; Single nucleotide polymorphism array assay; PCR; Next-generation sequencing assay; Sanger sequencing assay | |||
| Experiment for Drug Resistance |
Southern blot analysis; Spectral karyotyping assay | |||
| Mechanism Description | FLT3-mutated patients treated with AC220, sorafenib, or sunitinib commonly relapse with new, resistant FLT3 D835 or F691 mutations within the preexisting FLT3-ITD allele, and one third of the patients who discontinued therapy for any reason also have acquired such mutations. | |||
| Key Molecule: Receptor-type tyrosine-protein kinase FLT3 (FLT3) | [5] | |||
| Resistant Disease | Acute myeloid leukemia [ICD-11: 2A60.0] | |||
| Molecule Alteration | Missense mutation | p.D835 |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Experiment for Molecule Alteration |
FISH assay; Comparative genomic hybridization array assay; Single nucleotide polymorphism array assay; PCR; Next-generation sequencing assay; Sanger sequencing assay | |||
| Experiment for Drug Resistance |
Southern blot analysis; Spectral karyotyping assay | |||
| Mechanism Description | FLT3-mutated patients treated with AC220, sorafenib, or sunitinib commonly relapse with new, resistant FLT3 D835 or F691 mutations within the preexisting FLT3-ITD allele, and one third of the patients who discontinued therapy for any reason also have acquired such mutations. | |||
| Key Molecule: Receptor-type tyrosine-protein kinase FLT3 (FLT3) | [4] | |||
| Resistant Disease | Acute myeloid leukemia [ICD-11: 2A60.0] | |||
| Molecule Alteration | Mutation | p.Y842C |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | MOLM-14 cells | Peripheral blood | Homo sapiens (Human) | CVCL_7916 |
| Experiment for Drug Resistance |
MTS assay | |||
| Mechanism Description | The quizartinib IC50 values of cells with Y842C mutation was 106, the quizartinib IC50 value of cells without mutation was 1. | |||
| Key Molecule: Receptor-type tyrosine-protein kinase FLT3 (FLT3) | [4] | |||
| Resistant Disease | Acute myeloid leukemia [ICD-11: 2A60.0] | |||
| Molecule Alteration | Missense mutation | p.D835Y |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | MOLM-14 cells | Peripheral blood | Homo sapiens (Human) | CVCL_7916 |
| Experiment for Drug Resistance |
MTS assay | |||
| Mechanism Description | The quizartinib IC50 values of cells with D835Y mutation was 183, the quizartinib IC50 value of cells without mutation was 1. | |||
| Key Molecule: Receptor-type tyrosine-protein kinase FLT3 (FLT3) | [4] | |||
| Resistant Disease | Acute myeloid leukemia [ICD-11: 2A60.0] | |||
| Molecule Alteration | Missense mutation | p.D835V |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | MOLM-14 cells | Peripheral blood | Homo sapiens (Human) | CVCL_7916 |
| Experiment for Drug Resistance |
MTS assay | |||
| Mechanism Description | The quizartinib IC50 values of cells with D835V mutation was 563, the quizartinib IC50 value of cells without mutation was 1. | |||
| Key Molecule: Receptor-type tyrosine-protein kinase FLT3 (FLT3) | [4] | |||
| Resistant Disease | Acute myeloid leukemia [ICD-11: 2A60.0] | |||
| Molecule Alteration | Missense mutation | p.D835I |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | MOLM-14 cells | Peripheral blood | Homo sapiens (Human) | CVCL_7916 |
| Experiment for Drug Resistance |
MTS assay | |||
| Mechanism Description | The quizartinib IC50 values of cells with D835I mutation was 718, the quizartinib IC50 value of cells without mutation was 1. | |||
| Key Molecule: Receptor-type tyrosine-protein kinase FLT3 (FLT3) | [4] | |||
| Resistant Disease | Acute myeloid leukemia [ICD-11: 2A60.0] | |||
| Molecule Alteration | Missense mutation | p.D835F |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | MOLM-14 cells | Peripheral blood | Homo sapiens (Human) | CVCL_7916 |
| Experiment for Drug Resistance |
MTS assay | |||
| Mechanism Description | The quizartinib IC50 values of cells with D835F mutation was 1474, the quizartinib IC50 value of cells without mutation was 1. | |||
| Key Molecule: Receptor-type tyrosine-protein kinase FLT3 (FLT3) | [4] | |||
| Resistant Disease | Acute myeloid leukemia [ICD-11: 2A60.0] | |||
| Molecule Alteration | Frameshift mutation | p.D835Del |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | MOLM-14 cells | Peripheral blood | Homo sapiens (Human) | CVCL_7916 |
| Experiment for Drug Resistance |
MTS assay | |||
| Mechanism Description | The quizartinib IC50 values of cells with D835Del mutation was 320, the quizartinib IC50 value of cells without mutation was 1. | |||
| Key Molecule: Receptor-type tyrosine-protein kinase FLT3 (FLT3) | [4] | |||
| Resistant Disease | Acute myeloid leukemia [ICD-11: 2A60.0] | |||
| Molecule Alteration | Mutation | p.F691L |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | MOLM-14 cells | Peripheral blood | Homo sapiens (Human) | CVCL_7916 |
| Experiment for Drug Resistance |
MTS assay | |||
| Mechanism Description | The gatekeeper mutation F691L confers resistance to specific FLT3 inhibitors such as quizartinib. | |||
| Key Molecule: Receptor-type tyrosine-protein kinase FLT3 (FLT3) | [4] | |||
| Resistant Disease | Acute myeloid leukemia [ICD-11: 2A60.0] | |||
| Molecule Alteration | Missense mutation | p.F691L |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | MOLM-14 cells | Peripheral blood | Homo sapiens (Human) | CVCL_7916 |
| MV4-11 cells | Peripheral blood | Homo sapiens (Human) | CVCL_0064 | |
| Experiment for Drug Resistance |
MTS assay | |||
| Mechanism Description | The multiple mutations that can confer resistance to quizartinib and pexidartinib. The gatekeeper mutation F691L was the most common mutation in all protocols involving quizartinib; it was rather frequent even with pexidartinib alone. | |||
| Key Molecule: Receptor-type tyrosine-protein kinase FLT3 (FLT3) | [4] | |||
| Resistant Disease | Acute myeloid leukemia [ICD-11: 2A60.0] | |||
| Molecule Alteration | Missense mutation | p.F691L |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | MOLM-14 cells | Peripheral blood | Homo sapiens (Human) | CVCL_7916 |
| MV4-11 cells | Peripheral blood | Homo sapiens (Human) | CVCL_0064 | |
| Experiment for Drug Resistance |
MTS assay | |||
| Mechanism Description | The multiple mutations that can confer resistance to quizartinib and pexidartinib. The gatekeeper mutation F691L was the most common mutation in all protocols involving quizartinib; it was rather frequent even with pexidartinib alone. | |||
|
|
||||
| Key Molecule: Catenin beta-1 (CTNNB1) | [6] | |||
| Resistant Disease | Acute myeloid leukemia [ICD-11: 2A60.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | SPHK1/S1P signaling pathway | Regulation | N.A. | |
| In Vitro Model | MOLM-13 cells | Peripheral blood | Homo sapiens (Human) | CVCL_2119 |
| MV-4-11 cells | Peripheral blood | Homo sapiens (Human) | CVCL_0064 | |
| In Vivo Model | AML mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Lipidomics profiling assay; RNA sequencing assay; qRT-PCR; ELISA assay | |||
| Experiment for Drug Resistance |
Western blot assay; Immunofluorescence assay | |||
| Mechanism Description | We demonstrate that long-term sorafenib or quizartinib treatment upregulates SPHK1/sphingosine-1-phosphate (S1P) signaling, which in turn positively modulates beta-catenin signaling to counteract TKI-mediated suppression of FLT3-mutated AML cells via the S1P2 receptor. Genetic or pharmacological inhibition of SPHK1 potently enhanced the TKI-mediated inhibition of proliferation and apoptosis induction in FLT3-mutated AML cells in vitro. SPHK1 knockdown enhanced sorafenib efficacy and improved survival of AML-xenografted mice. Mechanistically, targeting the SPHK1/S1P/S1P2 signaling synergizes with FLT3 TKIs to inhibit beta-catenin activity by activating the protein phosphatase 2 A (PP2A)-glycogen synthase kinase 3beta (GSK3beta) pathway. | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
