Molecule Information
General Information of the Molecule (ID: Mol01262)
| Name |
Growth arrest specific 5 (GAS5)
,Homo sapiens
|
||||
|---|---|---|---|---|---|
| Synonyms |
GAS5
Click to Show/Hide
|
||||
| Molecule Type |
LncRNA
|
||||
| Gene Name |
HANR
|
||||
| Gene ID | |||||
| Location |
chr1:173858559-173868882[-]
|
||||
| Ensembl ID | |||||
| HGNC ID | |||||
| Click to Show/Hide the Complete Species Lineage | |||||
Type(s) of Resistant Mechanism of This Molecule
Drug Resistance Data Categorized by Drug
Approved Drug(s)
11 drug(s) in total
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Lung adenocarcinoma [ICD-11: 2C25.0] | [1] | |||
| Sensitive Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | |||
| Sensitive Drug | Gefitinib | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Lung cancer [ICD-11: 2C25] | |||
| The Specified Disease | Lung adenocarcinoma | |||
| The Studied Tissue | Lung | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 4.78E-12 Fold-change: 6.09E-01 Z-score: 7.06E+00 |
|||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell invasion | Inhibition | hsa05200 | ||
| Cell migration | Inhibition | hsa04670 | ||
| Cell proliferation | Inhibition | hsa05200 | ||
| EGFR signaling pathway | Inhibition | hsa01521 | ||
| In Vitro Model | H1975 cells | Lung | Homo sapiens (Human) | CVCL_1511 |
| A549 cells | Lung | Homo sapiens (Human) | CVCL_0023 | |
| H1299 cells | Lung | Homo sapiens (Human) | CVCL_0060 | |
| HCC827 cells | Lung | Homo sapiens (Human) | CVCL_2063 | |
| 16HBE cells | Lung | Homo sapiens (Human) | CVCL_0112 | |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
MTT assay; EdU assay | |||
| Mechanism Description | GAS5 was significantly downregulated in lung adenocarcinoma tissues compared with the paired adjacent non-tumorous tissue samples. Furthermore, lower GAS5 expression levels were associated with larger tumor sizes, poor tumor differentiation, and advanced pathological stages. However, GAS5 was almost equally expressed between benign tumors compared with the adjacent normal tissues. GAS5 was also overexpressed in EGFR-TkI sensitive cell lines compared with the resistant cell line. Using MTT, EdU incorporation, and colony formation assays, we showed that GAS5-expressing A549 cells displayed an elevated level of cell death. In addition to its pro-apoptotic effect in the A549 cell line, GAS5 overexpression also suppressed the growth of A549-derived tumors in nude mice treated with gefitinib. GAS5 overexpression was inversely correlated with the expression of the EGFR pathway and IGF-1R proteins. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Prostate cancer [ICD-11: 2C82.0] | [2] | |||
| Sensitive Disease | Prostate cancer [ICD-11: 2C82.0] | |||
| Sensitive Drug | Temsirolimus | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Prostate cancer [ICD-11: 2C82] | |||
| The Specified Disease | Prostate adenocarcinoma | |||
| The Studied Tissue | Prostate | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.54E-18 Fold-change: 5.36E-01 Z-score: 9.11E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell proliferation | Inhibition | hsa05200 | |
| In Vitro Model | DU-145 cells | Prostate | Homo sapiens (Human) | CVCL_0105 |
| LNCaP cells | Prostate | Homo sapiens (Human) | CVCL_0395 | |
| PC3 cells | Prostate | Homo sapiens (Human) | CVCL_0035 | |
| 22RV1 cells | Prostate | Homo sapiens (Human) | CVCL_1045 | |
| PNT2C2 cells | Prostate | Homo sapiens (Human) | CVCL_4889 | |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
GAS5 assay; MTS assay | |||
| Mechanism Description | First generation mTORC1, combined mTORC1/mTORC2 and dual PI3k/mTOR inhibitors all increased cellular GAS5 levels and inhibited culture growth in androgen-dependent (LNCaP) and androgen-sensitive (22Rv1) cell lines, but not in androgen-independent (PC-3 and DU 145) cell lines. The latter exhibited low endogenous GAS5 expression, and GAS5 silencing in LNCaP and 22Rv1 cells decreased the sensitivity to mTOR inhibitors, whereas transfection of GAS5 LncRNA sensitized PC-3 and DU 145 cells to these agents. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Mantle cell lymphoma [ICD-11: 2A85.0] | [13] | |||
| Resistant Disease | Mantle cell lymphoma [ICD-11: 2A85.0] | |||
| Resistant Drug | Temsirolimus | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell proliferation | Activation | hsa05200 | ||
| mTOR signaling pathway | Regulation | N.A. | ||
| In Vitro Model | Z138 cells | Peripheral blood | Homo sapiens (Human) | CVCL_B077 |
| Jeko-1 cells | Blood | Homo sapiens (Human) | CVCL_1865 | |
| Experiment for Molecule Alteration |
RT-PCR | |||
| Experiment for Drug Resistance |
Nigrosin exclusion analysis | |||
| Mechanism Description | Small interfering RNAs (sirRNAs) targeting GAS5 protect the cell viability and proliferation of jeko-1 and z-138 cells from the inhibitory effects of mTOR inhibitors result in rapamycin resistance. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Bladder urothelial carcinoma [ICD-11: 2C94.2] | [3] | |||
| Resistant Disease | Bladder urothelial carcinoma [ICD-11: 2C94.2] | |||
| Resistant Drug | Doxorubicin | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Bladder cancer [ICD-11: 2C94] | |||
| The Specified Disease | Bladder urothelial carcinoma | |||
| The Studied Tissue | Bladder | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.49E-03 Fold-change: 3.89E-01 Z-score: 3.36E+00 |
|||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | J82 cells | Bladder | Homo sapiens (Human) | CVCL_0359 |
| T24 cells | Bladder | Homo sapiens (Human) | CVCL_0554 | |
| T24/DOX cells | Bladder | Homo sapiens (Human) | CVCL_0554 | |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
MTT assay; Dual-color flow cytometric method; Annexin V-FITC apoptosis assay | |||
| Mechanism Description | Long noncoding RNA GAS5 inhibits malignant proliferation and chemotherapy resistance to doxorubicin in bladder transitional cell carcinoma. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Pancreatic cancer [ICD-11: 2C10.3] | [5] | |||
| Resistant Disease | Pancreatic cancer [ICD-11: 2C10.3] | |||
| Resistant Drug | Fluorouracil | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Pancreatic cancer [ICD-11: 2C10] | |||
| The Specified Disease | Pancreatic adenocarcinoma | |||
| The Studied Tissue | Pancreas | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 6.05E-15 Fold-change: -8.27E-01 Z-score: -8.25E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Hippo signaling pathway | Inhibition | hsa04390 | |
| In Vitro Model | SW1990 cells | Pancreas | Homo sapiens (Human) | CVCL_1723 |
| 5-FU cells | Colon | Homo sapiens (Human) | CVCL_1846 | |
| PATU8988 | Pancreas | Homo sapiens (Human) | CVCL_1847 | |
| PATU8988 cells | Pancreas | Homo sapiens (Human) | CVCL_1846 | |
| SW1990/GEM cells | Pancreas | Homo sapiens (Human) | CVCL_ZW98 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | GAS5 regualtes Hippo signaling pathway via miR181c-5p to antagonize the development of multidrug resistance in pancreatic cancer cells. GAS5 regulated chemoresistance and Hippo pathway of pancreatic cancer cells via miR181c-5p/Hippo. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Cervical cancer [ICD-11: 2C77.0] | [6] | |||
| Resistant Disease | Cervical cancer [ICD-11: 2C77.0] | |||
| Resistant Drug | Cisplatin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Cervical cancer [ICD-11: 2C77] | |||
| The Specified Disease | Cervical & endocervical cancer | |||
| The Studied Tissue | Cervix Uteri | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 2.12E-02 Fold-change: -8.47E-01 Z-score: -2.67E+00 |
|||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | PI3K/AKT signaling pathway | Activation | hsa04151 | |
| In Vitro Model | Hela cells | Cervix uteri | Homo sapiens (Human) | CVCL_0030 |
| Siha cells | Cervix uteri | Homo sapiens (Human) | CVCL_0032 | |
| Caski cells | Uterus | Homo sapiens (Human) | CVCL_1100 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | The low level of GAS5 can down-regulate PTEN by interacting with miR21 because PTEN is one of the genes in the PI3k/Akt/mTOR pathway that can be regulated by GAS5 negatively. The low expression of PTEN activates the PI3k/Akt pathway. | |||
| Disease Class: Cervical cancer [ICD-11: 2C77.0] | [7] | |||
| Resistant Disease | Cervical cancer [ICD-11: 2C77.0] | |||
| Resistant Drug | Cisplatin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell invasion | Activation | hsa05200 | ||
| Cell migration | Activation | hsa04670 | ||
| STAT3 signaling pathway | Activation | hsa04550 | ||
| In Vitro Model | Hela cells | Cervix uteri | Homo sapiens (Human) | CVCL_0030 |
| Siha cells | Cervix uteri | Homo sapiens (Human) | CVCL_0032 | |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
MTT assay; Flow cytometry assay; Colony formation assay | |||
| Mechanism Description | Down-regulation of LncRNA GAS5 strengthen cisplatin-induced apoptosis in cervical cancer by regulating STAT3 signaling via miR-21. | |||
| Disease Class: Non-small cell lung cancer [ICD-11: 2C25.Y] | [8] | |||
| Resistant Disease | Non-small cell lung cancer [ICD-11: 2C25.Y] | |||
| Resistant Drug | Cisplatin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | miR21/PTEN signaling pathway | Regulation | N.A. | |
| In Vitro Model | A549 cells | Lung | Homo sapiens (Human) | CVCL_0023 |
| H460 cells | Lung | Homo sapiens (Human) | CVCL_0459 | |
| H1299 cells | Lung | Homo sapiens (Human) | CVCL_0060 | |
| Sk-MES-1 cells | Lung | Homo sapiens (Human) | CVCL_0630 | |
| NCI-H358 cells | Lung | Homo sapiens (Human) | CVCL_1559 | |
| 16HBE cells | Lung | Homo sapiens (Human) | CVCL_0112 | |
| H157 cells | Lung | Homo sapiens (Human) | CVCL_2458 | |
| Experiment for Molecule Alteration |
RT-PCR | |||
| Experiment for Drug Resistance |
MTT assay; Soft agar assay | |||
| Mechanism Description | GAS5 could compete with PTEN for miR21 binding, GAS5 downregulation can induce trastuzumab resistance of breast cancer. | |||
| Disease Class: Non-small cell lung cancer [ICD-11: 2C25.Y] | [8] | |||
| Resistant Disease | Non-small cell lung cancer [ICD-11: 2C25.Y] | |||
| Resistant Drug | Cisplatin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | AKT signaling pathway | Regulation | N.A. | |
| In Vitro Model | A549 cells | Lung | Homo sapiens (Human) | CVCL_0023 |
| H460 cells | Lung | Homo sapiens (Human) | CVCL_0459 | |
| H1299 cells | Lung | Homo sapiens (Human) | CVCL_0060 | |
| Sk-MES-1 cells | Lung | Homo sapiens (Human) | CVCL_0630 | |
| NCI-H358 cells | Lung | Homo sapiens (Human) | CVCL_1559 | |
| 16HBE cells | Lung | Homo sapiens (Human) | CVCL_0112 | |
| H157 cells | Lung | Homo sapiens (Human) | CVCL_2458 | |
| Experiment for Molecule Alteration |
qPCR | |||
| Experiment for Drug Resistance |
MTT assay; Soft agar assay | |||
| Mechanism Description | GAS5 could compete with PTEN for miR21 binding, GAS5 downregulation can induce trastuzumab resistance of breast cancer By negatively regulating the intracellular levels of PI3k, PTEN exerts a suppressive effect on tumor through AkT pathway. GAS5 regulated NSCLC chemo-sensitivity to DDP-based therapy through PTEN pathway. | |||
| Disease Class: Non-small cell lung cancer [ICD-11: 2C25.Y] | [9] | |||
| Resistant Disease | Non-small cell lung cancer [ICD-11: 2C25.Y] | |||
| Resistant Drug | Cisplatin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| In Vitro Model | A549 cells | Lung | Homo sapiens (Human) | CVCL_0023 |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | GAS5 downregulation is associated with cisplatin resistance in NSCLC. GAS5 can inhibit autophagy and therefore enhance cisplatin sensitivity in NSCLC cells. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Epithelial ovarian cancer [ICD-11: 2B5D.0] | [10] | |||
| Sensitive Disease | Epithelial ovarian cancer [ICD-11: 2B5D.0] | |||
| Sensitive Drug | Cisplatin | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell formation | Inhibition | hsa05200 | ||
| Cell invasion | Inhibition | hsa05200 | ||
| MAPK signaling pathway | Regulation | N.A. | ||
| In Vitro Model | HEY cells | Ovary | Homo sapiens (Human) | CVCL_0297 |
| SkOV3 cells | Ovary | Homo sapiens (Human) | CVCL_0532 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
RT-qPCR | |||
| Experiment for Drug Resistance |
CCK8 assay; Flow cytometry assay | |||
| Mechanism Description | GAS5 might regulate PARP1 expression by recruiting the transcription factor E2F4 to its promoter, and then affect the MAPk pathway activity and enhance sensitivity to DDP of OC both in vitro and in vivo. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Prostate cancer [ICD-11: 2C82.0] | [11] | |||
| Resistant Disease | Prostate cancer [ICD-11: 2C82.0] | |||
| Resistant Drug | Docetaxel | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| In Vitro Model | PC3 cells | Prostate | Homo sapiens (Human) | CVCL_0035 |
| 22RV1 cells | Prostate | Homo sapiens (Human) | CVCL_1045 | |
| Experiment for Molecule Alteration |
RT-PCR | |||
| Experiment for Drug Resistance |
Fluorescence microscopy test apoptosis assay | |||
| Mechanism Description | Transient expression of GAS5 enhances apoptosis and decreases the survival of 22Rv1 cells, forced variation of GAS5 gene expression can modulate cellular responses to various apoptotic stimuli, including a range of chemotherapeutic drugs. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Prostate cancer [ICD-11: 2C82.0] | [2] | |||
| Sensitive Disease | Prostate cancer [ICD-11: 2C82.0] | |||
| Sensitive Drug | Everolimus | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell proliferation | Inhibition | hsa05200 | |
| In Vitro Model | DU-145 cells | Prostate | Homo sapiens (Human) | CVCL_0105 |
| LNCaP cells | Prostate | Homo sapiens (Human) | CVCL_0395 | |
| PC3 cells | Prostate | Homo sapiens (Human) | CVCL_0035 | |
| 22RV1 cells | Prostate | Homo sapiens (Human) | CVCL_1045 | |
| PNT2C2 cells | Prostate | Homo sapiens (Human) | CVCL_4889 | |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
GAS5 assay; MTS assay | |||
| Mechanism Description | First generation mTORC1, combined mTORC1/mTORC2 and dual PI3k/mTOR inhibitors all increased cellular GAS5 levels and inhibited culture growth in androgen-dependent (LNCaP) and androgen-sensitive (22Rv1) cell lines, but not in androgen-independent (PC-3 and DU 145) cell lines. The latter exhibited low endogenous GAS5 expression, and GAS5 silencing in LNCaP and 22Rv1 cells decreased the sensitivity to mTOR inhibitors, whereas transfection of GAS5 LncRNA sensitized PC-3 and DU 145 cells to these agents. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Pancreatic cancer [ICD-11: 2C10.3] | [12] | |||
| Sensitive Disease | Pancreatic cancer [ICD-11: 2C10.3] | |||
| Sensitive Drug | Gemcitabine | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell invasion | Inhibition | hsa05200 | |
| Cell migration | Inhibition | hsa04670 | ||
| Cell proliferation | Inhibition | hsa05200 | ||
| miR221/SOCS3 signaling pathway | Regulation | N.A. | ||
| In Vitro Model | BxPC-3 cells | Pancreas | Homo sapiens (Human) | CVCL_0186 |
| PANC-1 cells | Pancreas | Homo sapiens (Human) | CVCL_0480 | |
| Capan-2 cells | Pancreas | Homo sapiens (Human) | CVCL_0026 | |
| AsPC-1 cells | Pancreas | Homo sapiens (Human) | CVCL_0152 | |
| SW1990 cells | Pancreas | Homo sapiens (Human) | CVCL_1723 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis; RT-qPCR | |||
| Experiment for Drug Resistance |
CCK8 assay; Flow cytometry assay | |||
| Mechanism Description | Overexpression of GAS5 inhibits proliferation, migration, and chemotherapy resistance by suppressing the emt and tumor stem cell-like properties. LncRNA GAS5 functioned as a competing endogenous RNA for miR-221, and it suppressed cell growth, metastasis, and gemcitabine resistance in PC by regulating the miR-221/SOCS3 pathway mediating EMT and tumor stem cell self-renewal. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Prostate cancer [ICD-11: 2C82.0] | [11] | |||
| Resistant Disease | Prostate cancer [ICD-11: 2C82.0] | |||
| Resistant Drug | Mitoxantrone | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| In Vitro Model | PC3 cells | Prostate | Homo sapiens (Human) | CVCL_0035 |
| 22RV1 cells | Prostate | Homo sapiens (Human) | CVCL_1045 | |
| Experiment for Molecule Alteration |
RT-PCR | |||
| Experiment for Drug Resistance |
Fluorescence microscopy test apoptosis assay | |||
| Mechanism Description | Transient expression of GAS5 enhances apoptosis and decreases the survival of 22Rv1 cells, forced variation of GAS5 gene expression can modulate cellular responses to various apoptotic stimuli, including a range of chemotherapeutic drugs. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Mantle cell lymphoma [ICD-11: 2A85.0] | [13] | |||
| Resistant Disease | Mantle cell lymphoma [ICD-11: 2A85.0] | |||
| Resistant Drug | Sirolimus | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell proliferation | Activation | hsa05200 | ||
| mTOR signaling pathway | Regulation | N.A. | ||
| In Vitro Model | Z138 cells | Peripheral blood | Homo sapiens (Human) | CVCL_B077 |
| Jeko-1 cells | Blood | Homo sapiens (Human) | CVCL_1865 | |
| Experiment for Molecule Alteration |
RT-PCR | |||
| Experiment for Drug Resistance |
Nigrosin exclusion analysis | |||
| Mechanism Description | Small interfering RNAs (sirRNAs) targeting GAS5 protect the cell viability and proliferation of jeko-1 and z-138 cells from the inhibitory effects of mTOR inhibitors result in rapamycin resistance. | |||
| Disease Class: Mantle cell lymphoma [ICD-11: 2A85.0] | [13] | |||
| Resistant Disease | Mantle cell lymphoma [ICD-11: 2A85.0] | |||
| Resistant Drug | Sirolimus | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell proliferation | Activation | hsa05200 | ||
| mTOR signaling pathway | Regulation | N.A. | ||
| In Vitro Model | Z138 cells | Peripheral blood | Homo sapiens (Human) | CVCL_B077 |
| Jeko-1 cells | Blood | Homo sapiens (Human) | CVCL_1865 | |
| Experiment for Molecule Alteration |
RT-PCR | |||
| Experiment for Drug Resistance |
Nigrosin exclusion analysis | |||
| Mechanism Description | Small interfering RNAs (sirRNAs) targeting GAS5 protect the cell viability and proliferation of jeko-1 and z-138 cells from the inhibitory effects of mTOR inhibitors result in rapamycin resistance. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Prostate cancer [ICD-11: 2C82.0] | [2] | |||
| Sensitive Disease | Prostate cancer [ICD-11: 2C82.0] | |||
| Sensitive Drug | Sirolimus | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell proliferation | Inhibition | hsa05200 | |
| In Vitro Model | DU-145 cells | Prostate | Homo sapiens (Human) | CVCL_0105 |
| LNCaP cells | Prostate | Homo sapiens (Human) | CVCL_0395 | |
| PC3 cells | Prostate | Homo sapiens (Human) | CVCL_0035 | |
| 22RV1 cells | Prostate | Homo sapiens (Human) | CVCL_1045 | |
| PNT2C2 cells | Prostate | Homo sapiens (Human) | CVCL_4889 | |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
GAS5 assay; MTS assay | |||
| Mechanism Description | First generation mTORC1, combined mTORC1/mTORC2 and dual PI3k/mTOR inhibitors all increased cellular GAS5 levels and inhibited culture growth in androgen-dependent (LNCaP) and androgen-sensitive (22Rv1) cell lines, but not in androgen-independent (PC-3 and DU 145) cell lines. The latter exhibited low endogenous GAS5 expression, and GAS5 silencing in LNCaP and 22Rv1 cells decreased the sensitivity to mTOR inhibitors, whereas transfection of GAS5 LncRNA sensitized PC-3 and DU 145 cells to these agents. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: HER2 positive breast cancer [ICD-11: 2C60.8] | [14] | |||
| Resistant Disease | HER2 positive breast cancer [ICD-11: 2C60.8] | |||
| Resistant Drug | Trastuzumab | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell proliferation | Activation | hsa05200 | |
| mTOR signaling pathway | Activation | hsa04150 | ||
| In Vitro Model | SkBR3 cells | Breast | Homo sapiens (Human) | CVCL_0033 |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Down-regulation of LncRNA GAS5 causes trastuzumab resistance in breast cancer.Expression of the LncRNA GAS5 was decreased in trastuzumab-resistant SkBR-3/Tr cells and in breast cancer tissue from trastuzumab-treated patients. GAS5 suppresses cancer proliferation by acting as a molecular sponge for miR-21, leading to the de-repression of phosphatase and tensin homologs (PTEN), the endogenous target of miR-21. Moreover, mTOR activation associated with reduced GAS5 expression was required to suppress PTEN. This work identifies GAS5 as a novel prognostic marker and candidate drug target for HER2-positive breast cancer. | |||
Investigative Drug(s)
5 drug(s) in total
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Melanoma [ICD-11: 2C30.0] | [4] | |||
| Sensitive Disease | Melanoma [ICD-11: 2C30.0] | |||
| Sensitive Drug | Orthocresol | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Melanoma [ICD-11: 2C30] | |||
| The Specified Disease | Skin cutaneous melanoma | |||
| The Studied Tissue | Skin | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 3.29E-02 Fold-change: 1.53E-01 Z-score: 2.14E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell proliferation | Inhibition | hsa05200 | ||
| In Vitro Model | A375 cells | Skin | Homo sapiens (Human) | CVCL_0132 |
| A431 cells | Skin | Homo sapiens (Human) | CVCL_0037 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
MTT assay; Flow cytometry assay | |||
| Mechanism Description | 2-O-Methylmagnolol upregulates the long non-coding RNA, GAS5, and enhances apoptosis in skin cancer cells. Overexpression of LncRNA GAS5 inhibited cell proliferation and promoted cell apoptosis in skin cancer cells. | |||
| Disease Class: Skin squamous cell carcinoma [ICD-11: 2C31.2] | [4] | |||
| Sensitive Disease | Skin squamous cell carcinoma [ICD-11: 2C31.2] | |||
| Sensitive Drug | Orthocresol | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell proliferation | Inhibition | hsa05200 | ||
| In Vitro Model | A375 cells | Skin | Homo sapiens (Human) | CVCL_0132 |
| A431 cells | Skin | Homo sapiens (Human) | CVCL_0037 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
MTT assay; Flow cytometry assay | |||
| Mechanism Description | 2-O-Methylmagnolol upregulates the long non-coding RNA, GAS5, and enhances apoptosis in skin cancer cells. Overexpression of LncRNA GAS5 inhibited cell proliferation and promoted cell apoptosis in skin cancer cells. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Prostate cancer [ICD-11: 2C82.0] | [15] | |||
| Resistant Disease | Prostate cancer [ICD-11: 2C82.0] | |||
| Resistant Drug | Alpha-solanine | |||
| Molecule Alteration | Down-regulation | Expression |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | GAS5/miR-18a signaling pathway | Activation | hsa05206 | |
| In Vitro Model | RWPE-1 cells | Prostate | Homo sapiens (Human) | CVCL_3791 |
| DU145 cells | Prostate | Homo sapiens (Human) | CVCL_0105 | |
| Experiment for Molecule Alteration |
Overexpression assay | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | Long noncoding RNA GAS5 modulates alpha-Solanine-induced radiosensitivity by negatively regulating miR-18a in human prostate cancer cells. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Hepatic fibrosis [ICD-11: DB93.0] | [15] | |||
| Resistant Disease | Hepatic fibrosis [ICD-11: DB93.0] | |||
| Resistant Drug | Carbon tetrachloride | |||
| Molecule Alteration | Down-regulation | Interaction |
||
| Experimental Note | Discovered Using In-vivo Testing Model | |||
| In Vitro Model | HSC cells | N.A. | N.A. | N.A. |
| In Vivo Model | Rat hepatic fibrosis model | Rattus norvegicus | ||
| Experiment for Molecule Alteration |
qRT-PCR; Western bloting analysis; Dual luciferase assay; RNA pull down assay | |||
| Mechanism Description | LncRNA GAS5/miR-23a may bring molecular targets for hepatic fibrosis therapy.LncRNA GAS5 restrains CCl4-induced hepatic fibrosis by targeting miR-23a through the PTEN/PI3K/Akt signaling pathway. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Cardiac microvascular endothelial cells injury [ICD-11: BE2Z.Y] | [15] | |||
| Resistant Disease | Cardiac microvascular endothelial cells injury [ICD-11: BE2Z.Y] | |||
| Resistant Drug | Homocysteine | |||
| Molecule Alteration | Down-regulation | Interaction |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | ECs | N.A. | Drosophila melanogaster (Fruit fly) | CVCL_Z893 |
| CMECs | N.A. | N.A. | N.A. | |
| Experiment for Molecule Alteration |
Overexpression assay | |||
| Experiment for Drug Resistance |
MTT assay; EdU assay; TUNEL staining assay; Caspase-3 activity detection; Dual luciferase reporter gene assay | |||
| Mechanism Description | Long-chain noncoding RNA GAS5 mediates oxidative stress in cardiac microvascular endothelial cells injury. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Prostate cancer [ICD-11: 2C82.0] | [11] | |||
| Resistant Disease | Prostate cancer [ICD-11: 2C82.0] | |||
| Resistant Drug | Nutlin-3 | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| In Vitro Model | PC3 cells | Prostate | Homo sapiens (Human) | CVCL_0035 |
| 22RV1 cells | Prostate | Homo sapiens (Human) | CVCL_1045 | |
| Experiment for Molecule Alteration |
RT-PCR | |||
| Experiment for Drug Resistance |
Fluorescence microscopy test apoptosis assay | |||
| Mechanism Description | Transient expression of GAS5 enhances apoptosis and decreases the survival of 22Rv1 cells, forced variation of GAS5 gene expression can modulate cellular responses to various apoptotic stimuli, including a range of chemotherapeutic drugs. | |||
Clinical Trial Drug(s)
2 drug(s) in total
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Prostate cancer [ICD-11: 2C82.0] | [2] | |||
| Sensitive Disease | Prostate cancer [ICD-11: 2C82.0] | |||
| Sensitive Drug | Dactolisib | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell proliferation | Inhibition | hsa05200 | |
| In Vitro Model | DU-145 cells | Prostate | Homo sapiens (Human) | CVCL_0105 |
| LNCaP cells | Prostate | Homo sapiens (Human) | CVCL_0395 | |
| PC3 cells | Prostate | Homo sapiens (Human) | CVCL_0035 | |
| 22RV1 cells | Prostate | Homo sapiens (Human) | CVCL_1045 | |
| PNT2C2 cells | Prostate | Homo sapiens (Human) | CVCL_4889 | |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
GAS5 assay; MTS assay | |||
| Mechanism Description | First generation mTORC1, combined mTORC1/mTORC2 and dual PI3k/mTOR inhibitors all increased cellular GAS5 levels and inhibited culture growth in androgen-dependent (LNCaP) and androgen-sensitive (22Rv1) cell lines, but not in androgen-independent (PC-3 and DU 145) cell lines. The latter exhibited low endogenous GAS5 expression, and GAS5 silencing in LNCaP and 22Rv1 cells decreased the sensitivity to mTOR inhibitors, whereas transfection of GAS5 LncRNA sensitized PC-3 and DU 145 cells to these agents. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Prostate cancer [ICD-11: 2C82.0] | [2] | |||
| Sensitive Disease | Prostate cancer [ICD-11: 2C82.0] | |||
| Sensitive Drug | AZD-8055 | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell proliferation | Inhibition | hsa05200 | |
| In Vitro Model | DU-145 cells | Prostate | Homo sapiens (Human) | CVCL_0105 |
| LNCaP cells | Prostate | Homo sapiens (Human) | CVCL_0395 | |
| PC3 cells | Prostate | Homo sapiens (Human) | CVCL_0035 | |
| 22RV1 cells | Prostate | Homo sapiens (Human) | CVCL_1045 | |
| PNT2C2 cells | Prostate | Homo sapiens (Human) | CVCL_4889 | |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
GAS5 assay; MTS assay | |||
| Mechanism Description | First generation mTORC1, combined mTORC1/mTORC2 and dual PI3k/mTOR inhibitors all increased cellular GAS5 levels and inhibited culture growth in androgen-dependent (LNCaP) and androgen-sensitive (22Rv1) cell lines, but not in androgen-independent (PC-3 and DU 145) cell lines. The latter exhibited low endogenous GAS5 expression, and GAS5 silencing in LNCaP and 22Rv1 cells decreased the sensitivity to mTOR inhibitors, whereas transfection of GAS5 LncRNA sensitized PC-3 and DU 145 cells to these agents. | |||
Discontinued Drug(s)
1 drug(s) in total
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Lung adenocarcinoma [ICD-11: 2C25.0] | [15] | |||
| Resistant Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | |||
| Resistant Drug | Urethane | |||
| Molecule Alteration | Down-regulation | Interaction |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | 16HBE cells | Lung | Homo sapiens (Human) | CVCL_0112 |
| In Vivo Model | BALB/c nude mice model | Mus musculus | ||
| Experiment for Molecule Alteration |
Overexpression assay | |||
| Experiment for Drug Resistance |
EdU assay | |||
| Mechanism Description | Low Long Noncoding RNA Growth Arrest-Specific Transcript 5 Expression in the Exosomes of Lung Cancer Cells Promotes Tumor Angiogenesis. | |||
Disease- and Tissue-specific Abundances of This Molecule
ICD Disease Classification 02

| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Pancreas | |
| The Specified Disease | Pancreatic adenocarcinoma | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.85E-20; Fold-change: 4.28E-02 | |
|
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |
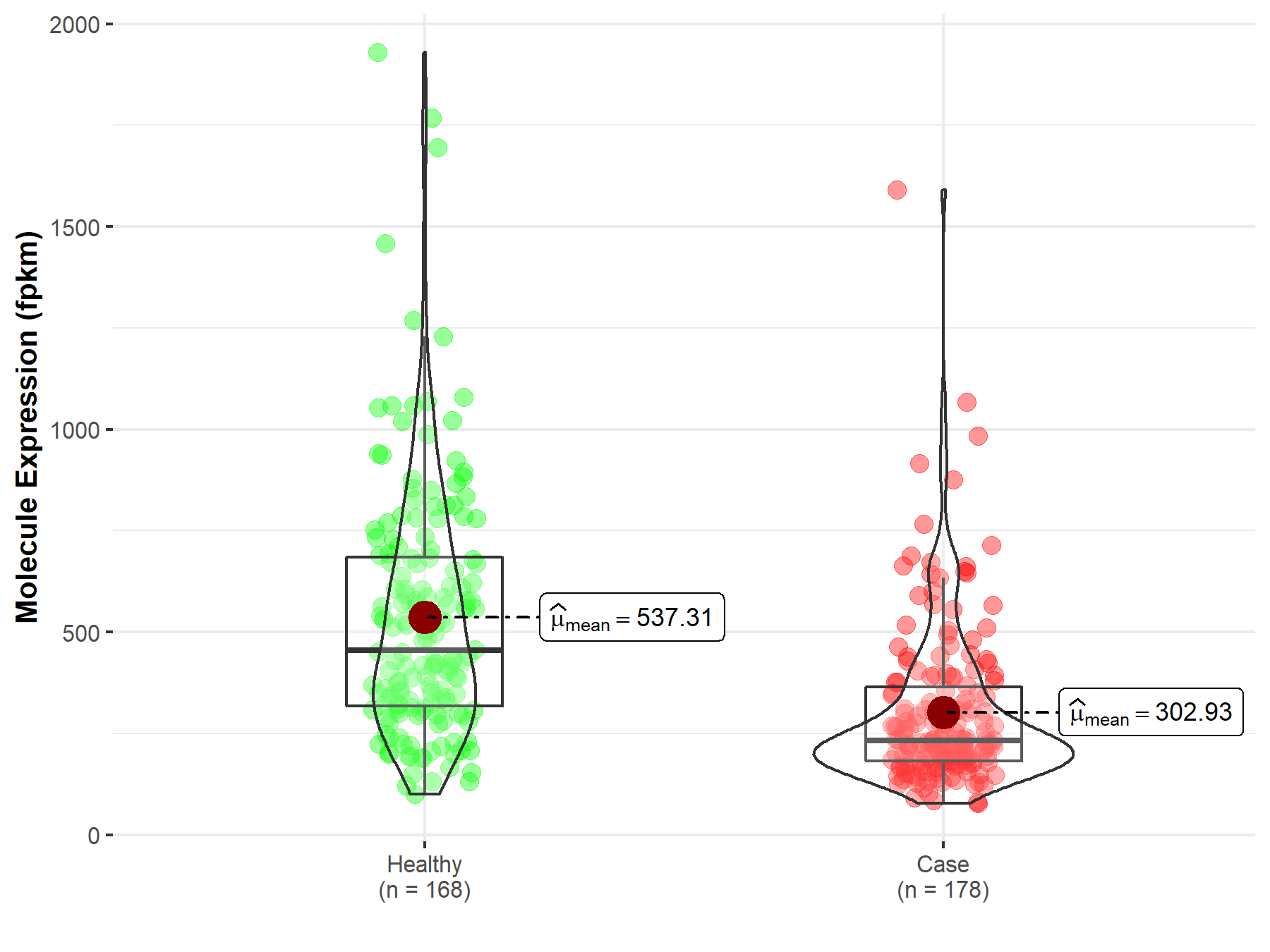
|
Click to View the Clearer Original Diagram |
| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Lung | |
| The Specified Disease | Lung adenocarcinoma | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.08E-07; Fold-change: -1.65E-02 | |
|
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |
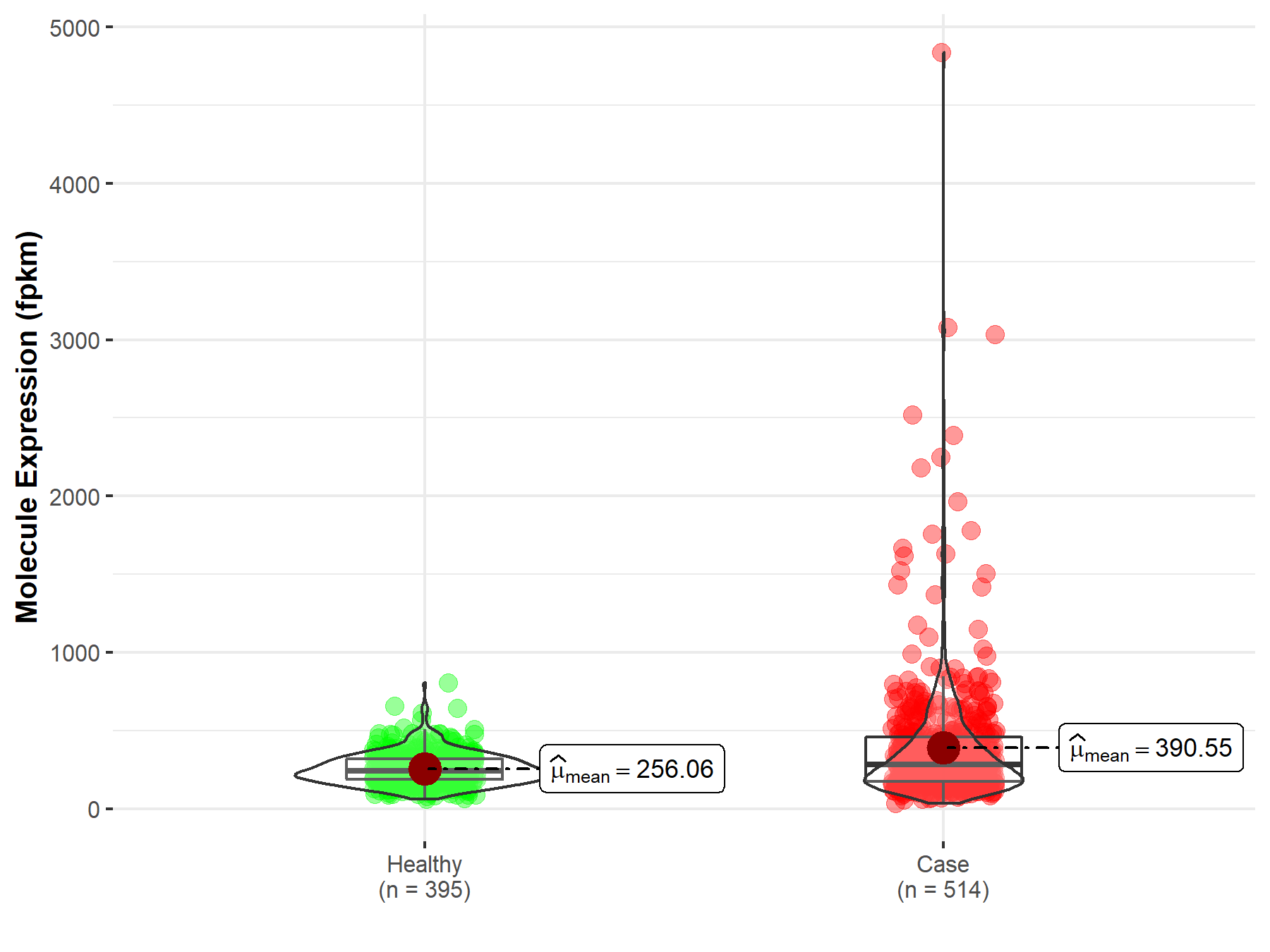
|
Click to View the Clearer Original Diagram |
| The Studied Tissue | Lung | |
| The Specified Disease | Lung squamous cell carcinoma | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.80E-04; Fold-change: -1.04E-02 | |
|
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |
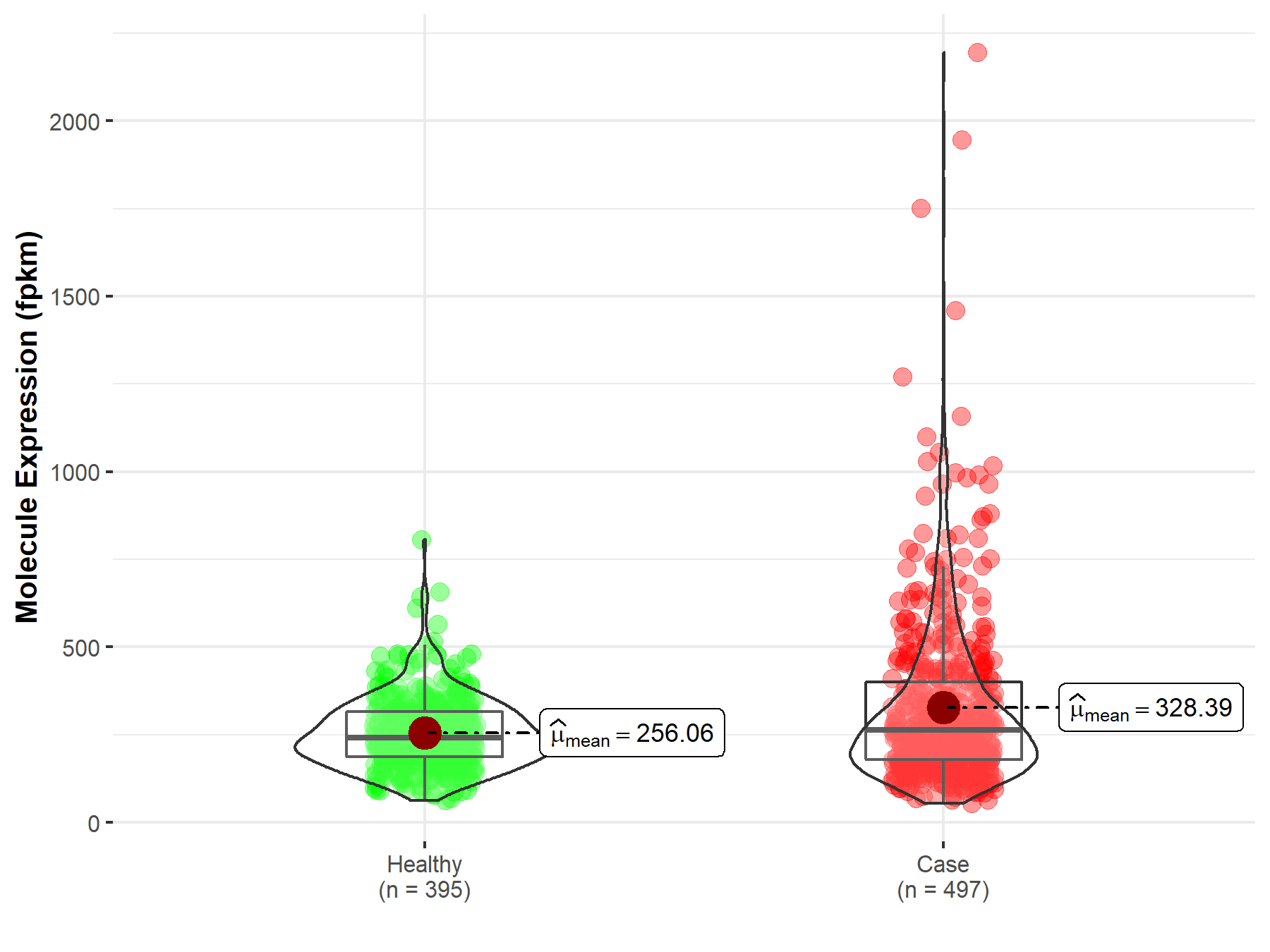
|
Click to View the Clearer Original Diagram |
| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Skin | |
| The Specified Disease | Skin cutaneous melanoma | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 3.82E-06; Fold-change: 1.17E-02 | |
|
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |
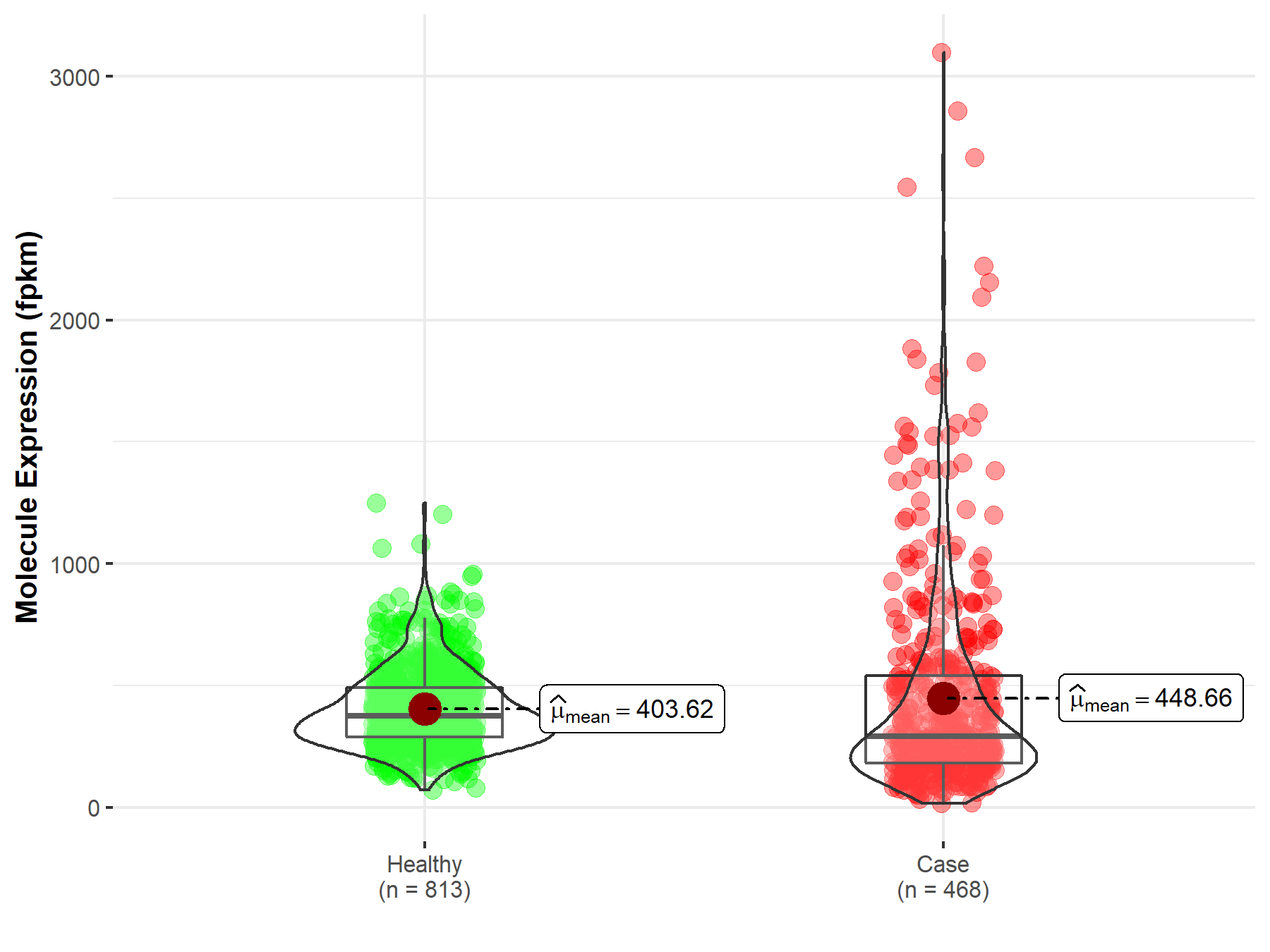
|
Click to View the Clearer Original Diagram |
| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Cervix uteri | |
| The Specified Disease | Cervical and endocervical cancer | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.40E-04; Fold-change: 4.91E-02 | |
|
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |
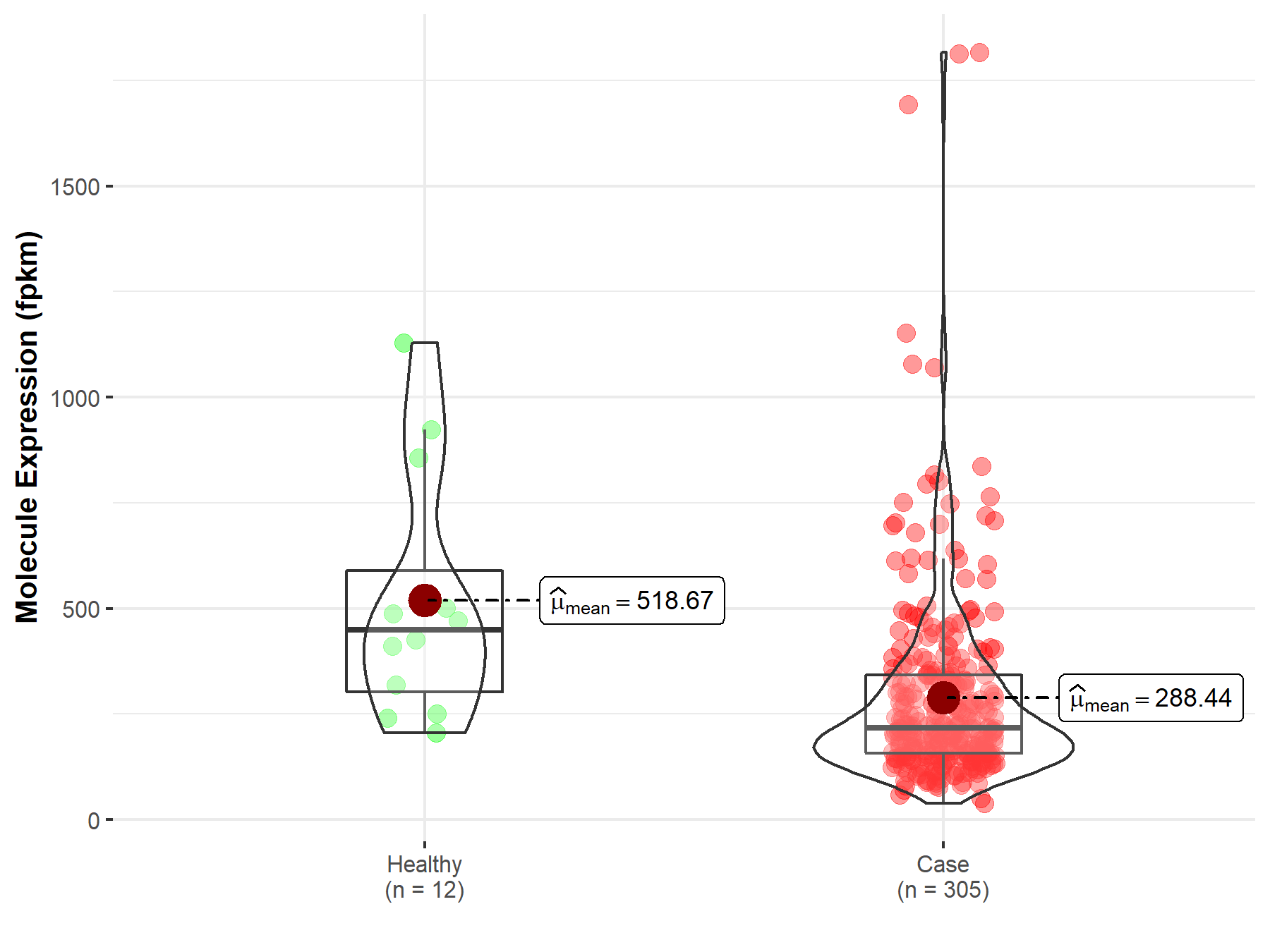
|
Click to View the Clearer Original Diagram |
| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Prostate | |
| The Specified Disease | Prostate adenocarcinoma | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 4.67E-11; Fold-change: -2.27E-02 | |
|
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |
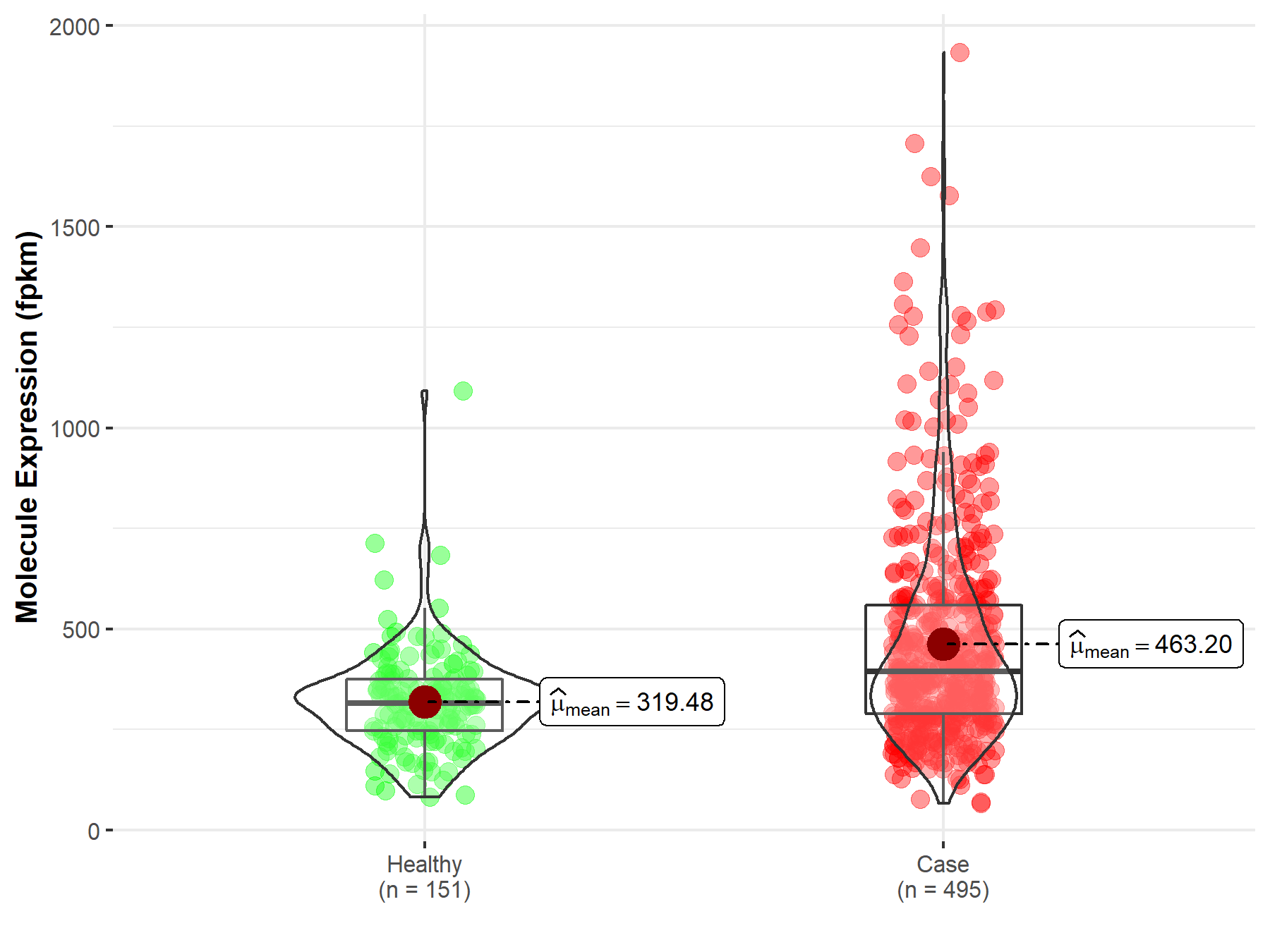
|
Click to View the Clearer Original Diagram |
| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Bladder | |
| The Specified Disease | Bladder urothelial carcinoma | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 2.10E-01; Fold-change: -1.18E-02 | |
|
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |
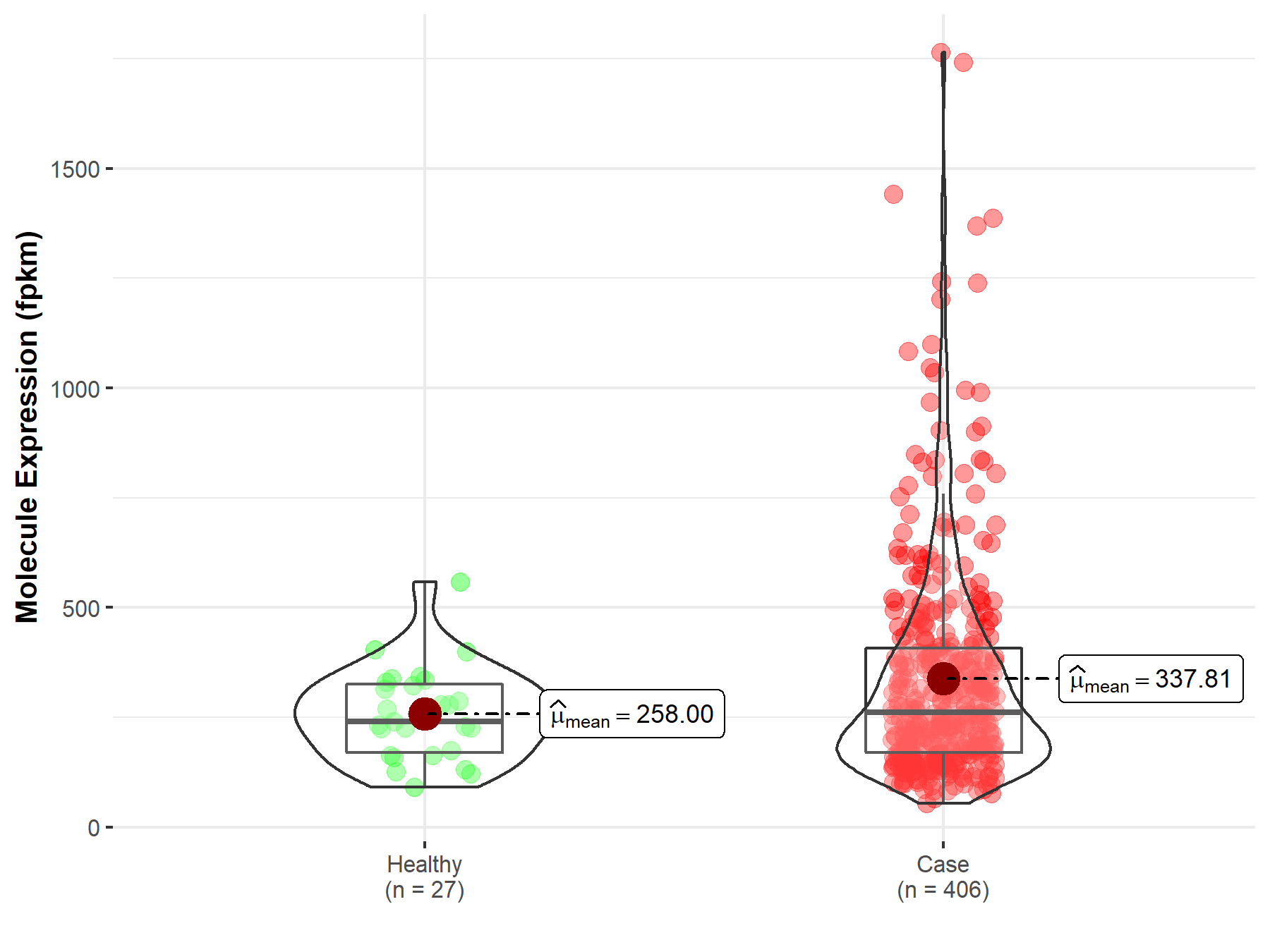
|
Click to View the Clearer Original Diagram |
Tissue-specific Molecule Abundances in Healthy Individuals

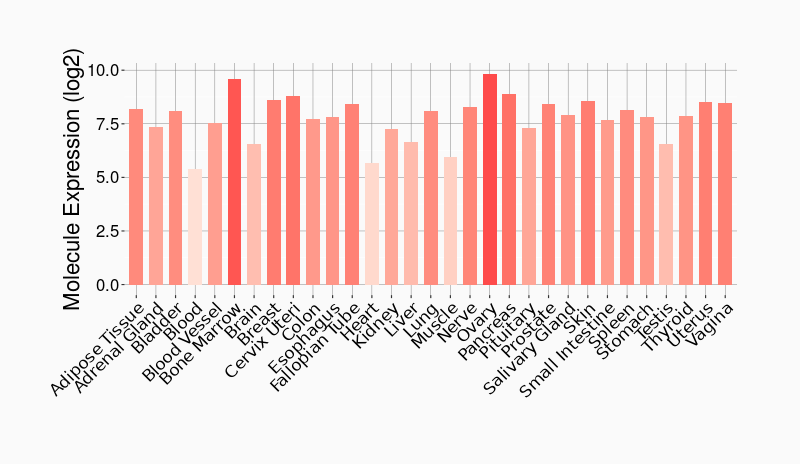
|
||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
