Drug Information
Drug (ID: DG00395) and It's Reported Resistant Information
| Name |
Orthocresol
|
||||
|---|---|---|---|---|---|
| Synonyms |
O-cresol; 2-Methylphenol; 95-48-7; Orthocresol; 2-hydroxytoluene; Phenol, 2-methyl-; 2-Cresol; o-methylphenol; o-Cresylic acid; o-Oxytoluene; o-Toluol; o-Hydroxytoluene; o-Methylphenylol; 1-Hydroxy-2-methylbenzene; ortho-cresol; o-Kresol; 2-methyl phenol; Cresol, ortho-; Cresol, o-; o-Kresol [German]; 2-Hydroxy-1-methylbenzene; Cresylic acid; 2-methyl-phenol; Cresol, o-isomer; hydroxy toluene; Phenol, methyl-; UNII-YW84DH5I7U; NSC 23076; Cresol mixture of isomers; 1-Methyl-2-hydroxybenzene; YW84DH5I7U; CHEBI:28054; MFCD00002226; TOLUENE,2-HYDROXY (ORTHO-CRESOL); o-Cresol [UN2076] [Poison, Corrosive]; DSSTox_CID_1808; Cresol mixture of isomers;Methylphenol;Tricresol; DSSTox_RID_76341; WLN: QR B1; DSSTox_GSID_21808; CAS-95-48-7; Orthocresol [NF]; Cresols are organic compounds which are methylphenols. They are a widely occurring natural and manufactured group of aromatic organic compounds, which are categorized as phenols.; CCRIS 646; FEMA No. 3480; HSDB 1813; EINECS 202-423-8; ortho cresol; Methyl phenol; 2-methyiphenol; AI3-00137; JZ0; O-Cresol,(S); Carvacrol derivative, 9; o-Cresol, >=99%; bmse000433; UN 2076 (Related); EC 202-423-8; 2-Methylphenol (o-cresol); ortho-cresol,2-methylphenol; SCHEMBL16002; MLS002454426; o-Cresol, analytical standard; BIDD:ER0677; CHEMBL46931; 3C7H8O; DTXSID8021808; FEMA 3480; BDBM248166; HMS2268O24; LABOTEST-BB LTBB001400; ZINC901022; o-Cresol, for synthesis, 99.3%; 2-Methylphenol, analytical standard; NSC23076; NSC36809; Tox21_202305; Tox21_300021; NSC-23076; NSC-36809; STL194295; o-Cresol, ReagentPlus(R), >=99%; AKOS000119021; AS00217; MCULE-4124485112; NCGC00091534-01; NCGC00091534-02; NCGC00091534-03; NCGC00091534-04; NCGC00254140-01; NCGC00259854-01; o-Cresol, SAJ first grade, >=97.0%; SMR001252248; 2-Methylphenol 100 microg/mL in Methanol; FT-0656046; Z3651; 7520-EP2277867A2; 7520-EP2280003A2; 7520-EP2292227A2; 7520-EP2295426A1; 7520-EP2295427A1; 7520-EP2295435A1; 7520-EP2298751A2; 7520-EP2302015A1; 7520-EP2305683A1; 7520-EP2308861A1; 7520-EP2311839A1; 7520-EP2314584A1; 7520-EP2314589A1; 7520-EP2316470A2; 7520-EP2316832A1; 7520-EP2316833A1; 7520-EP2316837A1; C01542; 19034-EP2311811A1; 19034-EP2314576A1; 28670-EP2279750A1; 28670-EP2284146A2; 28670-EP2284147A2; 28670-EP2284165A1; 28670-EP2287159A1; 28670-EP2305673A1; 28670-EP2308838A1; 28670-EP2308865A1; 28670-EP2308877A1; 28670-EP2311815A1; 28670-EP2311834A1; 28670-EP2314579A1; 28670-EP2372017A1; 45193-EP2311821A1; 93911-EP2269977A2; 93911-EP2305625A1; 1-Hydroxyl 2-Methyl Benzene, 2-Hydroxyl Toluene; Q312708; J-006098; J-523819; F0001-2271; UNII-3JYG22FD73 component QWVGKYWNOKOFNN-UHFFFAOYSA-N; UNII-GF3CGH8D7Z component QWVGKYWNOKOFNN-UHFFFAOYSA-N
Click to Show/Hide
|
||||
| Structure |
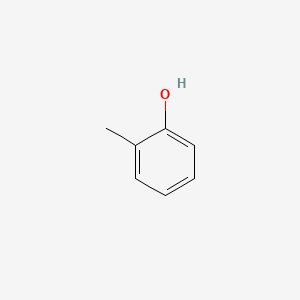
|
||||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
C7H8O
|
||||
| IsoSMILES |
CC1=CC=CC=C1O
|
||||
| InChI |
1S/C7H8O/c1-6-4-2-3-5-7(6)8/h2-5,8H,1H3
|
||||
| InChIKey |
QWVGKYWNOKOFNN-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-02: Benign/in-situ/malignant neoplasm
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Growth arrest specific 5 (GAS5) | [1] | |||
| Sensitive Disease | Melanoma [ICD-11: 2C30.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Melanoma [ICD-11: 2C30] | |||
| The Specified Disease | Skin cutaneous melanoma | |||
| The Studied Tissue | Skin | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 3.29E-02 Fold-change: 1.53E-01 Z-score: 2.14E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell proliferation | Inhibition | hsa05200 | ||
| In Vitro Model | A375 cells | Skin | Homo sapiens (Human) | CVCL_0132 |
| A431 cells | Skin | Homo sapiens (Human) | CVCL_0037 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
MTT assay; Flow cytometry assay | |||
| Mechanism Description | 2-O-Methylmagnolol upregulates the long non-coding RNA, GAS5, and enhances apoptosis in skin cancer cells. Overexpression of LncRNA GAS5 inhibited cell proliferation and promoted cell apoptosis in skin cancer cells. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Growth arrest specific 5 (GAS5) | [1] | |||
| Sensitive Disease | Skin squamous cell carcinoma [ICD-11: 2C31.2] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell proliferation | Inhibition | hsa05200 | ||
| In Vitro Model | A375 cells | Skin | Homo sapiens (Human) | CVCL_0132 |
| A431 cells | Skin | Homo sapiens (Human) | CVCL_0037 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
MTT assay; Flow cytometry assay | |||
| Mechanism Description | 2-O-Methylmagnolol upregulates the long non-coding RNA, GAS5, and enhances apoptosis in skin cancer cells. Overexpression of LncRNA GAS5 inhibited cell proliferation and promoted cell apoptosis in skin cancer cells. | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
