Molecule Information
General Information of the Molecule (ID: Mol01143)
| Name |
Adenylate cyclase-stimulating G alpha protein (GNAS)
,Homo sapiens
|
||||
|---|---|---|---|---|---|
| Synonyms |
GNAS; GNAS1; GSP; Adenylate cyclase-stimulating G alpha protein; Guanine nucleotide-binding protein G(s) subunit alpha isoforms short
Click to Show/Hide
|
||||
| Molecule Type |
Protein
|
||||
| Gene Name |
GNAS
|
||||
| Gene ID | |||||
| Location |
chr20:58839718-58911192[+]
|
||||
| Sequence |
MGCLGNSKTEDQRNEEKAQREANKKIEKQLQKDKQVYRATHRLLLLGAGESGKSTIVKQM
RILHVNGFNGEGGEEDPQAARSNSDGEKATKVQDIKNNLKEAIETIVAAMSNLVPPVELA NPENQFRVDYILSVMNVPDFDFPPEFYEHAKALWEDEGVRACYERSNEYQLIDCAQYFLD KIDVIKQADYVPSDQDLLRCRVLTSGIFETKFQVDKVNFHMFDVGGQRDERRKWIQCFND VTAIIFVVASSSYNMVIREDNQTNRLQEALNLFKSIWNNRWLRTISVILFLNKQDLLAEK VLAGKSKIEDYFPEFARYTTPEDATPEPGEDPRVTRAKYFIRDEFLRISTASGDGRHYCY PHFTCAVDTENIRRVFNDCRDIIQRMHLRQYELL Click to Show/Hide
|
||||
| 3D-structure |
|
||||
| Function |
Guanine nucleotide-binding proteins (G proteins) function as transducers in numerous signaling pathways controlled by G protein-coupled receptors (GPCRs)
Click to Show/Hide
|
||||
| Uniprot ID | |||||
| Ensembl ID | |||||
| HGNC ID | |||||
| Click to Show/Hide the Complete Species Lineage | |||||
Type(s) of Resistant Mechanism of This Molecule
Drug Resistance Data Categorized by Drug
Approved Drug(s)
4 drug(s) in total
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: HER2 positive breast cancer [ICD-11: 2C60.8] | [1] | |||
| Resistant Disease | HER2 positive breast cancer [ICD-11: 2C60.8] | |||
| Resistant Drug | Lapatinib | |||
| Molecule Alteration | Missense mutation | p.R216L |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Plasma | Blood | Homo sapiens (Human) | N.A. |
| Experiment for Molecule Alteration |
Next-generation sequencing assay; Circulating-free DNA assay | |||
| Experiment for Drug Resistance |
Positron emission tomography/Computed tomography assay | |||
| Mechanism Description | Seven genes, including epidermal growth factor receptor (EGFR), G protein subunit alpha S (GNAS), HRas proto-oncogene (HRAS), mutL homolog 1 (MLH1), cadherin 1 (CDH1), neuroblastoma RAS viral oncogene homolog (NRAS), and NOTCH1, that only occurred mutations in the resistant group were associated with the resistance of targeted therapy. | |||
| Disease Class: HER2 positive breast cancer [ICD-11: 2C60.8] | [1] | |||
| Resistant Disease | HER2 positive breast cancer [ICD-11: 2C60.8] | |||
| Resistant Drug | Lapatinib | |||
| Molecule Alteration | Missense mutation | p.R216C |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Plasma | Blood | Homo sapiens (Human) | N.A. |
| Experiment for Molecule Alteration |
Next-generation sequencing assay; Circulating-free DNA assay | |||
| Experiment for Drug Resistance |
Positron emission tomography/Computed tomography assay | |||
| Mechanism Description | Seven genes, including epidermal growth factor receptor (EGFR), G protein subunit alpha S (GNAS), HRas proto-oncogene (HRAS), mutL homolog 1 (MLH1), cadherin 1 (CDH1), neuroblastoma RAS viral oncogene homolog (NRAS), and NOTCH1, that only occurred mutations in the resistant group were associated with the resistance of targeted therapy. | |||
| Disease Class: HER2 positive breast cancer [ICD-11: 2C60.8] | [1] | |||
| Resistant Disease | HER2 positive breast cancer [ICD-11: 2C60.8] | |||
| Resistant Drug | Lapatinib | |||
| Molecule Alteration | Missense mutation | p.R186H |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Plasma | Blood | Homo sapiens (Human) | N.A. |
| Experiment for Molecule Alteration |
Next-generation sequencing assay; Circulating-free DNA assay | |||
| Experiment for Drug Resistance |
Positron emission tomography/Computed tomography assay | |||
| Mechanism Description | Seven genes, including epidermal growth factor receptor (EGFR), G protein subunit alpha S (GNAS), HRas proto-oncogene (HRAS), mutL homolog 1 (MLH1), cadherin 1 (CDH1), neuroblastoma RAS viral oncogene homolog (NRAS), and NOTCH1, that only occurred mutations in the resistant group were associated with the resistance of targeted therapy. | |||
| Disease Class: HER2 positive breast cancer [ICD-11: 2C60.8] | [1] | |||
| Resistant Disease | HER2 positive breast cancer [ICD-11: 2C60.8] | |||
| Resistant Drug | Lapatinib | |||
| Molecule Alteration | Missense mutation | p.N203S |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Plasma | Blood | Homo sapiens (Human) | N.A. |
| Experiment for Molecule Alteration |
Next-generation sequencing assay; Circulating-free DNA assay | |||
| Experiment for Drug Resistance |
Positron emission tomography/Computed tomography assay | |||
| Mechanism Description | Seven genes, including epidermal growth factor receptor (EGFR), G protein subunit alpha S (GNAS), HRas proto-oncogene (HRAS), mutL homolog 1 (MLH1), cadherin 1 (CDH1), neuroblastoma RAS viral oncogene homolog (NRAS), and NOTCH1, that only occurred mutations in the resistant group were associated with the resistance of targeted therapy. | |||
| Disease Class: HER2 positive breast cancer [ICD-11: 2C60.8] | [1] | |||
| Resistant Disease | HER2 positive breast cancer [ICD-11: 2C60.8] | |||
| Resistant Drug | Lapatinib | |||
| Molecule Alteration | Missense mutation | p.M206V |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Plasma | Blood | Homo sapiens (Human) | N.A. |
| Experiment for Molecule Alteration |
Next-generation sequencing assay; Circulating-free DNA assay | |||
| Experiment for Drug Resistance |
Positron emission tomography/Computed tomography assay | |||
| Mechanism Description | Seven genes, including epidermal growth factor receptor (EGFR), G protein subunit alpha S (GNAS), HRas proto-oncogene (HRAS), mutL homolog 1 (MLH1), cadherin 1 (CDH1), neuroblastoma RAS viral oncogene homolog (NRAS), and NOTCH1, that only occurred mutations in the resistant group were associated with the resistance of targeted therapy. | |||
| Disease Class: HER2 positive breast cancer [ICD-11: 2C60.8] | [1] | |||
| Resistant Disease | HER2 positive breast cancer [ICD-11: 2C60.8] | |||
| Resistant Drug | Lapatinib | |||
| Molecule Alteration | Missense mutation | p.D214N |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Plasma | Blood | Homo sapiens (Human) | N.A. |
| Experiment for Molecule Alteration |
Next-generation sequencing assay; Circulating-free DNA assay | |||
| Experiment for Drug Resistance |
Positron emission tomography/Computed tomography assay | |||
| Mechanism Description | Seven genes, including epidermal growth factor receptor (EGFR), G protein subunit alpha S (GNAS), HRas proto-oncogene (HRAS), mutL homolog 1 (MLH1), cadherin 1 (CDH1), neuroblastoma RAS viral oncogene homolog (NRAS), and NOTCH1, that only occurred mutations in the resistant group were associated with the resistance of targeted therapy. | |||
| Disease Class: HER2 positive breast cancer [ICD-11: 2C60.8] | [1] | |||
| Resistant Disease | HER2 positive breast cancer [ICD-11: 2C60.8] | |||
| Resistant Drug | Lapatinib | |||
| Molecule Alteration | Missense mutation | p.D181G |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Plasma | Blood | Homo sapiens (Human) | N.A. |
| Experiment for Molecule Alteration |
Next-generation sequencing assay; Circulating-free DNA assay | |||
| Experiment for Drug Resistance |
Positron emission tomography/Computed tomography assay | |||
| Mechanism Description | Seven genes, including epidermal growth factor receptor (EGFR), G protein subunit alpha S (GNAS), HRas proto-oncogene (HRAS), mutL homolog 1 (MLH1), cadherin 1 (CDH1), neuroblastoma RAS viral oncogene homolog (NRAS), and NOTCH1, that only occurred mutations in the resistant group were associated with the resistance of targeted therapy. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: HER2 positive breast cancer [ICD-11: 2C60.8] | [1] | |||
| Resistant Disease | HER2 positive breast cancer [ICD-11: 2C60.8] | |||
| Resistant Drug | Pertuzumab | |||
| Molecule Alteration | Missense mutation | p.R216L |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Plasma | Blood | Homo sapiens (Human) | N.A. |
| Experiment for Molecule Alteration |
Next-generation sequencing assay; Circulating-free DNA assay | |||
| Experiment for Drug Resistance |
Positron emission tomography/Computed tomography assay | |||
| Mechanism Description | Seven genes, including epidermal growth factor receptor (EGFR), G protein subunit alpha S (GNAS), HRas proto-oncogene (HRAS), mutL homolog 1 (MLH1), cadherin 1 (CDH1), neuroblastoma RAS viral oncogene homolog (NRAS), and NOTCH1, that only occurred mutations in the resistant group were associated with the resistance of targeted therapy. | |||
| Disease Class: HER2 positive breast cancer [ICD-11: 2C60.8] | [1] | |||
| Resistant Disease | HER2 positive breast cancer [ICD-11: 2C60.8] | |||
| Resistant Drug | Pertuzumab | |||
| Molecule Alteration | Missense mutation | p.R216C |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Plasma | Blood | Homo sapiens (Human) | N.A. |
| Experiment for Molecule Alteration |
Next-generation sequencing assay; Circulating-free DNA assay | |||
| Experiment for Drug Resistance |
Positron emission tomography/Computed tomography assay | |||
| Mechanism Description | Seven genes, including epidermal growth factor receptor (EGFR), G protein subunit alpha S (GNAS), HRas proto-oncogene (HRAS), mutL homolog 1 (MLH1), cadherin 1 (CDH1), neuroblastoma RAS viral oncogene homolog (NRAS), and NOTCH1, that only occurred mutations in the resistant group were associated with the resistance of targeted therapy. | |||
| Disease Class: HER2 positive breast cancer [ICD-11: 2C60.8] | [1] | |||
| Resistant Disease | HER2 positive breast cancer [ICD-11: 2C60.8] | |||
| Resistant Drug | Pertuzumab | |||
| Molecule Alteration | Missense mutation | p.R186H |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Plasma | Blood | Homo sapiens (Human) | N.A. |
| Experiment for Molecule Alteration |
Next-generation sequencing assay; Circulating-free DNA assay | |||
| Experiment for Drug Resistance |
Positron emission tomography/Computed tomography assay | |||
| Mechanism Description | Seven genes, including epidermal growth factor receptor (EGFR), G protein subunit alpha S (GNAS), HRas proto-oncogene (HRAS), mutL homolog 1 (MLH1), cadherin 1 (CDH1), neuroblastoma RAS viral oncogene homolog (NRAS), and NOTCH1, that only occurred mutations in the resistant group were associated with the resistance of targeted therapy. | |||
| Disease Class: HER2 positive breast cancer [ICD-11: 2C60.8] | [1] | |||
| Resistant Disease | HER2 positive breast cancer [ICD-11: 2C60.8] | |||
| Resistant Drug | Pertuzumab | |||
| Molecule Alteration | Missense mutation | p.N203S |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Plasma | Blood | Homo sapiens (Human) | N.A. |
| Experiment for Molecule Alteration |
Next-generation sequencing assay; Circulating-free DNA assay | |||
| Experiment for Drug Resistance |
Positron emission tomography/Computed tomography assay | |||
| Mechanism Description | Seven genes, including epidermal growth factor receptor (EGFR), G protein subunit alpha S (GNAS), HRas proto-oncogene (HRAS), mutL homolog 1 (MLH1), cadherin 1 (CDH1), neuroblastoma RAS viral oncogene homolog (NRAS), and NOTCH1, that only occurred mutations in the resistant group were associated with the resistance of targeted therapy. | |||
| Disease Class: HER2 positive breast cancer [ICD-11: 2C60.8] | [1] | |||
| Resistant Disease | HER2 positive breast cancer [ICD-11: 2C60.8] | |||
| Resistant Drug | Pertuzumab | |||
| Molecule Alteration | Missense mutation | p.M206V |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Plasma | Blood | Homo sapiens (Human) | N.A. |
| Experiment for Molecule Alteration |
Next-generation sequencing assay; Circulating-free DNA assay | |||
| Experiment for Drug Resistance |
Positron emission tomography/Computed tomography assay | |||
| Mechanism Description | Seven genes, including epidermal growth factor receptor (EGFR), G protein subunit alpha S (GNAS), HRas proto-oncogene (HRAS), mutL homolog 1 (MLH1), cadherin 1 (CDH1), neuroblastoma RAS viral oncogene homolog (NRAS), and NOTCH1, that only occurred mutations in the resistant group were associated with the resistance of targeted therapy. | |||
| Disease Class: HER2 positive breast cancer [ICD-11: 2C60.8] | [1] | |||
| Resistant Disease | HER2 positive breast cancer [ICD-11: 2C60.8] | |||
| Resistant Drug | Pertuzumab | |||
| Molecule Alteration | Missense mutation | p.D214N |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Plasma | Blood | Homo sapiens (Human) | N.A. |
| Experiment for Molecule Alteration |
Next-generation sequencing assay; Circulating-free DNA assay | |||
| Experiment for Drug Resistance |
Positron emission tomography/Computed tomography assay | |||
| Mechanism Description | Seven genes, including epidermal growth factor receptor (EGFR), G protein subunit alpha S (GNAS), HRas proto-oncogene (HRAS), mutL homolog 1 (MLH1), cadherin 1 (CDH1), neuroblastoma RAS viral oncogene homolog (NRAS), and NOTCH1, that only occurred mutations in the resistant group were associated with the resistance of targeted therapy. | |||
| Disease Class: HER2 positive breast cancer [ICD-11: 2C60.8] | [1] | |||
| Resistant Disease | HER2 positive breast cancer [ICD-11: 2C60.8] | |||
| Resistant Drug | Pertuzumab | |||
| Molecule Alteration | Missense mutation | p.D181G |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Plasma | Blood | Homo sapiens (Human) | N.A. |
| Experiment for Molecule Alteration |
Next-generation sequencing assay; Circulating-free DNA assay | |||
| Experiment for Drug Resistance |
Positron emission tomography/Computed tomography assay | |||
| Mechanism Description | Seven genes, including epidermal growth factor receptor (EGFR), G protein subunit alpha S (GNAS), HRas proto-oncogene (HRAS), mutL homolog 1 (MLH1), cadherin 1 (CDH1), neuroblastoma RAS viral oncogene homolog (NRAS), and NOTCH1, that only occurred mutations in the resistant group were associated with the resistance of targeted therapy. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: HER2 positive breast cancer [ICD-11: 2C60.8] | [1] | |||
| Resistant Disease | HER2 positive breast cancer [ICD-11: 2C60.8] | |||
| Resistant Drug | Trastuzumab | |||
| Molecule Alteration | Missense mutation | p.R216L |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Plasma | Blood | Homo sapiens (Human) | N.A. |
| Experiment for Molecule Alteration |
Next-generation sequencing assay; Circulating-free DNA assay | |||
| Experiment for Drug Resistance |
Positron emission tomography/Computed tomography assay | |||
| Mechanism Description | Seven genes, including epidermal growth factor receptor (EGFR), G protein subunit alpha S (GNAS), HRas proto-oncogene (HRAS), mutL homolog 1 (MLH1), cadherin 1 (CDH1), neuroblastoma RAS viral oncogene homolog (NRAS), and NOTCH1, that only occurred mutations in the resistant group were associated with the resistance of targeted therapy. | |||
| Disease Class: HER2 positive breast cancer [ICD-11: 2C60.8] | [1] | |||
| Resistant Disease | HER2 positive breast cancer [ICD-11: 2C60.8] | |||
| Resistant Drug | Trastuzumab | |||
| Molecule Alteration | Missense mutation | p.R216C |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Plasma | Blood | Homo sapiens (Human) | N.A. |
| Experiment for Molecule Alteration |
Next-generation sequencing assay; Circulating-free DNA assay | |||
| Experiment for Drug Resistance |
Positron emission tomography/Computed tomography assay | |||
| Mechanism Description | Seven genes, including epidermal growth factor receptor (EGFR), G protein subunit alpha S (GNAS), HRas proto-oncogene (HRAS), mutL homolog 1 (MLH1), cadherin 1 (CDH1), neuroblastoma RAS viral oncogene homolog (NRAS), and NOTCH1, that only occurred mutations in the resistant group were associated with the resistance of targeted therapy. | |||
| Disease Class: HER2 positive breast cancer [ICD-11: 2C60.8] | [1] | |||
| Resistant Disease | HER2 positive breast cancer [ICD-11: 2C60.8] | |||
| Resistant Drug | Trastuzumab | |||
| Molecule Alteration | Missense mutation | p.R186H |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Plasma | Blood | Homo sapiens (Human) | N.A. |
| Experiment for Molecule Alteration |
Next-generation sequencing assay; Circulating-free DNA assay | |||
| Experiment for Drug Resistance |
Positron emission tomography/Computed tomography assay | |||
| Mechanism Description | Seven genes, including epidermal growth factor receptor (EGFR), G protein subunit alpha S (GNAS), HRas proto-oncogene (HRAS), mutL homolog 1 (MLH1), cadherin 1 (CDH1), neuroblastoma RAS viral oncogene homolog (NRAS), and NOTCH1, that only occurred mutations in the resistant group were associated with the resistance of targeted therapy. | |||
| Disease Class: HER2 positive breast cancer [ICD-11: 2C60.8] | [1] | |||
| Resistant Disease | HER2 positive breast cancer [ICD-11: 2C60.8] | |||
| Resistant Drug | Trastuzumab | |||
| Molecule Alteration | Missense mutation | p.N203S |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Plasma | Blood | Homo sapiens (Human) | N.A. |
| Experiment for Molecule Alteration |
Next-generation sequencing assay; Circulating-free DNA assay | |||
| Experiment for Drug Resistance |
Positron emission tomography/Computed tomography assay | |||
| Mechanism Description | Seven genes, including epidermal growth factor receptor (EGFR), G protein subunit alpha S (GNAS), HRas proto-oncogene (HRAS), mutL homolog 1 (MLH1), cadherin 1 (CDH1), neuroblastoma RAS viral oncogene homolog (NRAS), and NOTCH1, that only occurred mutations in the resistant group were associated with the resistance of targeted therapy. | |||
| Disease Class: HER2 positive breast cancer [ICD-11: 2C60.8] | [1] | |||
| Resistant Disease | HER2 positive breast cancer [ICD-11: 2C60.8] | |||
| Resistant Drug | Trastuzumab | |||
| Molecule Alteration | Missense mutation | p.M206V |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Plasma | Blood | Homo sapiens (Human) | N.A. |
| Experiment for Molecule Alteration |
Next-generation sequencing assay; Circulating-free DNA assay | |||
| Experiment for Drug Resistance |
Positron emission tomography/Computed tomography assay | |||
| Mechanism Description | Seven genes, including epidermal growth factor receptor (EGFR), G protein subunit alpha S (GNAS), HRas proto-oncogene (HRAS), mutL homolog 1 (MLH1), cadherin 1 (CDH1), neuroblastoma RAS viral oncogene homolog (NRAS), and NOTCH1, that only occurred mutations in the resistant group were associated with the resistance of targeted therapy. | |||
| Disease Class: HER2 positive breast cancer [ICD-11: 2C60.8] | [1] | |||
| Resistant Disease | HER2 positive breast cancer [ICD-11: 2C60.8] | |||
| Resistant Drug | Trastuzumab | |||
| Molecule Alteration | Missense mutation | p.D214N |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Plasma | Blood | Homo sapiens (Human) | N.A. |
| Experiment for Molecule Alteration |
Next-generation sequencing assay; Circulating-free DNA assay | |||
| Experiment for Drug Resistance |
Positron emission tomography/Computed tomography assay | |||
| Mechanism Description | Seven genes, including epidermal growth factor receptor (EGFR), G protein subunit alpha S (GNAS), HRas proto-oncogene (HRAS), mutL homolog 1 (MLH1), cadherin 1 (CDH1), neuroblastoma RAS viral oncogene homolog (NRAS), and NOTCH1, that only occurred mutations in the resistant group were associated with the resistance of targeted therapy. | |||
| Disease Class: HER2 positive breast cancer [ICD-11: 2C60.8] | [1] | |||
| Resistant Disease | HER2 positive breast cancer [ICD-11: 2C60.8] | |||
| Resistant Drug | Trastuzumab | |||
| Molecule Alteration | Missense mutation | p.D181G |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Plasma | Blood | Homo sapiens (Human) | N.A. |
| Experiment for Molecule Alteration |
Next-generation sequencing assay; Circulating-free DNA assay | |||
| Experiment for Drug Resistance |
Positron emission tomography/Computed tomography assay | |||
| Mechanism Description | Seven genes, including epidermal growth factor receptor (EGFR), G protein subunit alpha S (GNAS), HRas proto-oncogene (HRAS), mutL homolog 1 (MLH1), cadherin 1 (CDH1), neuroblastoma RAS viral oncogene homolog (NRAS), and NOTCH1, that only occurred mutations in the resistant group were associated with the resistance of targeted therapy. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: HER2 positive breast cancer [ICD-11: 2C60.8] | [1] | |||
| Resistant Disease | HER2 positive breast cancer [ICD-11: 2C60.8] | |||
| Resistant Drug | Trastuzumab emtansine | |||
| Molecule Alteration | Missense mutation | p.R216L |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Plasma | Blood | Homo sapiens (Human) | N.A. |
| Experiment for Molecule Alteration |
Next-generation sequencing assay; Circulating-free DNA assay | |||
| Experiment for Drug Resistance |
Positron emission tomography/Computed tomography assay | |||
| Mechanism Description | Seven genes, including epidermal growth factor receptor (EGFR), G protein subunit alpha S (GNAS), HRas proto-oncogene (HRAS), mutL homolog 1 (MLH1), cadherin 1 (CDH1), neuroblastoma RAS viral oncogene homolog (NRAS), and NOTCH1, that only occurred mutations in the resistant group were associated with the resistance of targeted therapy. | |||
| Disease Class: HER2 positive breast cancer [ICD-11: 2C60.8] | [1] | |||
| Resistant Disease | HER2 positive breast cancer [ICD-11: 2C60.8] | |||
| Resistant Drug | Trastuzumab emtansine | |||
| Molecule Alteration | Missense mutation | p.R216C |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Plasma | Blood | Homo sapiens (Human) | N.A. |
| Experiment for Molecule Alteration |
Next-generation sequencing assay; Circulating-free DNA assay | |||
| Experiment for Drug Resistance |
Positron emission tomography/Computed tomography assay | |||
| Mechanism Description | Seven genes, including epidermal growth factor receptor (EGFR), G protein subunit alpha S (GNAS), HRas proto-oncogene (HRAS), mutL homolog 1 (MLH1), cadherin 1 (CDH1), neuroblastoma RAS viral oncogene homolog (NRAS), and NOTCH1, that only occurred mutations in the resistant group were associated with the resistance of targeted therapy. | |||
| Disease Class: HER2 positive breast cancer [ICD-11: 2C60.8] | [1] | |||
| Resistant Disease | HER2 positive breast cancer [ICD-11: 2C60.8] | |||
| Resistant Drug | Trastuzumab emtansine | |||
| Molecule Alteration | Missense mutation | p.R186H |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Plasma | Blood | Homo sapiens (Human) | N.A. |
| Experiment for Molecule Alteration |
Next-generation sequencing assay; Circulating-free DNA assay | |||
| Experiment for Drug Resistance |
Positron emission tomography/Computed tomography assay | |||
| Mechanism Description | Seven genes, including epidermal growth factor receptor (EGFR), G protein subunit alpha S (GNAS), HRas proto-oncogene (HRAS), mutL homolog 1 (MLH1), cadherin 1 (CDH1), neuroblastoma RAS viral oncogene homolog (NRAS), and NOTCH1, that only occurred mutations in the resistant group were associated with the resistance of targeted therapy. | |||
| Disease Class: HER2 positive breast cancer [ICD-11: 2C60.8] | [1] | |||
| Resistant Disease | HER2 positive breast cancer [ICD-11: 2C60.8] | |||
| Resistant Drug | Trastuzumab emtansine | |||
| Molecule Alteration | Missense mutation | p.N203S |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Plasma | Blood | Homo sapiens (Human) | N.A. |
| Experiment for Molecule Alteration |
Next-generation sequencing assay; Circulating-free DNA assay | |||
| Experiment for Drug Resistance |
Positron emission tomography/Computed tomography assay | |||
| Mechanism Description | Seven genes, including epidermal growth factor receptor (EGFR), G protein subunit alpha S (GNAS), HRas proto-oncogene (HRAS), mutL homolog 1 (MLH1), cadherin 1 (CDH1), neuroblastoma RAS viral oncogene homolog (NRAS), and NOTCH1, that only occurred mutations in the resistant group were associated with the resistance of targeted therapy. | |||
| Disease Class: HER2 positive breast cancer [ICD-11: 2C60.8] | [1] | |||
| Resistant Disease | HER2 positive breast cancer [ICD-11: 2C60.8] | |||
| Resistant Drug | Trastuzumab emtansine | |||
| Molecule Alteration | Missense mutation | p.M206V |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Plasma | Blood | Homo sapiens (Human) | N.A. |
| Experiment for Molecule Alteration |
Next-generation sequencing assay; Circulating-free DNA assay | |||
| Experiment for Drug Resistance |
Positron emission tomography/Computed tomography assay | |||
| Mechanism Description | Seven genes, including epidermal growth factor receptor (EGFR), G protein subunit alpha S (GNAS), HRas proto-oncogene (HRAS), mutL homolog 1 (MLH1), cadherin 1 (CDH1), neuroblastoma RAS viral oncogene homolog (NRAS), and NOTCH1, that only occurred mutations in the resistant group were associated with the resistance of targeted therapy. | |||
| Disease Class: HER2 positive breast cancer [ICD-11: 2C60.8] | [1] | |||
| Resistant Disease | HER2 positive breast cancer [ICD-11: 2C60.8] | |||
| Resistant Drug | Trastuzumab emtansine | |||
| Molecule Alteration | Missense mutation | p.D214N |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Plasma | Blood | Homo sapiens (Human) | N.A. |
| Experiment for Molecule Alteration |
Next-generation sequencing assay; Circulating-free DNA assay | |||
| Experiment for Drug Resistance |
Positron emission tomography/Computed tomography assay | |||
| Mechanism Description | Seven genes, including epidermal growth factor receptor (EGFR), G protein subunit alpha S (GNAS), HRas proto-oncogene (HRAS), mutL homolog 1 (MLH1), cadherin 1 (CDH1), neuroblastoma RAS viral oncogene homolog (NRAS), and NOTCH1, that only occurred mutations in the resistant group were associated with the resistance of targeted therapy. | |||
| Disease Class: HER2 positive breast cancer [ICD-11: 2C60.8] | [1] | |||
| Resistant Disease | HER2 positive breast cancer [ICD-11: 2C60.8] | |||
| Resistant Drug | Trastuzumab emtansine | |||
| Molecule Alteration | Missense mutation | p.D181G |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Plasma | Blood | Homo sapiens (Human) | N.A. |
| Experiment for Molecule Alteration |
Next-generation sequencing assay; Circulating-free DNA assay | |||
| Experiment for Drug Resistance |
Positron emission tomography/Computed tomography assay | |||
| Mechanism Description | Seven genes, including epidermal growth factor receptor (EGFR), G protein subunit alpha S (GNAS), HRas proto-oncogene (HRAS), mutL homolog 1 (MLH1), cadherin 1 (CDH1), neuroblastoma RAS viral oncogene homolog (NRAS), and NOTCH1, that only occurred mutations in the resistant group were associated with the resistance of targeted therapy. | |||
Investigative Drug(s)
2 drug(s) in total
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [2] | |||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Sensitive Drug | Pyridone 6 | |||
| Molecule Alteration | Missense mutation | p.R201L (c.602G>T) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Human liver cancer tissue | N.A. | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Mechanism Description | The missense mutation p.R201L (c.602G>T) in gene GNAS cause the sensitivity of JAK inhibitors by unusual activation of pro-survival pathway | |||
| Disease Class: Solid tumour/cancer [ICD-11: 2A00-2F9Z] | [2] | |||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Sensitive Drug | Pyridone 6 | |||
| Molecule Alteration | Missense mutation | p.R201H (c.602G>A) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Human liver cancer tissue | N.A. | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Mechanism Description | The missense mutation p.R201H (c.602G>A) in gene GNAS cause the sensitivity of JAK inhibitors by unusual activation of pro-survival pathway | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Albright hereditary osteodystrophy syndrome [ICD-11: LD44.20] | [3] | |||
| Resistant Disease | Albright hereditary osteodystrophy syndrome [ICD-11: LD44.20] | |||
| Resistant Drug | Thyrotropin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Mechanism Description | Heterozygous germline mutations in the gene encoding the alpha subunit of G stimulatory protein (Gsalpha, GNAS1) cause hypocalcemia and hyperphosphatemia due to impaired signaling transduction from the parathormone receptor (pseudohypoparathyroidism, PHP Ia). Haploinsufficiency for GNAS1 also explains the resistance to other hormones, specifically gonadotropins and TSH. | |||
Disease- and Tissue-specific Abundances of This Molecule
ICD Disease Classification 02

| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Breast tissue | |
| The Specified Disease | Breast cancer | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 8.66E-59; Fold-change: 5.44E-01; Z-score: 1.45E+00 | |
| The Expression Level of Disease Section Compare with the Adjacent Tissue | p-value: 1.93E-07; Fold-change: 2.18E-01; Z-score: 7.42E-01 | |
|
Molecule expression in the normal tissue adjacent to the diseased tissue of patients
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |
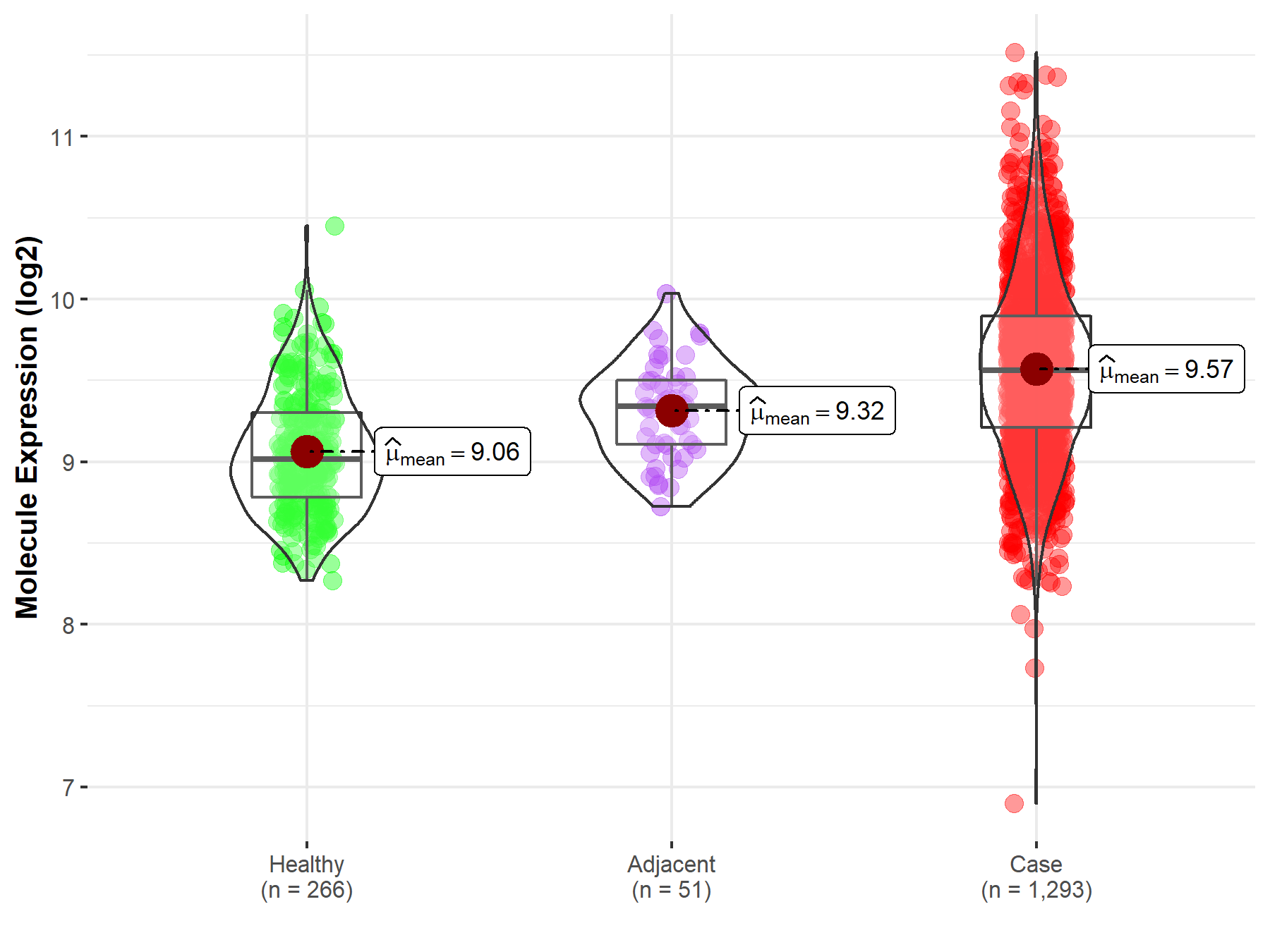
|
Click to View the Clearer Original Diagram |
Tissue-specific Molecule Abundances in Healthy Individuals

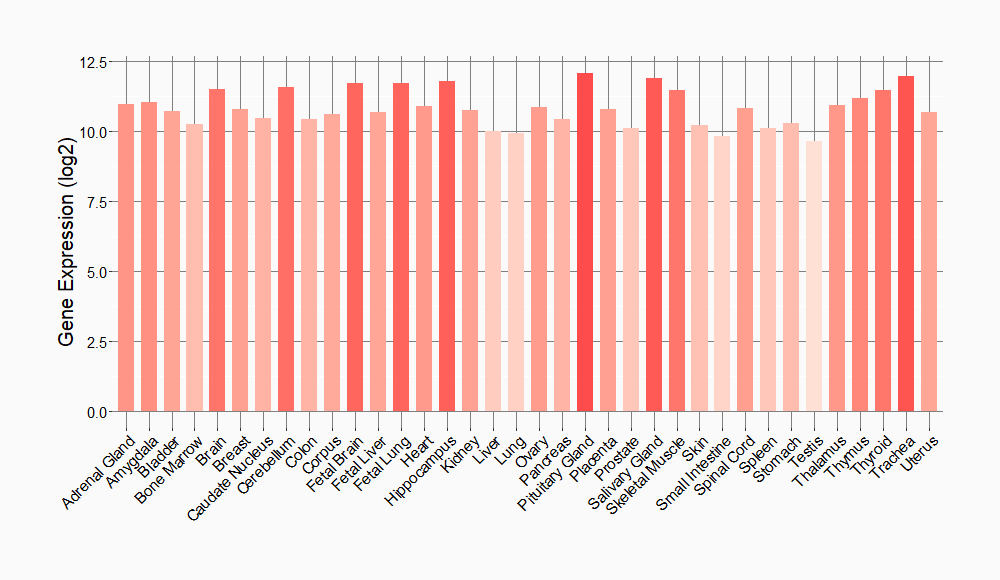
|
||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
