Molecule Information
General Information of the Molecule (ID: Mol00663)
| Name |
Tubulin beta-3 chain (TUBB3)
,Homo sapiens
|
||||
|---|---|---|---|---|---|
| Synonyms |
Tubulin beta-4 chain; Tubulin beta-III; TUBB4
Click to Show/Hide
|
||||
| Molecule Type |
Protein
|
||||
| Gene Name |
TUBB3
|
||||
| Gene ID | |||||
| Location |
chr16:89921392-89938761[+]
|
||||
| Sequence |
MREIVHIQAGQCGNQIGAKFWEVISDEHGIDPSGNYVGDSDLQLERISVYYNEASSHKYV
PRAILVDLEPGTMDSVRSGAFGHLFRPDNFIFGQSGAGNNWAKGHYTEGAELVDSVLDVV RKECENCDCLQGFQLTHSLGGGTGSGMGTLLISKVREEYPDRIMNTFSVVPSPKVSDTVV EPYNATLSIHQLVENTDETYCIDNEALYDICFRTLKLATPTYGDLNHLVSATMSGVTTSL RFPGQLNADLRKLAVNMVPFPRLHFFMPGFAPLTARGSQQYRALTVPELTQQMFDAKNMM AACDPRHGRYLTVATVFRGRMSMKEVDEQMLAIQSKNSSYFVEWIPNNVKVAVCDIPPRG LKMSSTFIGNSTAIQELFKRISEQFTAMFRRKAFLHWYTGEGMDEMEFTEAESNMNDLVS EYQQYQDATAEEEGEMYEDDEEESEAQGPK Click to Show/Hide
|
||||
| 3D-structure |
|
||||
| Function |
Tubulin is the major constituent of microtubules. It binds two moles of GTP, one at an exchangeable site on the beta chain and one at a non-exchangeable site on the alpha chain. TUBB3 plays a critical role in proper axon guidance and maintenance. Binding of NTN1/Netrin-1 to its receptor UNC5C might cause dissociation of UNC5C from polymerized TUBB3 in microtubules and thereby lead to increased microtubule dynamics and axon repulsion. Plays a role in dorsal root ganglion axon projection towards the spinal cord.
Click to Show/Hide
|
||||
| Uniprot ID | |||||
| Ensembl ID | |||||
| HGNC ID | |||||
| Click to Show/Hide the Complete Species Lineage | |||||
Type(s) of Resistant Mechanism of This Molecule
Drug Resistance Data Categorized by Drug
Approved Drug(s)
6 drug(s) in total
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Ovarian cancer [ICD-11: 2C73.0] | [1] | |||
| Resistant Disease | Ovarian cancer [ICD-11: 2C73.0] | |||
| Resistant Drug | Paclitaxel | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Ovarian cancer [ICD-11: 2C73] | |||
| The Specified Disease | Ovarian cancer | |||
| The Studied Tissue | Ovarian tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 7.94E-02 Fold-change: 8.18E-02 Z-score: 2.01E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell proliferation | Inhibition | hsa05200 | ||
| In Vitro Model | OVCAR3 cells | Ovary | Homo sapiens (Human) | CVCL_0465 |
| MES-OV cells | Ovary | Homo sapiens (Human) | CVCL_CZ92 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
SRB colorimetric assay; Flow cytometry assay | |||
| Mechanism Description | The miR-200 family has major roles in EMT and taxane resistance in taxane selected ovarian cancer cell variants, and that re-introduction of miR-200s was not sufficient to fully reverse the mesenchymal phenotype in these variants. Although miR-200s were able to restore paclitaxel sensitivity in one of the variants, they did not do so in the other, and produced resistance to carboplatin in both. The divergent effects of miR-200s on taxane and carboplatin cytotoxicity should be further investigated in ovarian cancers. miR-200c and miR-141 mimics conferred resistance to carboplatin in MES-OV/TP cells, similar to OVCAR-3/TP, but sensitized MES-OV to paclitaxel. Several genes involved in balancing oxidative stress were altered in OVCAR-3/TP 200c141 cells compared to controls. The miR-200 family plays major, cell-context dependent roles in regulating EMT and sensitivity to carboplatin and paclitaxel in OVCAR-3 and MES-OV cells. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: KRAS mutant breast cancer [ICD-11: 2C60.10] | [4] | |||
| Sensitive Disease | KRAS mutant breast cancer [ICD-11: 2C60.10] | |||
| Sensitive Drug | Paclitaxel | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell invasion | Inhibition | hsa05200 | ||
| Cell migration | Inhibition | hsa04670 | ||
| MEK/ERK /PI3K/AKT signaling pathway | Inhibition | hsa04151 | ||
| In Vitro Model | BxPC-3 cells | Pancreas | Homo sapiens (Human) | CVCL_0186 |
| PANC-1 cells | Pancreas | Homo sapiens (Human) | CVCL_0480 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Flow cytometry assay | |||
| Mechanism Description | Let-7b repletion selectively sensitized kRAS mutant tumor cells to the cytotoxicity of paclitaxel and gemcitabine. Transfection of let-7b mimic downregulated the expression of mutant but not wild-type kRAS. Combination of let-7b mimic with paclitaxel or gemcitabine diminished MEk/ERk and PI3k/AkT signaling concurrently, triggered the onset of apoptosis, and reverted the epithelial-mesenchymal transition in kRAS mutant tumor cells. In addition, let-7b repletion downregulated the expression of beta-tubulin III and ribonucleotide reductase subunit M2, two proteins known to mediate tumor resistance to paclitaxel and gemcitabine, respectively. Let-7 may represent a new class of chemosensitizer for the treatment of kRAS mutant tumors. | |||
| Disease Class: kRAS mutant non-small cell lung cancer [ICD-11: 2C25.9] | [4] | |||
| Sensitive Disease | kRAS mutant non-small cell lung cancer [ICD-11: 2C25.9] | |||
| Sensitive Drug | Paclitaxel | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell invasion | Inhibition | hsa05200 | ||
| Cell migration | Inhibition | hsa04670 | ||
| MEK/ERK /PI3K/AKT signaling pathway | Inhibition | hsa04151 | ||
| In Vitro Model | BxPC-3 cells | Pancreas | Homo sapiens (Human) | CVCL_0186 |
| PANC-1 cells | Pancreas | Homo sapiens (Human) | CVCL_0480 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Flow cytometry assay | |||
| Mechanism Description | Let-7b repletion selectively sensitized kRAS mutant tumor cells to the cytotoxicity of paclitaxel and gemcitabine. Transfection of let-7b mimic downregulated the expression of mutant but not wild-type kRAS. Combination of let-7b mimic with paclitaxel or gemcitabine diminished MEk/ERk and PI3k/AkT signaling concurrently, triggered the onset of apoptosis, and reverted the epithelial-mesenchymal transition in kRAS mutant tumor cells. In addition, let-7b repletion downregulated the expression of beta-tubulin III and ribonucleotide reductase subunit M2, two proteins known to mediate tumor resistance to paclitaxel and gemcitabine, respectively. Let-7 may represent a new class of chemosensitizer for the treatment of kRAS mutant tumors. | |||
| Disease Class: KRAS mutant pancreatic ductal adenocarcinoma [ICD-11: 2C10.5] | [4] | |||
| Sensitive Disease | KRAS mutant pancreatic ductal adenocarcinoma [ICD-11: 2C10.5] | |||
| Sensitive Drug | Paclitaxel | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell invasion | Inhibition | hsa05200 | ||
| Cell migration | Inhibition | hsa04670 | ||
| MEK/ERK /PI3K/AKT signaling pathway | Inhibition | hsa04151 | ||
| In Vitro Model | BxPC-3 cells | Pancreas | Homo sapiens (Human) | CVCL_0186 |
| PANC-1 cells | Pancreas | Homo sapiens (Human) | CVCL_0480 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Flow cytometry assay | |||
| Mechanism Description | Let-7b repletion selectively sensitized kRAS mutant tumor cells to the cytotoxicity of paclitaxel and gemcitabine. Transfection of let-7b mimic downregulated the expression of mutant but not wild-type kRAS. Combination of let-7b mimic with paclitaxel or gemcitabine diminished MEk/ERk and PI3k/AkT signaling concurrently, triggered the onset of apoptosis, and reverted the epithelial-mesenchymal transition in kRAS mutant tumor cells. In addition, let-7b repletion downregulated the expression of beta-tubulin III and ribonucleotide reductase subunit M2, two proteins known to mediate tumor resistance to paclitaxel and gemcitabine, respectively. Let-7 may represent a new class of chemosensitizer for the treatment of kRAS mutant tumors. | |||
| Disease Class: Ovarian cancer [ICD-11: 2C73.0] | [5] | |||
| Sensitive Disease | Ovarian cancer [ICD-11: 2C73.0] | |||
| Sensitive Drug | Paclitaxel | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell adhesion | Inhibition | hsa04514 | |
| Cell apoptosis | Inhibition | hsa04210 | ||
| In Vitro Model | HEY cells | Ovary | Homo sapiens (Human) | CVCL_0297 |
| SkOV3 cells | Ovary | Homo sapiens (Human) | CVCL_0532 | |
| OVCA433 cells | Ovary | Homo sapiens (Human) | CVCL_0475 | |
| OV 1847 cells | Breast | Homo sapiens (Human) | CVCL_D703 | |
| OVCA 420 cells | Breast | Homo sapiens (Human) | CVCL_3935 | |
| In Vivo Model | (NOD) /SCID nude mouse xenograft model | . | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Overexpression of TUBB3 is thought to result in resistance to taxanes is by enhancement of the dynamic instability of microtubules, thereby counteracting the activity of microtubule targeting agents. Transient restoration of miR-200c using miRNA mimics cause a significant decrease in TUBB3 levels, thus results in the resistance to taxanes. | |||
| Disease Class: Endometrial cancer [ICD-11: 2C76.1] | [3] | |||
| Sensitive Disease | Endometrial cancer [ICD-11: 2C76.1] | |||
| Sensitive Drug | Paclitaxel | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell migration | Inhibition | hsa04670 | |
| In Vitro Model | Hec50 cells | Endometrium | Homo sapiens (Human) | CVCL_2929 |
| Experiment for Molecule Alteration |
Immunoblotting analysis | |||
| Experiment for Drug Resistance |
ELISA assay | |||
| Mechanism Description | Low or absent miR-200c results in aberrant expression of ZEB1 and consequent repression of E-cadherin. Reinstatement of miR-200c to such cells restores E-cadherin and dramatically reduces migration and invasion. One such gene, class IIIbeta-tubulin (TUBB3), which encodes a tubulin isotype normally found only in neuronal cells, is a direct target of miR-200c. Restoration of miR-200c increases sensitivity to microtubule-targeting agents by up to 85%. Since expression of TUBB3 is a common mechanism of resistance to microtubule-binding chemotherapeutic agents in many types of solid tumors, the ability of miR-200c to restore chemosensitivity to such agents may be explained by its ability to reduce TUBB3. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Prostate cancer [ICD-11: 2C82.0] | [2] | |||
| Resistant Disease | Prostate cancer [ICD-11: 2C82.0] | |||
| Resistant Drug | Cabazitaxel | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Prostate cancer [ICD-11: 2C82] | |||
| The Specified Disease | Prostate cancer | |||
| The Studied Tissue | Prostate | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 2.82E-01 Fold-change: 2.20E-02 Z-score: 1.10E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | CAL27 cells | Oral | Homo sapiens (Human) | CVCL_1107 |
| LOVO cells | Colon | Homo sapiens (Human) | CVCL_0399 | |
| BxPC-3 cells | Pancreas | Homo sapiens (Human) | CVCL_0186 | |
| C4-2 cells | Prostate | Homo sapiens (Human) | CVCL_4782 | |
| HuTu80 cells | Small intestine | Homo sapiens (Human) | CVCL_1301 | |
| DU145-DR cells | Brain | Homo sapiens (Human) | CVCL_4Y36 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | TUBB3 expression was upregulated in DTX-resistant and CBZ-resistant cells. TUBB3 knockdown re-sensitized DTX-resistant cells to DTX and CBZ-resistant cells to CBZ. Additionally, TUBB3 knockdown re-sensitized DTX-resistant cell lines to CBZ, indicating that TUBB3 mediates cross-resistance between DTX and CBZ. Knockdown of TUBB3 enhanced PTEN expression, and PTEN knockout enhanced TUBB3 expression. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Prostate cancer [ICD-11: 2C82.0] | [2] | |||
| Sensitive Disease | Prostate cancer [ICD-11: 2C82.0] | |||
| Sensitive Drug | Cabazitaxel | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | CAL27 cells | Oral | Homo sapiens (Human) | CVCL_1107 |
| LOVO cells | Colon | Homo sapiens (Human) | CVCL_0399 | |
| BxPC-3 cells | Pancreas | Homo sapiens (Human) | CVCL_0186 | |
| C4-2 cells | Prostate | Homo sapiens (Human) | CVCL_4782 | |
| HuTu80 cells | Small intestine | Homo sapiens (Human) | CVCL_1301 | |
| DU145-DR cells | Brain | Homo sapiens (Human) | CVCL_4Y36 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | TUBB3 expression was upregulated in DTX-resistant and CBZ-resistant cells. TUBB3 knockdown re-sensitized DTX-resistant cells to DTX and CBZ-resistant cells to CBZ. Additionally, TUBB3 knockdown re-sensitized DTX-resistant cell lines to CBZ, indicating that TUBB3 mediates cross-resistance between DTX and CBZ. Knockdown of TUBB3 enhanced PTEN expression, and PTEN knockout enhanced TUBB3 expression. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Ovarian cancer [ICD-11: 2C73.0] | [1] | |||
| Resistant Disease | Ovarian cancer [ICD-11: 2C73.0] | |||
| Resistant Drug | Carboplatin | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell proliferation | Inhibition | hsa05200 | ||
| In Vitro Model | OVCAR3 cells | Ovary | Homo sapiens (Human) | CVCL_0465 |
| MES-OV cells | Ovary | Homo sapiens (Human) | CVCL_CZ92 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
SRB colorimetric assay; Flow cytometry assay | |||
| Mechanism Description | The miR-200 family has major roles in EMT and taxane resistance in taxane selected ovarian cancer cell variants, and that re-introduction of miR-200s was not sufficient to fully reverse the mesenchymal phenotype in these variants. Although miR-200s were able to restore paclitaxel sensitivity in one of the variants, they did not do so in the other, and produced resistance to carboplatin in both. The divergent effects of miR-200s on taxane and carboplatin cytotoxicity should be further investigated in ovarian cancers. miR-200c and miR-141 mimics conferred resistance to carboplatin in MES-OV/TP cells, similar to OVCAR-3/TP, but sensitized MES-OV to paclitaxel. Several genes involved in balancing oxidative stress were altered in OVCAR-3/TP 200c141 cells compared to controls. The miR-200 family plays major, cell-context dependent roles in regulating EMT and sensitivity to carboplatin and paclitaxel in OVCAR-3 and MES-OV cells. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Endometrial cancer [ICD-11: 2C76.1] | [3] | |||
| Sensitive Disease | Endometrial cancer [ICD-11: 2C76.1] | |||
| Sensitive Drug | Epothilone B | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell migration | Inhibition | hsa04670 | |
| In Vitro Model | Hec50 cells | Endometrium | Homo sapiens (Human) | CVCL_2929 |
| Experiment for Molecule Alteration |
Immunoblotting analysis | |||
| Experiment for Drug Resistance |
ELISA assay | |||
| Mechanism Description | Low or absent miR-200c results in aberrant expression of ZEB1 and consequent repression of E-cadherin. Reinstatement of miR-200c to such cells restores E-cadherin and dramatically reduces migration and invasion. One such gene, class IIIbeta-tubulin (TUBB3), which encodes a tubulin isotype normally found only in neuronal cells, is a direct target of miR-200c. Restoration of miR-200c increases sensitivity to microtubule-targeting agents by up to 85%. Since expression of TUBB3 is a common mechanism of resistance to microtubule-binding chemotherapeutic agents in many types of solid tumors, the ability of miR-200c to restore chemosensitivity to such agents may be explained by its ability to reduce TUBB3. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: KRAS mutant breast cancer [ICD-11: 2C60.10] | [4] | |||
| Sensitive Disease | KRAS mutant breast cancer [ICD-11: 2C60.10] | |||
| Sensitive Drug | Gemcitabine | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell invasion | Inhibition | hsa05200 | ||
| Cell migration | Inhibition | hsa04670 | ||
| MEK/ERK /PI3K/AKT signaling pathway | Inhibition | hsa04151 | ||
| In Vitro Model | BxPC-3 cells | Pancreas | Homo sapiens (Human) | CVCL_0186 |
| PANC-1 cells | Pancreas | Homo sapiens (Human) | CVCL_0480 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Flow cytometry assay | |||
| Mechanism Description | Let-7b repletion selectively sensitized kRAS mutant tumor cells to the cytotoxicity of paclitaxel and gemcitabine. Transfection of let-7b mimic downregulated the expression of mutant but not wild-type kRAS. Combination of let-7b mimic with paclitaxel or gemcitabine diminished MEk/ERk and PI3k/AkT signaling concurrently, triggered the onset of apoptosis, and reverted the epithelial-mesenchymal transition in kRAS mutant tumor cells. In addition, let-7b repletion downregulated the expression of beta-tubulin III and ribonucleotide reductase subunit M2, two proteins known to mediate tumor resistance to paclitaxel and gemcitabine, respectively. Let-7 may represent a new class of chemosensitizer for the treatment of kRAS mutant tumors. | |||
| Disease Class: kRAS mutant non-small cell lung cancer [ICD-11: 2C25.9] | [4] | |||
| Sensitive Disease | kRAS mutant non-small cell lung cancer [ICD-11: 2C25.9] | |||
| Sensitive Drug | Gemcitabine | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell invasion | Inhibition | hsa05200 | ||
| Cell migration | Inhibition | hsa04670 | ||
| MEK/ERK /PI3K/AKT signaling pathway | Inhibition | hsa04151 | ||
| In Vitro Model | BxPC-3 cells | Pancreas | Homo sapiens (Human) | CVCL_0186 |
| PANC-1 cells | Pancreas | Homo sapiens (Human) | CVCL_0480 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Flow cytometry assay | |||
| Mechanism Description | Let-7b repletion selectively sensitized kRAS mutant tumor cells to the cytotoxicity of paclitaxel and gemcitabine. Transfection of let-7b mimic downregulated the expression of mutant but not wild-type kRAS. Combination of let-7b mimic with paclitaxel or gemcitabine diminished MEk/ERk and PI3k/AkT signaling concurrently, triggered the onset of apoptosis, and reverted the epithelial-mesenchymal transition in kRAS mutant tumor cells. In addition, let-7b repletion downregulated the expression of beta-tubulin III and ribonucleotide reductase subunit M2, two proteins known to mediate tumor resistance to paclitaxel and gemcitabine, respectively. Let-7 may represent a new class of chemosensitizer for the treatment of kRAS mutant tumors. | |||
| Disease Class: KRAS mutant pancreatic ductal adenocarcinoma [ICD-11: 2C10.5] | [4] | |||
| Sensitive Disease | KRAS mutant pancreatic ductal adenocarcinoma [ICD-11: 2C10.5] | |||
| Sensitive Drug | Gemcitabine | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell invasion | Inhibition | hsa05200 | ||
| Cell migration | Inhibition | hsa04670 | ||
| MEK/ERK /PI3K/AKT signaling pathway | Inhibition | hsa04151 | ||
| In Vitro Model | BxPC-3 cells | Pancreas | Homo sapiens (Human) | CVCL_0186 |
| PANC-1 cells | Pancreas | Homo sapiens (Human) | CVCL_0480 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Flow cytometry assay | |||
| Mechanism Description | Let-7b repletion selectively sensitized kRAS mutant tumor cells to the cytotoxicity of paclitaxel and gemcitabine. Transfection of let-7b mimic downregulated the expression of mutant but not wild-type kRAS. Combination of let-7b mimic with paclitaxel or gemcitabine diminished MEk/ERk and PI3k/AkT signaling concurrently, triggered the onset of apoptosis, and reverted the epithelial-mesenchymal transition in kRAS mutant tumor cells. In addition, let-7b repletion downregulated the expression of beta-tubulin III and ribonucleotide reductase subunit M2, two proteins known to mediate tumor resistance to paclitaxel and gemcitabine, respectively. Let-7 may represent a new class of chemosensitizer for the treatment of kRAS mutant tumors. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Endometrial cancer [ICD-11: 2C76.1] | [3] | |||
| Sensitive Disease | Endometrial cancer [ICD-11: 2C76.1] | |||
| Sensitive Drug | Vincristine | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell migration | Inhibition | hsa04670 | |
| In Vitro Model | Hec50 cells | Endometrium | Homo sapiens (Human) | CVCL_2929 |
| Experiment for Molecule Alteration |
Immunoblotting analysis | |||
| Experiment for Drug Resistance |
ELISA assay | |||
| Mechanism Description | Low or absent miR-200c results in aberrant expression of ZEB1 and consequent repression of E-cadherin. Reinstatement of miR-200c to such cells restores E-cadherin and dramatically reduces migration and invasion. One such gene, class IIIbeta-tubulin (TUBB3), which encodes a tubulin isotype normally found only in neuronal cells, is a direct target of miR-200c. Restoration of miR-200c increases sensitivity to microtubule-targeting agents by up to 85%. Since expression of TUBB3 is a common mechanism of resistance to microtubule-binding chemotherapeutic agents in many types of solid tumors, the ability of miR-200c to restore chemosensitivity to such agents may be explained by its ability to reduce TUBB3. | |||
Disease- and Tissue-specific Abundances of This Molecule
ICD Disease Classification 02

| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Pancreas | |
| The Specified Disease | Pancreatic cancer | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 2.16E-02; Fold-change: 6.56E-01; Z-score: 7.86E-01 | |
| The Expression Level of Disease Section Compare with the Adjacent Tissue | p-value: 1.26E-07; Fold-change: 8.73E-01; Z-score: 1.31E+00 | |
|
Molecule expression in the normal tissue adjacent to the diseased tissue of patients
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |

|
Click to View the Clearer Original Diagram |
| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Lung | |
| The Specified Disease | Lung cancer | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 5.46E-09; Fold-change: 2.83E-01; Z-score: 7.20E-01 | |
| The Expression Level of Disease Section Compare with the Adjacent Tissue | p-value: 1.54E-02; Fold-change: 1.70E-01; Z-score: 3.42E-01 | |
|
Molecule expression in the normal tissue adjacent to the diseased tissue of patients
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |

|
Click to View the Clearer Original Diagram |
| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Breast tissue | |
| The Specified Disease | Breast cancer | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 4.29E-15; Fold-change: 3.01E-01; Z-score: 5.85E-01 | |
| The Expression Level of Disease Section Compare with the Adjacent Tissue | p-value: 3.00E-03; Fold-change: 2.36E-01; Z-score: 3.84E-01 | |
|
Molecule expression in the normal tissue adjacent to the diseased tissue of patients
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |

|
Click to View the Clearer Original Diagram |
| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Ovary | |
| The Specified Disease | Ovarian cancer | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 7.94E-02; Fold-change: 3.53E-01; Z-score: 4.28E-01 | |
| The Expression Level of Disease Section Compare with the Adjacent Tissue | p-value: 7.32E-01; Fold-change: 1.00E+00; Z-score: 6.01E-01 | |
|
Molecule expression in the normal tissue adjacent to the diseased tissue of patients
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |

|
Click to View the Clearer Original Diagram |
| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Prostate | |
| The Specified Disease | Prostate cancer | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 2.82E-01; Fold-change: 9.49E-03; Z-score: 1.43E-02 | |
|
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |

|
Click to View the Clearer Original Diagram |
Tissue-specific Molecule Abundances in Healthy Individuals

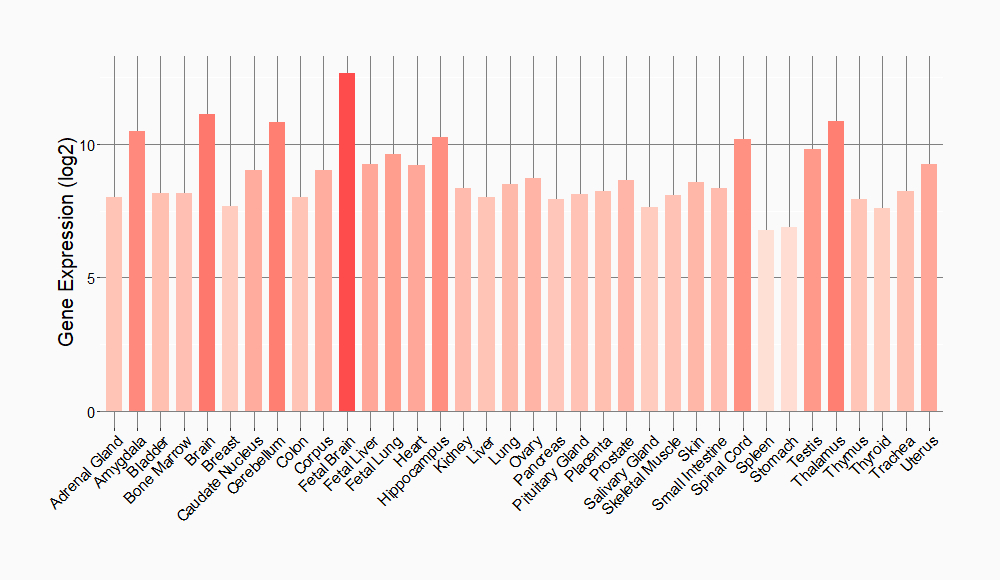
|
||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
