Drug Information
Drug (ID: DG01497) and It's Reported Resistant Information
| Name |
Rigosertib
|
||||
|---|---|---|---|---|---|
| Synonyms |
Rigosertib; 592542-59-1; UNII-67DOW7F9GL; ON 01910; ON-01910; 67DOW7F9GL; 2-[2-methoxy-5-[[(E)-2-(2,4,6-trimethoxyphenyl)ethenyl]sulfonylmethyl]anilino]acetic acid; (E)-2-((2-methoxy-5-(((2,4,6-trimethoxystyryl)sulfonyl)methyl)phenyl)amino)acetic acid; N-[2-Methoxy-5-({[(E)-2-(2,4,6-Trimethoxyphenyl)ethenyl]sulfonyl}methyl)phenyl]glycine; ON-01910.Na; Rigosertib [USAN:INN]; rigosertibum; 6FS; Rigosertib (USAN); Estybon (proposed trade name); SCHEMBL498623; SCHEMBL498624; GTPL7833; CHEMBL1241855; ON01910.Na; DTXSID30207984; 2-[2-methoxy-5-[2-(2,4,6-trimethoxyphenyl)ethenylsulfonylmethyl]anilino]acetic acid; CHEBI:124939; CHEBI:145417; BCP08296; EX-A4346; ZINC3942646; 4006AH; BDBM50060917; AKOS015966442; DB12146; N-[2-Methoxy-5-[[[(1E)-2-(2,4,6-trimethoxyphenyl)ethenyl]sulfonyl]methyl]phenyl]glycine; (E)-ON 01910; 2-[[2-methoxy-5-[[(E)-2-(2,4,6-trimethoxyphenyl)ethenyl]sulfonylmethyl]phenyl]amino]acetic Acid; AC-32479; AS-35249; ON-01910ON-01910; D10154; ON-01910;ON01910;ON 01910; BRD-K55187425-236-01-1; Q21099552; (e)-2-(5-((2,4,6-trimethoxystyrylsulfonyl)methyl)-2-methoxyphenylamino)acetic acid; [2-methoxy-5-({[(E)-2-(2,4,6-trimethoxyphenyl)ethenyl]sulfonyl}methyl)anilino]acetic acid; [2-methoxy-5-({[2-(2,4,6-trimethoxyphenyl)ethenyl]sulfonyl}methyl)anilino]acetic acid; N-(2-Methoxy-5-((((1E)-2-(2,4,6-trimethoxyphenyl)ethenyl)sulfonyl)methyl) phenyl)glycine; N-[2-methoxy-5-({[2-(2,4,6-trimethoxyphenyl)ethenyl]sulfonyl}methyl)phenyl]glycine; Glycine, N-(2-methoxy-5-((((1E)-2-(2,4,6-trimethoxyphenyl)ethenyl)sulfonyl) methyl)phenyl)-; Glycine, N-[2-Methoxy-5-[[[(1E)-2-(2,4,6-triMethoxyphenyl)ethenyl]sulfonyl]Methyl]phenyl]-; ON-01910; ; ; 2-[2-Methoxy-5-[[(E)-2-(2,4,6-trimethoxyphenyl)ethenyl]sulfonylmethyl]anilino]acetic acid
Click to Show/Hide
|
||||
| Indication |
In total 3 Indication(s)
|
||||
| Structure |
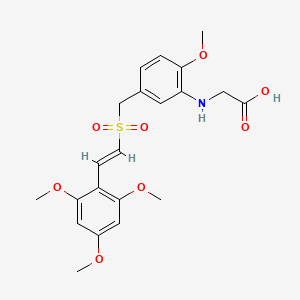
|
||||
| Target | Calcineurin (PPP3CA) | PP2BA_HUMAN | [1] | ||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
11
|
||||
| IsoSMILES |
COC1=C(C=C(C=C1)CS(=O)(=O)/C=C/C2=C(C=C(C=C2OC)OC)OC)NCC(=O)O
|
||||
| InChI |
InChI=1S/C21H25NO8S/c1-27-15-10-19(29-3)16(20(11-15)30-4)7-8-31(25,26)13-14-5-6-18(28-2)17(9-14)22-12-21(23)24/h5-11,22H,12-13H2,1-4H3,(H,23,24)/b8-7+
|
||||
| InChIKey |
OWBFCJROIKNMGD-BQYQJAHWSA-N
|
||||
| PubChem CID | |||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-02: Benign/in-situ/malignant neoplasm
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | |||||||||||||
|
|
|||||||||||||
| Key Molecule: GTPase Hras (HRAS) | [1] | ||||||||||||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | ||||||||||||
| Molecule Alteration | Missense mutation | p.G12V (c.35G>T) |
|||||||||||
| Wild Type Structure | Method: X-ray diffraction | Resolution: 1.98 Å | |||||||||||
| Mutant Type Structure | Method: X-ray diffraction | Resolution: 1.96 Å | |||||||||||
| Download The Information of Sequence | Download The Structure File | ||||||||||||
-
G
-
0
|
S
-
M
M
T
T
E
E
Y
Y
K
K
L
L
V
V
V
V
V
V
10
|
G
G
A
A
G
V
G
G
V
V
G
G
K
K
S
S
A
A
L
L
20
|
T
T
I
I
Q
Q
L
L
I
I
Q
Q
N
N
H
H
F
F
V
V
30
|
D
D
E
E
Y
Y
D
D
P
P
T
T
I
I
E
E
D
D
S
S
40
|
Y
Y
R
R
K
K
Q
Q
V
V
V
V
I
I
D
D
G
G
E
E
50
|
T
T
C
C
L
L
L
L
D
D
I
I
L
L
D
D
T
T
A
A
60
|
G
G
Q
Q
E
E
E
E
Y
Y
S
S
A
A
M
M
R
R
D
D
70
|
Q
Q
Y
Y
M
M
R
R
T
T
G
G
E
E
G
G
F
F
L
L
80
|
C
C
V
V
F
F
A
A
I
I
N
N
N
N
T
T
K
K
S
S
90
|
F
F
E
E
D
D
I
I
H
H
H
H
Y
Y
R
R
E
E
Q
Q
100
|
I
I
K
K
R
R
V
V
K
K
D
D
S
S
E
E
D
D
V
V
110
|
P
P
M
M
V
V
L
L
V
V
G
G
N
N
K
K
S
S
D
D
120
|
L
L
P
P
S
S
R
R
T
T
V
V
D
D
T
T
K
K
Q
Q
130
|
A
A
Q
Q
D
D
L
L
A
A
R
R
S
S
Y
Y
G
G
I
I
140
|
P
P
F
F
I
I
E
E
T
T
S
S
A
A
K
K
T
T
R
R
150
|
Q
Q
G
G
V
V
D
D
D
D
A
A
F
F
Y
Y
T
T
L
L
160
|
V
V
R
R
E
E
I
I
R
R
K
K
H
H
K
K
E
E
K
K
170
|
M
M
S
S
K
K
D
D
G
G
K
K
K
K
K
K
K
K
K
K
180
|
K
K
S
S
K
K
T
T
K
K
C
C
V
V
I
I
M
M
|
|||||||||||||
| Experimental Note | Revealed Based on the Cell Line Data | ||||||||||||
| Cell Pathway Regulation | RAS/RAF/MEK/ERK signaling pathway | Inhibition | hsa01521 | ||||||||||
| In Vitro Model | HCT116 cells | Colon | Homo sapiens (Human) | CVCL_0291 | |||||||||
| A549 cells | Lung | Homo sapiens (Human) | CVCL_0023 | ||||||||||
| Hela cells | Cervix uteri | Homo sapiens (Human) | CVCL_0030 | ||||||||||
| A431 cells | Skin | Homo sapiens (Human) | CVCL_0037 | ||||||||||
| HEK293T cells | Kidney | Homo sapiens (Human) | CVCL_0063 | ||||||||||
| MIA PaCa-2 cells | Pancreas | Homo sapiens (Human) | CVCL_0428 | ||||||||||
| WM1617 cells | Lymph node | Homo sapiens (Human) | CVCL_6791 | ||||||||||
| In Vivo Model | Female nu/nu mouse PDX model | Mus musculus | |||||||||||
| Mechanism Description | Rigosertib, a styryl-benzyl sulfone, acts as a RAS-mimetic and interacts with the RBDs of RAF kinases, resulting in their inability to bind to RAS, disruption of RAF activation, and inhibition of the RAS-RAF-MEK pathway. We also find that rigosertib binds to the RBDs of Ral-GDS and PI3Ks. | ||||||||||||
| Key Molecule: PI3-kinase alpha (PIK3CA) | [1] | ||||||||||||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | ||||||||||||
| Molecule Alteration | Missense mutation | p.H1047R (c.3140A>G) |
|||||||||||
| Wild Type Structure | Method: Electron microscopy | Resolution: 2.41 Å | |||||||||||
| Mutant Type Structure | Method: X-ray diffraction | Resolution: 2.61 Å | |||||||||||
| Download The Information of Sequence | Download The Structure File | ||||||||||||
-
M
-
S
-
Y
-
Y
-
H
-
H
-
H
-
-20
|
H
-
H
-
H
-
D
-
Y
-
D
-
I
-
P
-
T
-
T
-
-10
|
E
-
N
-
L
-
Y
-
F
-
Q
-
G
G
A
A
M
M
G
G
0
|
S
S
M
M
P
P
P
P
R
R
P
P
S
S
S
S
G
G
E
E
10
|
L
L
W
W
G
G
I
I
H
H
L
L
M
M
P
P
P
P
R
R
20
|
I
I
L
L
V
V
E
E
C
C
L
L
L
L
P
P
N
N
G
G
30
|
M
M
I
I
V
V
T
T
L
L
E
E
C
C
L
L
R
R
E
E
40
|
A
A
T
T
L
L
I
I
T
T
I
I
K
K
H
H
E
E
L
L
50
|
F
F
K
K
E
E
A
A
R
R
K
K
Y
Y
P
P
L
L
H
H
60
|
Q
Q
L
L
L
L
Q
Q
D
D
E
E
S
S
S
S
Y
Y
I
I
70
|
F
F
V
V
S
S
V
V
T
T
Q
Q
E
E
A
A
E
E
R
R
80
|
E
E
E
E
F
F
F
F
D
D
E
E
T
T
R
R
R
R
L
L
90
|
C
C
D
D
L
L
R
R
L
L
F
F
Q
Q
P
P
F
F
L
L
100
|
K
K
V
V
I
I
E
E
P
P
V
V
G
G
N
N
R
R
E
E
110
|
E
E
K
K
I
I
L
L
N
N
R
R
E
E
I
I
G
G
F
F
120
|
A
A
I
I
G
G
M
M
P
P
V
V
C
C
E
E
F
F
D
D
130
|
M
M
V
V
K
K
D
D
P
P
E
E
V
V
Q
Q
D
D
F
F
140
|
R
R
R
R
N
N
I
I
L
L
N
N
V
V
C
C
K
K
E
E
150
|
A
A
V
V
D
D
L
L
R
R
D
D
L
L
N
N
S
S
P
P
160
|
H
H
S
S
R
R
A
A
M
M
Y
Y
V
V
Y
Y
P
P
P
P
170
|
N
N
V
V
E
E
S
S
S
S
P
P
E
E
L
L
P
P
K
K
180
|
H
H
I
I
Y
Y
N
N
K
K
L
L
D
D
K
K
G
G
Q
Q
190
|
I
I
I
I
V
V
V
V
I
I
W
W
V
V
I
I
V
V
S
S
200
|
P
P
N
N
N
N
D
D
K
K
Q
Q
K
K
Y
Y
T
T
L
L
210
|
K
K
I
I
N
N
H
H
D
D
C
C
V
V
P
P
E
E
Q
Q
220
|
V
V
I
I
A
A
E
E
A
A
I
I
R
R
K
K
K
K
T
T
230
|
R
R
S
S
M
M
L
L
L
L
S
S
S
S
E
E
Q
Q
L
L
240
|
K
K
L
L
C
C
V
V
L
L
E
E
Y
Y
Q
Q
G
G
K
K
250
|
Y
Y
I
I
L
L
K
K
V
V
C
C
G
G
C
C
D
D
E
E
260
|
Y
Y
F
F
L
L
E
E
K
K
Y
Y
P
P
L
L
S
S
Q
Q
270
|
Y
Y
K
K
Y
Y
I
I
R
R
S
S
C
C
I
I
M
M
L
L
280
|
G
G
R
R
M
M
P
P
N
N
L
L
M
M
L
L
M
M
A
A
290
|
K
K
E
E
S
S
L
L
Y
Y
S
S
Q
Q
L
L
P
P
M
M
300
|
D
D
C
C
F
F
T
T
M
M
P
P
S
S
Y
Y
S
S
R
R
310
|
R
R
I
I
S
S
T
T
A
A
T
T
P
P
Y
Y
M
M
N
N
320
|
G
G
E
E
T
T
S
S
T
T
K
K
S
S
L
L
W
W
V
V
330
|
I
I
N
N
S
S
A
A
L
L
R
R
I
I
K
K
I
I
L
L
340
|
C
C
A
A
T
T
Y
Y
V
V
N
N
V
V
N
N
I
I
R
R
350
|
D
D
I
I
D
D
K
K
I
I
Y
Y
V
V
R
R
T
T
G
G
360
|
I
I
Y
Y
H
H
G
G
G
G
E
E
P
P
L
L
C
C
D
D
370
|
N
N
V
V
N
N
T
T
Q
Q
R
R
V
V
P
P
C
C
S
S
380
|
N
N
P
P
R
R
W
W
N
N
E
E
W
W
L
L
N
N
Y
Y
390
|
D
D
I
I
Y
Y
I
I
P
P
D
D
L
L
P
P
R
R
A
A
400
|
A
A
R
R
L
L
C
C
L
L
S
S
I
I
C
C
S
S
V
V
410
|
K
K
G
G
R
R
K
K
G
G
A
A
K
K
E
E
E
E
H
H
420
|
C
C
P
P
L
L
A
A
W
W
G
G
N
N
I
I
N
N
L
L
430
|
F
F
D
D
Y
Y
T
T
D
D
T
T
L
L
V
V
S
S
G
G
440
|
K
K
M
M
A
A
L
L
N
N
L
L
W
W
P
P
V
V
P
P
450
|
H
H
G
G
L
L
E
E
D
D
L
L
L
L
N
N
P
P
I
I
460
|
G
G
V
V
T
T
G
G
S
S
N
N
P
P
N
N
K
K
E
E
470
|
T
T
P
P
C
C
L
L
E
E
L
L
E
E
F
F
D
D
W
W
480
|
F
F
S
S
S
S
V
V
V
V
K
K
F
F
P
P
D
D
M
M
490
|
S
S
V
V
I
I
E
E
E
E
H
H
A
A
N
N
W
W
S
S
500
|
V
V
S
S
R
R
E
E
A
A
G
G
F
F
S
S
Y
Y
S
S
510
|
H
H
A
A
G
G
L
L
S
S
N
N
R
R
L
L
A
A
R
R
520
|
D
D
N
N
E
E
L
L
R
R
E
E
N
N
D
D
K
K
E
E
530
|
Q
Q
L
L
K
K
A
A
I
I
S
S
T
T
R
R
D
D
P
P
540
|
L
L
S
S
E
E
I
I
T
T
E
E
Q
Q
E
E
K
K
D
D
550
|
F
F
L
L
W
W
S
S
H
H
R
R
H
H
Y
Y
C
C
V
V
560
|
T
T
I
I
P
P
E
E
I
I
L
L
P
P
K
K
L
L
L
L
570
|
L
L
S
S
V
V
K
K
W
W
N
N
S
S
R
R
D
D
E
E
580
|
V
V
A
A
Q
Q
M
M
Y
Y
C
C
L
L
V
V
K
K
D
D
590
|
W
W
P
P
P
P
I
I
K
K
P
P
E
E
Q
Q
A
A
M
M
600
|
E
E
L
L
L
L
D
D
C
C
N
N
Y
Y
P
P
D
D
P
P
610
|
M
M
V
V
R
R
G
G
F
F
A
A
V
V
R
R
C
C
L
L
620
|
E
E
K
K
Y
Y
L
L
T
T
D
D
D
D
K
K
L
L
S
S
630
|
Q
Q
Y
Y
L
L
I
I
Q
Q
L
L
V
V
Q
Q
V
V
L
L
640
|
K
K
Y
Y
E
E
Q
Q
Y
Y
L
L
D
D
N
N
L
L
L
L
650
|
V
V
R
R
F
F
L
L
L
L
K
K
K
K
A
A
L
L
T
T
660
|
N
N
Q
Q
R
R
I
I
G
G
H
H
F
F
F
F
F
F
W
W
670
|
H
H
L
L
K
K
S
S
E
E
M
M
H
H
N
N
K
K
T
T
680
|
V
V
S
S
Q
Q
R
R
F
F
G
G
L
L
L
L
L
L
E
E
690
|
S
S
Y
Y
C
C
R
R
A
A
C
C
G
G
M
M
Y
Y
L
L
700
|
K
K
H
H
L
L
N
N
R
R
Q
Q
V
V
E
E
A
A
M
M
710
|
E
E
K
K
L
L
I
I
N
N
L
L
T
T
D
D
I
I
L
L
720
|
K
K
Q
Q
E
E
K
K
K
K
D
D
E
E
T
T
Q
Q
K
K
730
|
V
V
Q
Q
M
M
K
K
F
F
L
L
V
V
E
E
Q
Q
M
M
740
|
R
R
R
R
P
P
D
D
F
F
M
M
D
D
A
A
L
L
Q
Q
750
|
G
G
F
F
L
L
S
S
P
P
L
L
N
N
P
P
A
A
H
H
760
|
Q
Q
L
L
G
G
N
N
L
L
R
R
L
L
E
E
E
E
C
C
770
|
R
R
I
I
M
M
S
S
S
S
A
A
K
K
R
R
P
P
L
L
780
|
W
W
L
L
N
N
W
W
E
E
N
N
P
P
D
D
I
I
M
M
790
|
S
S
E
E
L
L
L
L
F
F
Q
Q
N
N
N
N
E
E
I
I
800
|
I
I
F
F
K
K
N
N
G
G
D
D
D
D
L
L
R
R
Q
Q
810
|
D
D
M
M
L
L
T
T
L
L
Q
Q
I
I
I
I
R
R
I
I
820
|
M
M
E
E
N
N
I
I
W
W
Q
Q
N
N
Q
Q
G
G
L
L
830
|
D
D
L
L
R
R
M
M
L
L
P
P
Y
Y
G
G
C
C
L
L
840
|
S
S
I
I
G
G
D
D
C
C
V
V
G
G
L
L
I
I
E
E
850
|
V
V
V
V
R
R
N
N
S
S
H
H
T
T
I
I
M
M
Q
Q
860
|
I
I
Q
Q
C
C
K
K
G
G
G
G
L
L
K
K
G
G
A
A
870
|
L
L
Q
Q
F
F
N
N
S
S
H
H
T
T
L
L
H
H
Q
Q
880
|
W
W
L
L
K
K
D
D
K
K
N
N
K
K
G
G
E
E
I
I
890
|
Y
Y
D
D
A
A
A
A
I
I
D
D
L
L
F
F
T
T
R
R
900
|
S
S
C
C
A
A
G
G
Y
Y
C
C
V
V
A
A
T
T
F
F
910
|
I
I
L
L
G
G
I
I
G
G
D
D
R
R
H
H
N
N
S
S
920
|
N
N
I
I
M
M
V
V
K
K
D
D
D
D
G
G
Q
Q
L
L
930
|
F
F
H
H
I
I
D
D
F
F
G
G
H
H
F
F
L
L
D
D
940
|
H
H
K
K
K
K
K
K
K
K
F
F
G
G
Y
Y
K
K
R
R
950
|
E
E
R
R
V
V
P
P
F
F
V
V
L
L
T
T
Q
Q
D
D
960
|
F
F
L
L
I
I
V
V
I
I
S
S
K
K
G
G
A
A
Q
Q
970
|
E
E
C
C
T
T
K
K
T
T
R
R
E
E
F
F
E
E
R
R
980
|
F
F
Q
Q
E
E
M
M
C
C
Y
Y
K
K
A
A
Y
Y
L
L
990
|
A
A
I
I
R
R
Q
Q
H
H
A
A
N
N
L
L
F
F
I
I
1000
|
N
N
L
L
F
F
S
S
M
M
M
M
L
L
G
G
S
S
G
G
1010
|
M
M
P
P
E
E
L
L
Q
Q
S
S
F
F
D
D
D
D
I
I
1020
|
A
A
Y
Y
I
I
R
R
K
K
T
T
L
L
A
A
L
L
D
D
1030
|
K
K
T
T
E
E
Q
Q
E
E
A
A
L
L
E
E
Y
Y
F
F
1040
|
M
M
K
K
Q
Q
M
M
N
N
D
D
A
A
H
R
H
H
G
G
1050
|
G
G
W
W
T
T
T
T
K
K
M
M
D
D
W
W
I
I
F
F
1060
|
H
H
T
T
I
I
K
K
Q
Q
H
H
A
A
L
L
N
N
|
|||||||||||||
| Experimental Note | Revealed Based on the Cell Line Data | ||||||||||||
| Cell Pathway Regulation | RAS/RAF/MEK/ERK signaling pathway | Inhibition | hsa01521 | ||||||||||
| In Vitro Model | HCT116 cells | Colon | Homo sapiens (Human) | CVCL_0291 | |||||||||
| A549 cells | Lung | Homo sapiens (Human) | CVCL_0023 | ||||||||||
| Hela cells | Cervix uteri | Homo sapiens (Human) | CVCL_0030 | ||||||||||
| A431 cells | Skin | Homo sapiens (Human) | CVCL_0037 | ||||||||||
| HEK293T cells | Kidney | Homo sapiens (Human) | CVCL_0063 | ||||||||||
| MIA PaCa-2 cells | Pancreas | Homo sapiens (Human) | CVCL_0428 | ||||||||||
| WM1617 cells | Lymph node | Homo sapiens (Human) | CVCL_6791 | ||||||||||
| In Vivo Model | Female nu/nu mouse PDX model | Mus musculus | |||||||||||
| Mechanism Description | Rigosertib, a styryl-benzyl sulfone, acts as a RAS-mimetic and interacts with the RBDs of RAF kinases, resulting in their inability to bind to RAS, disruption of RAF activation, and inhibition of the RAS-RAF-MEK pathway. We also find that rigosertib binds to the RBDs of Ral-GDS and PI3Ks. | ||||||||||||
| Key Molecule: PI3-kinase alpha (PIK3CA) | [1] | ||||||||||||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | ||||||||||||
| Molecule Alteration | Missense mutation | p.E545K (c.1633G>A) |
|||||||||||
| Experimental Note | Revealed Based on the Cell Line Data | ||||||||||||
| Cell Pathway Regulation | RAS/RAF/MEK/ERK signaling pathway | Inhibition | hsa01521 | ||||||||||
| In Vitro Model | HCT116 cells | Colon | Homo sapiens (Human) | CVCL_0291 | |||||||||
| A549 cells | Lung | Homo sapiens (Human) | CVCL_0023 | ||||||||||
| Hela cells | Cervix uteri | Homo sapiens (Human) | CVCL_0030 | ||||||||||
| A431 cells | Skin | Homo sapiens (Human) | CVCL_0037 | ||||||||||
| HEK293T cells | Kidney | Homo sapiens (Human) | CVCL_0063 | ||||||||||
| MIA PaCa-2 cells | Pancreas | Homo sapiens (Human) | CVCL_0428 | ||||||||||
| WM1617 cells | Lymph node | Homo sapiens (Human) | CVCL_6791 | ||||||||||
| In Vivo Model | Female nu/nu mouse PDX model | Mus musculus | |||||||||||
| Mechanism Description | Rigosertib, a styryl-benzyl sulfone, acts as a RAS-mimetic and interacts with the RBDs of RAF kinases, resulting in their inability to bind to RAS, disruption of RAF activation, and inhibition of the RAS-RAF-MEK pathway. We also find that rigosertib binds to the RBDs of Ral-GDS and PI3Ks. | ||||||||||||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: PI3-kinase alpha (PIK3CA) | [2] | |||
| Sensitive Disease | Head and neck squamous cell carcinoma [ICD-11: 2D42.1] | |||
| Molecule Alteration | Missense mutation | p.E545K (c.1633G>A) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | HNSCC cells | Neck | Homo sapiens (Human) | N.A. |
| Experiment for Molecule Alteration |
DNA sequencing assay | |||
| Experiment for Drug Resistance |
Sulforhodamine B colorimetric assay | |||
| Mechanism Description | The missense mutation p.E545K (c.1633G>A) in gene PIK3CA cause the sensitivity of Rigosertib by aberration of the drug's therapeutic target | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
