Drug Information
Drug (ID: DG01483) and It's Reported Resistant Information
| Name |
U0126
|
||||
|---|---|---|---|---|---|
| Synonyms |
U0126; 109511-58-2; U-0126; 1,4-Diamino-2,3-dicyano-1,4-bis(o-aminophenylmercapto)butadiene; U 0126; UNII-8027P94HLL; CHEBI:64208; (2Z,3Z)-2,3-bis(amino((2-aminophenyl)thio)methylene)succinonitrile; 8027P94HLL; C18H16N6S2; (2Z,3Z)-bis{amino[(2-aminophenyl)sulfanyl]methylidene}butanedinitrile; 109511-58-2 (free); 218601-62-8; FT-1069-1; (2z)-Bis{amino[(2-Aminophenyl)sulfanyl]methylidene}butanedinitrile; (2Z,3Z)-bis{amino[(2-aminophenyl)sulfanyl]methylene}succinonitrile; 1,4-Diamino-2,3-dicyano-1,4-bis[2-aminophenylthio]butadiene; (2Z,3Z)-2,3-bis[amino-(2-aminophenyl)sulfanylmethylidene]butanedinitrile; UO-126; BiomolKI_000002; BiomolKI2_000012; BMK1-B2; BSPBio_001224; CHEMBL34704; GTPL5282; Succinonitrile, bis(amino(o-aminophenylthio)methylene)-; CHEBI:90693; CHEBI:91463; Butanedinitrile, bis(amino((2-aminophenyl)thio)methylene)-; DTXSID10892034; HMS1362N05; HMS1792N05; HMS1990N05; HMS3403N05; HMS3414K05; HMS3678K05; AMY31125; BCP01851; EX-A1754; HY-12031A; (2Z,3Z)-2,3-bis[amino-(2-aminophenyl)sulfanyl-methylene]butanedinitrile; (2Z,3Z)-bis({amino[(2-aminophenyl)sulfanyl]methylidene})butanedinitrile; AKOS024456414; ZINC100007148; CCG-100606; LS40194; IDI1_002207; SMP2_000197; NCGC00025029-02; NCGC00025029-03; NCGC00025029-04; CS-0003351; U 126; V2422; A846574; A906530; SR-01000597365; J-002297; Q7863562; SR-01000597365-1; BRD-K18787491-001-04-5; BRD-K46419649-001-01-8; 2,3-Bis(amino((2-aminophenyl)thio)methylene)succinonitrile; Butanedinitrile,2,3-bis[amino[(2-aminophenyl)thio]methylene]-; (2Z,3Z)-2,3-Bis[amino[(2-aminophenyl)thio]methylene]butanedinitrile; Butanedinitrile, bis(amino((2-aminophenyl)thio)methylene)-, (2Z,3Z)-; 5BM
Click to Show/Hide
|
||||
| Indication |
In total 1 Indication(s)
|
||||
| Structure |
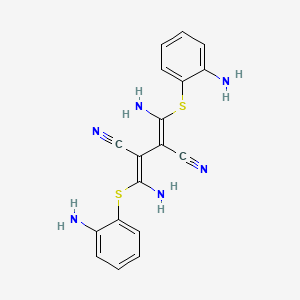
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Clinically Reported Resistance for This Drug
(1 diseases)
[2]
|
||||
| Target | Estrogen receptor (ESR) | ESR1_HUMAN | [2] | ||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
5
|
||||
| IsoSMILES |
C1=CC=C(C(=C1)N)S/C(=C(/C(=C(/SC2=CC=CC=C2N)\\N)/C#N)\\C#N)/N
|
||||
| InChI |
InChI=1S/C18H16N6S2/c19-9-11(17(23)25-15-7-3-1-5-13(15)21)12(10-20)18(24)26-16-8-4-2-6-14(16)22/h1-8H,21-24H2/b17-11+,18-12+
|
||||
| InChIKey |
DVEXZJFMOKTQEZ-JYFOCSDGSA-N
|
||||
| PubChem CID | |||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-02: Benign/in-situ/malignant neoplasm
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | |||||||||||||
|
|
|||||||||||||
| Key Molecule: Tyrosine-protein phosphatase non-receptor type 11 (PTPN11) | [1] | ||||||||||||
| Sensitive Disease | Acute lymphocytic leukemia [ICD-11: 2B33.0] | ||||||||||||
| Molecule Alteration | Missense mutation | p.E76K (c.226G>A) |
|||||||||||
| Wild Type Structure | Method: X-ray diffraction | Resolution: 1.70 Å | |||||||||||
| Mutant Type Structure | Method: X-ray diffraction | Resolution: 2.62 Å | |||||||||||
| Download The Information of Sequence | Download The Structure File | ||||||||||||
-
0
|
S
G
M
M
T
T
S
S
R
R
R
R
W
W
F
F
H
H
P
P
10
|
N
N
I
I
T
T
G
G
V
V
E
E
A
A
E
E
N
N
L
L
20
|
L
L
L
L
T
T
R
R
G
G
V
V
D
D
G
G
S
S
F
F
30
|
L
L
A
A
R
R
P
P
S
S
K
K
S
S
N
N
P
P
G
G
40
|
D
D
F
F
T
T
L
L
S
S
V
V
R
R
R
R
N
N
G
G
50
|
A
A
V
V
T
T
H
H
I
I
K
K
I
I
Q
Q
N
N
T
T
60
|
G
G
D
D
Y
Y
Y
Y
D
D
L
L
Y
Y
G
G
G
G
E
E
70
|
K
K
F
F
A
A
T
T
L
L
A
A
E
K
L
L
V
V
Q
Q
80
|
Y
Y
Y
Y
M
M
E
E
H
H
H
H
G
G
Q
Q
L
L
K
K
90
|
E
E
K
K
N
N
G
G
D
D
V
V
I
I
E
E
L
L
K
K
100
|
Y
Y
P
P
L
L
N
N
C
C
A
A
D
D
P
P
T
T
S
S
110
|
E
E
R
R
W
W
F
F
H
H
G
G
H
H
L
L
S
S
G
G
120
|
K
K
E
E
A
A
E
E
K
K
L
L
L
L
T
T
E
E
K
K
130
|
G
G
K
K
H
H
G
G
S
S
F
F
L
L
V
V
R
R
E
E
140
|
S
S
Q
Q
S
S
H
H
P
P
G
G
D
D
F
F
V
V
L
L
150
|
S
S
V
V
R
R
T
T
G
G
D
D
D
D
K
K
G
G
E
E
160
|
S
S
N
N
D
D
G
G
K
K
S
S
K
K
V
V
T
T
H
H
170
|
V
V
M
M
I
I
R
R
C
C
Q
Q
E
E
L
L
K
K
Y
Y
180
|
D
D
V
V
G
G
G
G
G
G
E
E
R
R
F
F
D
D
S
S
190
|
L
L
T
T
D
D
L
L
V
V
E
E
H
H
Y
Y
K
K
K
K
200
|
N
N
P
P
M
M
V
V
E
E
T
T
L
L
G
G
T
T
V
V
210
|
L
L
Q
Q
L
L
K
K
Q
Q
P
P
L
L
N
N
T
T
T
T
220
|
R
R
I
I
N
N
A
A
A
A
E
E
I
I
E
E
S
S
R
R
230
|
V
V
R
R
E
E
L
L
S
S
K
K
L
L
A
A
E
E
T
T
240
|
T
T
D
D
K
K
V
V
K
K
Q
Q
G
G
F
F
W
W
E
E
250
|
E
E
F
F
E
E
T
T
L
L
Q
Q
Q
Q
Q
Q
E
E
C
C
260
|
K
K
L
L
L
L
Y
Y
S
S
R
R
K
K
E
E
G
G
Q
Q
270
|
R
R
Q
Q
E
E
N
N
K
K
N
N
K
K
N
N
R
R
Y
Y
280
|
K
K
N
N
I
I
L
L
P
P
F
F
D
D
H
H
T
T
R
R
290
|
V
V
V
V
L
L
H
H
D
D
G
G
D
D
P
P
N
N
E
E
300
|
P
P
V
V
S
S
D
D
Y
Y
I
I
N
N
A
A
N
N
I
I
310
|
I
I
M
M
P
P
E
E
F
F
E
E
T
T
K
K
C
C
N
N
320
|
N
N
S
S
K
K
P
P
K
K
K
K
S
S
Y
Y
I
I
A
A
330
|
T
T
Q
Q
G
G
C
C
L
L
Q
Q
N
N
T
T
V
V
N
N
340
|
D
D
F
F
W
W
R
R
M
M
V
V
F
F
Q
Q
E
E
N
N
350
|
S
S
R
R
V
V
I
I
V
V
M
M
T
T
T
T
K
K
E
E
360
|
V
V
E
E
R
R
G
G
K
K
S
S
K
K
C
C
V
V
K
K
370
|
Y
Y
W
W
P
P
D
D
E
E
Y
Y
A
A
L
L
K
K
E
E
380
|
Y
Y
G
G
V
V
M
M
R
R
V
V
R
R
N
N
V
V
K
K
390
|
E
E
S
S
A
A
A
A
H
H
D
D
Y
Y
T
T
L
L
R
R
400
|
E
E
L
L
K
K
L
L
S
S
K
K
V
V
G
G
Q
Q
G
G
410
|
N
N
T
T
E
E
R
R
T
T
V
V
W
W
Q
Q
Y
Y
H
H
420
|
F
F
R
R
T
T
W
W
P
P
D
D
H
H
G
G
V
V
P
P
430
|
S
S
D
D
P
P
G
G
G
G
V
V
L
L
D
D
F
F
L
L
440
|
E
E
E
E
V
V
H
H
H
H
K
K
Q
Q
E
E
S
S
I
I
450
|
M
M
D
D
A
A
G
G
P
P
V
V
V
V
V
V
H
H
C
C
460
|
S
S
A
A
G
G
I
I
G
G
R
R
T
T
G
G
T
T
F
F
470
|
I
I
V
V
I
I
D
D
I
I
L
L
I
I
D
D
I
I
I
I
480
|
R
R
E
E
K
K
G
G
V
V
D
D
C
C
D
D
I
I
D
D
490
|
V
V
P
P
K
K
T
T
I
I
Q
Q
M
M
V
V
R
R
S
S
500
|
Q
Q
R
R
S
S
G
G
M
M
V
V
Q
Q
T
T
E
E
A
A
510
|
Q
Q
Y
Y
R
R
F
F
I
I
Y
Y
M
M
A
A
V
V
Q
Q
520
|
H
H
Y
Y
I
I
E
E
T
T
L
L
|
|||||||||||||
| Experimental Note | Revealed Based on the Cell Line Data | ||||||||||||
| Experiment for Molecule Alteration |
Immunoblotting analysis | ||||||||||||
| Experiment for Drug Resistance |
Flow cytometry assay | ||||||||||||
| Mechanism Description | The missense mutation p.E76K (c.226G>A) in gene PTPN11 cause the sensitivity of U0126 by unusual activation of pro-survival pathway | ||||||||||||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Serine/threonine-protein kinase B-raf (BRAF) | [2] | |||
| Resistant Disease | Melanoma [ICD-11: 2C30.0] | |||
| Molecule Alteration | Missense mutation | p.G469E (c.1406G>A) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Human melanoma tissue | N.A. | ||
| In Vivo Model | (SCID) CB-17 mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | The missense mutation p.G469E (c.1406G>A) in gene BRAF cause the resistance of U0126 by unusual activation of pro-survival pathway | |||
| Key Molecule: Serine/threonine-protein kinase B-raf (BRAF) | [2] | |||
| Resistant Disease | Melanoma [ICD-11: 2C30.0] | |||
| Molecule Alteration | Missense mutation | p.D594G (c.1781A>G) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Human melanoma tissue | N.A. | ||
| In Vivo Model | (SCID) CB-17 mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | The missense mutation p.D594G (c.1781A>G) in gene BRAF cause the resistance of U0126 by unusual activation of pro-survival pathway | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | |||||||||||||
|
|
|||||||||||||
| Key Molecule: Guanine nucleotide-binding protein alpha-q (GNAQ) | [3] | ||||||||||||
| Sensitive Disease | Uveal melanoma [ICD-11: 2D0Y.0] | ||||||||||||
| Molecule Alteration | Missense mutation | p.Q209L (c.626A>T) |
|||||||||||
| Wild Type Structure | Method: Electron microscopy | Resolution: 3.50 Å | |||||||||||
| Mutant Type Structure | Method: Electron microscopy | Resolution: 2.90 Å | |||||||||||
| Download The Information of Sequence | Download The Structure File | ||||||||||||
-
-
M
-
H
-
H
-
H
-
H
-
H
-
H
-
H
-
H
0
|
-
H
M
H
T
T
L
L
E
E
S
S
I
I
M
M
A
A
C
C
10
|
C
C
L
L
S
S
E
E
E
E
A
A
K
K
E
E
A
A
R
R
20
|
R
R
I
I
N
N
D
D
E
E
I
I
E
E
R
R
Q
Q
L
L
30
|
R
R
R
R
D
D
K
K
R
R
D
D
A
A
R
R
R
R
E
E
40
|
L
L
K
K
L
L
L
L
L
L
L
L
G
G
T
T
G
G
E
E
50
|
S
S
G
G
K
K
S
S
T
T
F
F
I
I
K
K
Q
Q
M
M
60
|
R
R
I
I
I
I
H
H
G
G
S
S
G
G
Y
Y
S
S
D
D
70
|
E
E
D
D
K
K
R
R
G
G
F
F
T
T
K
K
L
L
V
V
80
|
Y
Y
Q
Q
N
N
I
I
F
F
T
T
A
A
M
M
Q
Q
A
A
90
|
M
M
I
I
R
R
A
A
M
M
D
D
T
T
L
L
K
K
I
I
100
|
P
P
Y
Y
K
K
Y
Y
E
E
H
H
N
N
K
K
A
A
H
H
110
|
A
A
Q
Q
L
L
V
V
R
R
E
E
V
V
D
D
V
V
E
E
120
|
K
K
V
V
S
S
A
A
F
F
E
E
N
N
P
P
Y
Y
V
V
130
|
D
D
A
A
I
I
K
K
S
S
L
L
W
W
N
N
D
D
P
P
140
|
G
G
I
I
Q
Q
E
E
C
C
Y
Y
D
D
R
R
R
R
R
R
150
|
E
E
Y
Y
Q
Q
L
L
S
S
D
D
S
S
T
T
K
K
Y
Y
160
|
Y
Y
L
L
N
N
D
D
L
L
D
D
R
R
V
V
A
A
D
D
170
|
P
P
A
A
Y
Y
L
L
P
P
T
T
Q
Q
Q
Q
D
D
V
V
180
|
L
L
R
R
V
V
R
Q
V
V
P
P
T
T
T
T
G
G
I
I
190
|
I
I
E
E
Y
Y
P
P
F
F
D
D
L
L
Q
Q
S
S
V
V
200
|
I
I
F
F
R
R
M
M
V
V
D
D
V
V
G
G
G
G
Q
L
210
|
R
R
S
S
E
E
R
R
R
R
K
K
W
W
I
I
H
H
C
C
220
|
F
F
E
E
N
N
V
V
T
T
S
S
I
I
M
M
F
F
L
L
230
|
V
V
A
A
L
L
S
S
E
E
Y
Y
D
D
Q
Q
V
V
L
L
240
|
V
V
E
E
S
S
D
D
N
N
E
E
N
N
R
R
M
M
E
E
250
|
E
E
S
S
K
K
A
A
L
L
F
F
R
R
T
T
I
I
I
I
260
|
T
T
Y
Y
P
P
W
W
F
F
Q
Q
N
N
S
S
S
S
V
V
270
|
I
I
L
L
F
F
L
L
N
N
K
K
K
K
D
D
L
L
L
L
280
|
E
E
E
E
K
K
I
I
M
M
Y
Y
S
S
H
H
L
L
V
V
290
|
D
D
Y
Y
F
F
P
P
E
E
Y
Y
D
D
G
G
P
P
Q
Q
300
|
R
R
D
D
A
A
Q
Q
A
A
A
A
R
R
E
E
F
F
I
I
310
|
L
L
K
K
M
M
F
F
V
V
D
D
L
L
N
N
P
P
D
D
320
|
S
S
D
D
K
K
I
I
I
I
Y
Y
S
S
H
H
F
F
T
T
330
|
C
C
A
A
T
T
D
D
T
T
E
E
N
N
I
I
R
R
F
F
340
|
V
V
F
F
A
A
A
A
V
V
K
K
D
D
T
T
I
I
L
L
350
|
Q
Q
L
L
N
N
L
L
K
K
E
E
Y
Y
N
N
L
L
V
V
|
|||||||||||||
| Experimental Note | Revealed Based on the Cell Line Data | ||||||||||||
| In Vitro Model | Eyeball | N.A. | |||||||||||
| Experiment for Molecule Alteration |
DNA sequencing assay | ||||||||||||
| Experiment for Drug Resistance |
CyQUANT Cell (Invitrogen) proliferation assay | ||||||||||||
| Mechanism Description | The missense mutation p.Q209L (c.626A>T) in gene GNAQ cause the sensitivity of U0126 by unusual activation of pro-survival pathway | ||||||||||||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
