Drug Information
Drug (ID: DG01190) and It's Reported Resistant Information
| Name |
Midostaurin
|
||||
|---|---|---|---|---|---|
| Synonyms |
Midostaurin; PKC412; 120685-11-2; Cgp 41251; PKC-412; 4'-N-Benzoylstaurosporine; Benzoylstaurosporine; CGP-41251; RYDAPT; PKC 412; UNII-ID912S5VON; N-Benzoylstaurosporine; ID912S5VON; CHEMBL608533; CHEBI:63452; N-[(5S,6R,7R,9R)-6-methoxy-5-methyl-14-oxo-6,7,8,9,15,16-hexahydro-5H,14H-5,9-epoxy-4b,9a,15-triazadibenzo[b,h]cyclonona[1,2,3,4-jkl]cyclopenta[e]-as-indacen-7-yl]-N-methylbenzamide; Cgp 41 251; Midostaurin [USAN:INN]; CGP 41231; NSC-656576; Rydapt (TN); Midostaurin(PKC412); 4-N-benzoylstaurosporine; Staurosporine, N-Benzoyl; NVP-PKC412; Midostaurin (JAN/USAN/INN); GTPL5702; SCHEMBL8295379; HMS3229K17; EX-A1741; BDBM50326053; CGP-41521; MFCD00871372; NSC800791; s8064; AKOS024457372; ZINC100013130; CCG-101288; CS-3331; DB06595; NSC 656576; NSC-800791; NCGC00241102-01; NCGC00241102-02; NCGC00241102-05; NCGC00484987-03; AC-31929; Benzamide, N-(2,3,9,10,11,12-hexahydro-9-methoxy-8-methyl-1-oxo-8,12-epoxy-1H,8H-2,7b,12a-triazadibenzo(a,g)cyclonona(cde)trinden-10-yl)-N-methyl-, (8alpha,9beta,10beta,12alpha)-; HY-10230; N-((9S,10R,11R,13R)-10-methoxy-9-methyl-1-oxo-2,3,10,11,12,13-hexahydro-9,13-epoxy-1H,9H-diindolo(1,2,3-gh:3',2',1'-lm)pyrrolo(3,4-j)(1,7)benzodiazonin-11-yl)-N-methylbenzamide; C71714; D05029; J-004379; Q6842945; BRD-K13646352-001-01-2; [9S-(9 ,10 ,11 ,13 )]-N-(2,3,10,11,12,13-Hexahydro-10-methoxy-9-methyl-1-oxo-9,13-epoxy-1H,9H-diindolo[1,2,3-gh:3',2',1'-lm]pyrrolo[3,4-j][1,7]benzodiazonin-11-yl)-N-methylbenzamide; Benzamide, N-((9S,10R,11R,13R)-2,3,9,10,11,12-hexahydro-10-methoxy-9-methyl-1-oxo-9,13-epoxy-1H,9H-diindolo(1,2,3-gh:3',2',1'-lm)pyrrolo(3,4-j)(1,7)benzodiazonin-11-yl)-N-methyl-; N-[(2S,3R,4R,6R)-3-methoxy-2-methyl-16-oxo-29-oxa-1,7,17-triazaoctacyclo[12.12.2.1^{2,6}.0^{7,28}.0^{8,13}.0^{15,19}.0^{20,27}.0^{21,26}]nonacosa-8,10,12,14(28),15(19),20(27),21,23,25-nonaen-4-yl]-N-methylbenzamide; N-[(2S,3R,4R,6R)-3-methoxy-2-methyl-16-oxo-29-oxa-1,7,17-triazaoctacyclo[12.12.2.12,6.07,28.08,13.015,19.020,27.021,26]nonacosa-8,10,12,14,19,21,23,25,27-nonaen-4-yl]-N-methylbenzamide
Click to Show/Hide
|
||||
| Indication |
In total 5 Indication(s)
|
||||
| Structure |
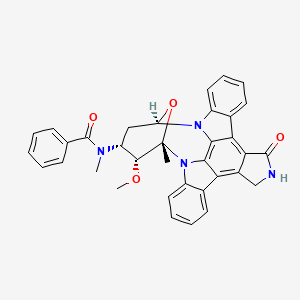
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Clinically Reported Resistance for This Drug
(1 diseases)
[2]
Disease(s) with Resistance Information Discovered by Cell Line Test for This Drug
(1 diseases)
[1]
|
||||
| Target | Fms-like tyrosine kinase 3 (FLT-3) | FLT3_HUMAN | [1] | ||
| Protein kinase C gamma (PRKCG) | KPCG_HUMAN | [1] | |||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
C35H30N4O4
|
||||
| IsoSMILES |
C[C@@]12[C@@H]([C@@H](C[C@@H](O1)N3C4=CC=CC=C4C5=C6C(=C7C8=CC=CC=C8N2C7=C53)CNC6=O)N(C)C(=O)C9=CC=CC=C9)OC
|
||||
| InChI |
1S/C35H30N4O4/c1-35-32(42-3)25(37(2)34(41)19-11-5-4-6-12-19)17-26(43-35)38-23-15-9-7-13-20(23)28-29-22(18-36-33(29)40)27-21-14-8-10-16-24(21)39(35)31(27)30(28)38/h4-16,25-26,32H,17-18H2,1-3H3,(H,36,40)/t25-,26-,32-,35+/m1/s1
|
||||
| InChIKey |
BMGQWWVMWDBQGC-IIFHNQTCSA-N
|
||||
| PubChem CID | |||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
| VARIDT ID | |||||
| INTEDE ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-02: Benign/in-situ/malignant neoplasm
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Receptor-type tyrosine-protein kinase FLT3 (FLT3) | [2] | |||
| Resistant Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Molecule Alteration | Missense mutation | p.S451F (c.1352C>T) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Bone marrow | N.A. | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Mechanism Description | The missense mutation p.S451F (c.1352C>T) in gene FLT3 cause the resistance of Midostaurin by unusual activation of pro-survival pathway | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Receptor-type tyrosine-protein kinase FLT3 (FLT3) | [3] | |||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Molecule Alteration | IF-deletion | p.I836delI (c.2508_2510delCAT) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 |
| Experiment for Molecule Alteration |
Immunoblotting analysis | |||
| Mechanism Description | The if-deletion p.I836delI (c.2508_2510delCAT) in gene FLT3 cause the sensitivity of Midostaurin by aberration of the drug's therapeutic target. | |||
| Key Molecule: Platelet-derived growth factor receptor alpha (PDGFRA) | [4] | |||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Molecule Alteration | Missense mutation | p.D842V (c.2525A>T) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | A375 cells | Skin | Homo sapiens (Human) | CVCL_0132 |
| THP-1 cells | Blood | Homo sapiens (Human) | CVCL_0006 | |
| Kasumi-1 cells | Peripheral blood | Homo sapiens (Human) | CVCL_0589 | |
| H1703 cells | Lung | Homo sapiens (Human) | CVCL_1490 | |
| HCT-116 cells | Colon | Homo sapiens (Human) | CVCL_0291 | |
| Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 | |
| HMC-1.2 cells | Blood | Homo sapiens (Human) | CVCL_H205 | |
| P815 cells | N.A. | Mus musculus (Mouse) | CVCL_2154 | |
| MV-4-11 cells | Peripheral blood | Homo sapiens (Human) | CVCL_0064 | |
| HMC-1.1 cells | Peripheral blood | Homo sapiens (Human) | CVCL_H206 | |
| EOL1 cells | Peripheral blood | Homo sapiens (Human) | CVCL_0258 | |
| CHO-K1 cells | Ovary | Cricetulus griseus (Chinese hamster) (Cricetulus barabensis griseus) | CVCL_0214 | |
| In Vivo Model | Female Hsd:Athymic Nude-Foxn1nu nude mouse xenograft model | Mus musculus | ||
| Experiment for Drug Resistance |
IC50 assay | |||
|
|
||||
| Key Molecule: Receptor-type tyrosine-protein kinase FLT3 (FLT3) | [2] | |||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Molecule Alteration | Missense mutation | p.Y572C (c.1715A>G) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Bone marrow | N.A. | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Mechanism Description | The missense mutation p.Y572C (c.1715A>G) in gene FLT3 cause the sensitivity of Midostaurin by unusual activation of pro-survival pathway | |||
| Key Molecule: Receptor-type tyrosine-protein kinase FLT3 (FLT3) | [2] | |||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Molecule Alteration | Missense mutation | p.V592G (c.1775T>G) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Bone marrow | N.A. | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Mechanism Description | The missense mutation p.V592G (c.1775T>G) in gene FLT3 cause the sensitivity of Midostaurin by unusual activation of pro-survival pathway | |||
| Key Molecule: Receptor-type tyrosine-protein kinase FLT3 (FLT3) | [2] | |||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Molecule Alteration | Missense mutation | p.R834Q (c.2501G>A) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Bone marrow | N.A. | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Mechanism Description | The missense mutation p.R834Q (c.2501G>A) in gene FLT3 cause the sensitivity of Midostaurin by unusual activation of pro-survival pathway | |||
| Key Molecule: Mast/stem cell growth factor receptor Kit (KIT) | [5] | |||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Molecule Alteration | Missense mutation | p.D816V (c.2447A>T) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 |
| M230 cells | Skin | Homo sapiens (Human) | CVCL_D749 | |
| Experiment for Molecule Alteration |
PCR | |||
| Experiment for Drug Resistance |
CellTiter-Glo assay; IC50 assay | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Mast/stem cell growth factor receptor Kit (KIT) | [6] | |||
| Sensitive Disease | Mast cell leukaemia [ICD-11: 2A21.2] | |||
| Molecule Alteration | Missense mutation | p.S476I (c.1427G>T) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Bone marrow | N.A. | ||
| Experiment for Molecule Alteration |
Histologic and immunophenotypic analysis | |||
| Experiment for Drug Resistance |
Examination of bone marrow smears assay | |||
| Key Molecule: Mast/stem cell growth factor receptor Kit (KIT) | [4] | |||
| Sensitive Disease | Mast cell neoplasm [ICD-11: 2A21.1] | |||
| Molecule Alteration | Missense mutation | p.V560G (c.1679T>G) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | A375 cells | Skin | Homo sapiens (Human) | CVCL_0132 |
| THP-1 cells | Blood | Homo sapiens (Human) | CVCL_0006 | |
| Kasumi-1 cells | Peripheral blood | Homo sapiens (Human) | CVCL_0589 | |
| H1703 cells | Lung | Homo sapiens (Human) | CVCL_1490 | |
| HCT-116 cells | Colon | Homo sapiens (Human) | CVCL_0291 | |
| Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 | |
| HMC-1.2 cells | Blood | Homo sapiens (Human) | CVCL_H205 | |
| P815 cells | N.A. | Mus musculus (Mouse) | CVCL_2154 | |
| MV-4-11 cells | Peripheral blood | Homo sapiens (Human) | CVCL_0064 | |
| HMC-1.1 cells | Peripheral blood | Homo sapiens (Human) | CVCL_H206 | |
| EOL1 cells | Peripheral blood | Homo sapiens (Human) | CVCL_0258 | |
| CHO-K1 cells | Ovary | Cricetulus griseus (Chinese hamster) (Cricetulus barabensis griseus) | CVCL_0214 | |
| In Vivo Model | Female Hsd:Athymic Nude-Foxn1nu nude mouse xenograft model | Mus musculus | ||
| Experiment for Drug Resistance |
IC50 assay | |||
| Key Molecule: Mast/stem cell growth factor receptor Kit (KIT) | [4] | |||
| Sensitive Disease | Mast cell neoplasm [ICD-11: 2A21.1] | |||
| Molecule Alteration | Missense mutation | p.D816Y (c.2446G>T) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Key Molecule: Mast/stem cell growth factor receptor Kit (KIT) | [7] | |||
| Sensitive Disease | Systemic mastocytosis [ICD-11: 2A21.3] | |||
| Molecule Alteration | Missense mutation | p.D816V (c.2447A>T) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Ras-related C3 botulinum toxin substrate 1 (RAC1) | [1] | |||
| Resistant Disease | Acute myeloid leukemia [ICD-11: 2A60.0] | |||
| Molecule Alteration | Function | Activation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | HEK293 cells | Kidney | Homo sapiens (Human) | CVCL_0045 |
| 786-O cells | Kidney | Homo sapiens (Human) | CVCL_1051 | |
| Experiment for Molecule Alteration |
RAC1 activation assay | |||
| Experiment for Drug Resistance |
CellTiter-Glo Luminescent Cell Viability Assay; Flow cytometric analysis | |||
| Mechanism Description | Midostaurin resistance can be overcome by a combination of midostaruin, the BCL-2 inhibitor venetoclax and the RAC1 inhibitor Eht1864 in midostaurin-resistant AML cell lines and primary samples, providing the first evidence of a potential new treatment approach to eradicate FLT3-ITD + AML. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Receptor-type tyrosine-protein kinase FLT3 (FLT3) | [8] | |||
| Sensitive Disease | Acute myeloid leukemia [ICD-11: 2A60.0] | |||
| Molecule Alteration | Missense mutation | p.Y842C (c.2525A>G) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Bone marrow | N.A. | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | The missense mutation p.Y842C (c.2525A>G) in gene FLT3 cause the sensitivity of Midostaurin by aberration of the drug's therapeutic target | |||
| Key Molecule: Receptor-type tyrosine-protein kinase FLT3 (FLT3) | [9] | |||
| Sensitive Disease | Acute myeloid leukemia [ICD-11: 2A60.0] | |||
| Molecule Alteration | Missense mutation | p.D835Y (c.2503G>T) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Bone marrow | N.A. | ||
| Mechanism Description | The missense mutation p.D835Y (c.2503G>T) in gene FLT3 cause the sensitivity of Midostaurin by aberration of the drug's therapeutic target | |||
|
|
||||
| Key Molecule: Mast/stem cell growth factor receptor Kit (KIT) | [10] | |||
| Sensitive Disease | Acute myeloid leukemia [ICD-11: 2A60.0] | |||
| Molecule Alteration | Missense mutation | p.D816V (c.2447A>T) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 |
| MV4-11 cells | Peripheral blood | Homo sapiens (Human) | CVCL_0064 | |
| MOLM14 cells | Peripheral blood | Homo sapiens (Human) | CVCL_7916 | |
| In Vivo Model | Female NCr-nude mouse model | Mus musculus | ||
| Experiment for Drug Resistance |
CellTiter-Glo assay; IC50 assay | |||
| Key Molecule: Mast/stem cell growth factor receptor Kit (KIT) | [4] | |||
| Sensitive Disease | Acute myeloid leukemia [ICD-11: 2A60.0] | |||
| Molecule Alteration | Missense mutation | p.N822K (c.2466T>G) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | A375 cells | Skin | Homo sapiens (Human) | CVCL_0132 |
| THP-1 cells | Blood | Homo sapiens (Human) | CVCL_0006 | |
| Kasumi-1 cells | Peripheral blood | Homo sapiens (Human) | CVCL_0589 | |
| H1703 cells | Lung | Homo sapiens (Human) | CVCL_1490 | |
| HCT-116 cells | Colon | Homo sapiens (Human) | CVCL_0291 | |
| Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 | |
| HMC-1.2 cells | Blood | Homo sapiens (Human) | CVCL_H205 | |
| P815 cells | N.A. | Mus musculus (Mouse) | CVCL_2154 | |
| MV-4-11 cells | Peripheral blood | Homo sapiens (Human) | CVCL_0064 | |
| HMC-1.1 cells | Peripheral blood | Homo sapiens (Human) | CVCL_H206 | |
| EOL1 cells | Peripheral blood | Homo sapiens (Human) | CVCL_0258 | |
| CHO-K1 cells | Ovary | Cricetulus griseus (Chinese hamster) (Cricetulus barabensis griseus) | CVCL_0214 | |
| In Vivo Model | Female Hsd:Athymic Nude-Foxn1nu nude mouse xenograft model | Mus musculus | ||
| Experiment for Drug Resistance |
IC50 assay | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Receptor-type tyrosine-protein kinase FLT3 (FLT3) | [10] | |||
| Sensitive Disease | Hematologic Cancer [ICD-11: MG24.Y] | |||
| Molecule Alteration | Missense mutation | p.D835Y (c.2503G>T) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 |
| MV4-11 cells | Peripheral blood | Homo sapiens (Human) | CVCL_0064 | |
| MOLM14 cells | Peripheral blood | Homo sapiens (Human) | CVCL_7916 | |
| In Vivo Model | Female NCr-nude mouse model | Mus musculus | ||
| Experiment for Drug Resistance |
CellTiter-Glo assay; IC50 assay | |||
|
|
||||
| Key Molecule: Mast/stem cell growth factor receptor Kit (KIT) | [10] | |||
| Sensitive Disease | Hematologic Cancer [ICD-11: MG24.Y] | |||
| Molecule Alteration | Missense mutation | p.D816V (c.2447A>T) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 |
| MV4-11 cells | Peripheral blood | Homo sapiens (Human) | CVCL_0064 | |
| MOLM14 cells | Peripheral blood | Homo sapiens (Human) | CVCL_7916 | |
| In Vivo Model | Female NCr-nude mouse model | Mus musculus | ||
| Experiment for Drug Resistance |
CellTiter-Glo assay; IC50 assay | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
