Drug Information
Drug (ID: DG01076) and It's Reported Resistant Information
| Name |
Unithiol
|
||||
|---|---|---|---|---|---|
| Synonyms |
Unithiol; 4076-02-2; Sodium 2,3-dimercapto-1-propanesulfonate; DMPS; Sodium 2,3-dimercaptopropane-1-sulfonate; UNITIOL; Dimaval; 1-Propanesulfonic acid, 2,3-dimercapto-, monosodium salt; sodium 2,3-dimercaptopropanesulfonate; sodium;2,3-bis(sulfanyl)propane-1-sulfonate; 2,3-Dimercapto-1-propanesulfonic acid sodium salt; sodium 2,3-disulfanylpropane-1-sulfonate; Sodium 2,3-dimercaptopropanesulphonate; Unithiolum; Sodium 2,3-dithiolpropanesulfonate; EINECS 223-796-3; 2,3-Dimercaptopropane sodium sulphonate; (+)-Dmps; (-)-Dmps; sodium 2,3-bis(sulfanyl)propane-1-sulfonate; C3H7O3S3Na; 2,3-Dimercaptopropanesulfonic acid sodium salt; C3H7NaO3S3.H2O; SCHEMBL164318; NIOSH/TZ6420050; NIOSH/TZ6420100; DTXSID40958410; AMY22488; MFCD00007523; STL372655; AKOS015898653; AKOS015967317; AS-64437; P323; 2,3-DIMERCAPTOPROPANESULFONIC SODIUM; DB-049647; FT-0609675; TZ64200500; TZ64201000; H10946; A923350; (+)-2,3-Dimercapto-1-propanesulfonate sodium salt; (-)-2,3-Dimercapto-1-propanesulfonate sodium salt; d-2,3-Dimercapto-1-propanesulfonic acid sodium salt; l-2,3-Dimercapto-1-propanesulfonic acid sodium salt; Q-201912; Q26841293; 207233-91-8 (. H2O); 1-Propanesulfonic acid, 2,3-dimercapto-, sodium salt, (+)-; 1-Propanesulfonic acid, 2,3-dimercapto-, sodium salt, (-)-; 37260-06-3
Click to Show/Hide
|
||||
| Indication |
In total 1 Indication(s)
|
||||
| Structure |
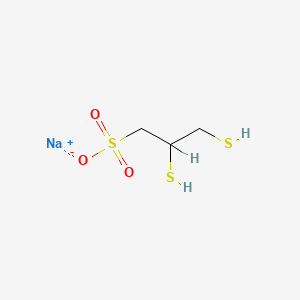
|
||||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
C3H7NaO3S3
|
||||
| IsoSMILES |
C(C(CS(=O)(=O)[O-])S)S.[Na+]
|
||||
| InChI |
1S/C3H8O3S3.Na/c4-9(5,6)2-3(8)1-7;/h3,7-8H,1-2H2,(H,4,5,6);/q;+1/p-1
|
||||
| InChIKey |
FGGPAWQCCGEWTJ-UHFFFAOYSA-M
|
||||
| PubChem CID | |||||
| TTD Drug ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-01: Infectious/parasitic diseases
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Vimentin 2, pseudogene (VIM2P) | [1] | |||
| Sensitive Disease | Pseudomonas aeruginosa infection [ICD-11: 1A00-1C4Z] | |||
| Molecule Alteration | Function | Inhibition |
||
| Experimental Note | Discovered Using In-vivo Testing Model | |||
| In Vitro Model | Schistosoma mansoni isolates | 6183 | ||
| SH-1-V1 cells | Esophagus | Homo sapiens (Human) | N.A. | |
| Experiment for Molecule Alteration |
Molecular modeling assay | |||
| Experiment for Drug Resistance |
Double-disk diffusion test assay | |||
| Mechanism Description | Disk diffusion and broth microdilution methods demonstrate that unithiol inhibits native MBLs NDM-1 and VIM-2 produced by carbapenem-resistant K. pneumoniae and P. aeruginosa bacterial strains. | |||
ICD-12: Respiratory system diseases
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Metallo-beta-lactamase type 2 (BLAN1) | [1] | |||
| Sensitive Disease | Klebsiella pneumoniae infection [ICD-11: CA40.1] | |||
| Molecule Alteration | Function | Inhibition |
||
| Experimental Note | Discovered Using In-vivo Testing Model | |||
| In Vitro Model | Klebsiella pneumoniae strain 409 | 573 | ||
| Klebsiella pneumoniae strain 410 | 573 | |||
| Experiment for Molecule Alteration |
Molecular modeling assay | |||
| Experiment for Drug Resistance |
Double-disk diffusion test assay | |||
| Mechanism Description | Disk diffusion and broth microdilution methods demonstrate that unithiol inhibits native MBLs NDM-1 and VIM-2 produced by carbapenem-resistant K. pneumoniae and P. aeruginosa bacterial strains. | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
