Drug Information
Drug (ID: DG00626) and It's Reported Resistant Information
| Name |
GDC-0623
|
||||
|---|---|---|---|---|---|
| Synonyms |
GDC-0623; 1168091-68-6; GDC 0623; RG 7421; GDC0623; UNII-HW67545I4Q; G-868; 5-((2-FLUORO-4-IODOPHENYL)AMINO)-N-(2-HYDROXYETHOXY)IMIDAZO[1,5-A]PYRIDINE-6-CARBOXAMIDE; 5-(2-fluoro-4-iodoanilino)-N-(2-hydroxyethoxy)imidazo[1,5-a]pyridine-6-carboxamide; HW67545I4Q; RG-7420; RG-7421; 5-[(2-FLUORO-4-IODOPHENYL)AMINO]-N-(2-HYDROXYETHOXY)IMIDAZO[1,5-A]PYRIDINE-6-CARBOXAMIDE; Imidazo[1,5-a]pyridine-6-carboxamide, 5-[(2-fluoro-4-iodophenyl)amino]-N-(2-hydroxyethoxy)-; MEK inhibitor 1; GTPL9909; SCHEMBL1615104; CHEMBL3330650; CHEBI:167659; AOB87143; BCP28689; EX-A2060; BDBM50025226; MFCD25976760; NSC778590; NSC800998; RG7421; s7553; ZINC43206499; AKOS027253679; CCG-269293; DB11982; NSC-778590; NSC-800998; SB16957; NCGC00351593-05; AS-55986; DA-33609; G868; HY-15610; FT-0769207; G 868; A858271; Q27280129; 5-(2-Fluoro-4-iodophenylamino)-imidazo[1,5-a]pyridine-6-carboxylic acid (2-hydroxyethoxy)-amide; 5-(2-Fluoro-4-iodophenylamino)-imidazo[15-a]pyridine-6-carboxylic acid (2-hydroxyethoxy)-amide; 5-(2-Fluoro-4-iodophenylamino)imidazo[1,5-a]pyridine-6-carboxylic acid N-(2-hydroxyethoxy)amide
Click to Show/Hide
|
||||
| Indication |
In total 1 Indication(s)
|
||||
| Structure |
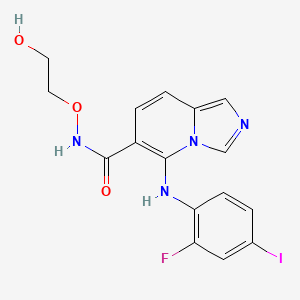
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Resistance Information Validated by in-vivo Model for This Drug
(1 diseases)
[1]
|
||||
| Target | ERK activator kinase (MEK) | NOUNIPROTAC | [1] | ||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
C16H14FIN4O3
|
||||
| IsoSMILES |
C1=CC(=C(C=C1I)F)NC2=C(C=CC3=CN=CN32)C(=O)NOCCO
|
||||
| InChI |
1S/C16H14FIN4O3/c17-13-7-10(18)1-4-14(13)20-15-12(16(24)21-25-6-5-23)3-2-11-8-19-9-22(11)15/h1-4,7-9,20,23H,5-6H2,(H,21,24)
|
||||
| InChIKey |
RFWVETIZUQEJEF-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
| TTD Drug ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-02: Benign/in-situ/malignant neoplasm
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | |||||||||||||
|
|
|||||||||||||
| Key Molecule: GTPase HRas (HRAS) | [1] | ||||||||||||
| Resistant Disease | Unclassified pleomorphic sarcoma [ICD-11: 2B54.0] | ||||||||||||
| Molecule Alteration | Missense mutation | p.G12V |
|||||||||||
| Wild Type Structure | Method: X-ray diffraction | Resolution: 1.98 Å | |||||||||||
| Mutant Type Structure | Method: X-ray diffraction | Resolution: 1.96 Å | |||||||||||
| Download The Information of Sequence | Download The Structure File | ||||||||||||
-
G
-
0
|
S
-
M
M
T
T
E
E
Y
Y
K
K
L
L
V
V
V
V
V
V
10
|
G
G
A
A
G
V
G
G
V
V
G
G
K
K
S
S
A
A
L
L
20
|
T
T
I
I
Q
Q
L
L
I
I
Q
Q
N
N
H
H
F
F
V
V
30
|
D
D
E
E
Y
Y
D
D
P
P
T
T
I
I
E
E
D
D
S
S
40
|
Y
Y
R
R
K
K
Q
Q
V
V
V
V
I
I
D
D
G
G
E
E
50
|
T
T
C
C
L
L
L
L
D
D
I
I
L
L
D
D
T
T
A
A
60
|
G
G
Q
Q
E
E
E
E
Y
Y
S
S
A
A
M
M
R
R
D
D
70
|
Q
Q
Y
Y
M
M
R
R
T
T
G
G
E
E
G
G
F
F
L
L
80
|
C
C
V
V
F
F
A
A
I
I
N
N
N
N
T
T
K
K
S
S
90
|
F
F
E
E
D
D
I
I
H
H
H
H
Y
Y
R
R
E
E
Q
Q
100
|
I
I
K
K
R
R
V
V
K
K
D
D
S
S
E
E
D
D
V
V
110
|
P
P
M
M
V
V
L
L
V
V
G
G
N
N
K
K
S
S
D
D
120
|
L
L
P
P
S
S
R
R
T
T
V
V
D
D
T
T
K
K
Q
Q
130
|
A
A
Q
Q
D
D
L
L
A
A
R
R
S
S
Y
Y
G
G
I
I
140
|
P
P
F
F
I
I
E
E
T
T
S
S
A
A
K
K
T
T
R
R
150
|
Q
Q
G
G
V
V
D
D
D
D
A
A
F
F
Y
Y
T
T
L
L
160
|
V
V
R
R
E
E
I
I
R
R
K
K
H
H
K
K
E
E
K
K
170
|
M
M
S
S
K
K
D
D
G
G
K
K
K
K
K
K
K
K
K
K
180
|
K
K
S
S
K
K
T
T
K
K
C
C
V
V
I
I
M
M
|
|||||||||||||
| Experimental Note | Discovered Using In-vivo Testing Model | ||||||||||||
| Cell Pathway Regulation | RAS signaling pathway | Activation | hsa04014 | ||||||||||
| In Vitro Model | HEK293T cells | Kidney | Homo sapiens (Human) | CVCL_0063 | |||||||||
| NIH3T3 cells | Embryo | Homo sapiens (Human) | CVCL_0594 | ||||||||||
| In Vivo Model | SCID/Beige mice model | Mus musculus | |||||||||||
| Experiment for Molecule Alteration |
qRT-PCR; Western blotting assay | ||||||||||||
| Experiment for Drug Resistance |
Cell viability assay | ||||||||||||
| Mechanism Description | Hras G12V mutation changed the drug target,impairing the ability to inhibit RAS-RAF-MEK-ERK signaling. | ||||||||||||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
