Drug Information
Drug (ID: DG00617) and It's Reported Resistant Information
| Name |
Cabozantinib
|
||||
|---|---|---|---|---|---|
| Synonyms |
Cabozantinib; 849217-68-1; Cometriq; XL184; XL-184; BMS-907351; XL 184; BMS 907351; Cabozantinib (XL184, BMS-907351); UNII-1C39JW444G; CHEBI:72317; XL-184 free base; BMS907351; 1C39JW444G; MFCD20926324; N'-[4-[(6,7-DIMETHOXY-4-QUINOLINYL)OXY]PHENYL]-N-(4-FLUOROPHENYL)-1,1-CYCLOPROPANEDICARBOXAMIDE; Cabometyx (TN); Cometriq (TN); 1-N-[4-(6,7-dimethoxyquinolin-4-yl)oxyphenyl]-1-N'-(4-fluorophenyl)cyclopropane-1,1-dicarboxamide; C28H24FN3O5; N-{4-[(6,7-dimethoxyquinolin-4-yl)oxy]phenyl}-N'-(4-fluorophenyl)cyclopropane-1,1- dicarboxamide; N-{4-[(6,7-dimethoxyquinolin-4-yl)oxy]phenyl}-N'-(4-fluorophenyl)cyclopropane-1,1-dicarboxamide; XL184 cpd; Cabozantinib [USAN:INN]; Carbozantinib; XL184 free base; Cabozantinib (USAN); Cabozantinib (free base); SCHEMBL360795; GTPL5887; Cabozantinib (BMS-907351); CHEMBL2105717; DTXSID10233968; EX-A075; QCR-122; SYN1138; XL184 free base - Cabozantinib; BCPP000308; BMS-907351 FREE BASE; HMS3654G06; XL-184 free base (Cabozantinib); AOB87755; BCP02591; 849217-68-1 (free base); BDBM50021574; NSC761068; NSC800066; s1119; ZINC70466416; AKOS025142112; BCP9000470; CCG-264678; CS-0278; DB08875; NSC-761068; NSC-800066; SB20062; XL-184 (Cabozantinib,BMS907351); XL-184,Cabozantinib, BMS-907351; NCGC00263164-01; NCGC00263164-14; NCGC00263164-17; 1,1-Cyclopropanedicarboxamide, N'-[4-[(6,7-dimethoxy-4-quinolinyl)oxy]phenyl]-N-(4-fluorophenyl)-; 1,1-Cyclopropanedicarboxamide,N-[4-[(6,7-dimethoxy-4-quinolinyl)oxy]phenyl]-N'-(4-fluorophenyl)-; AC-25082; AS-16277; HY-13016; n-(4-((6,7-dimethoxy-4-quinolinyl)oxy)phenyl)-n'-(4-fluorophenyl)-1,1-cyclopropanedicarboxamide; SY097158; DB-023624; FT-0664184; SW218093-3; X7477; D10062; AB01565831_02; Q795057; SR-01000941569; J-523016; SR-01000941569-1; BRD-K51544265-001-01-8; 1,1-Cyclopropanedicarboxamide, N'-(4-((6,7-dimethoxy-4-quinolinyl)oxy]phenyl]-N-(4- fluorophenyl)-; cyclopropane-1,1-dicarboxylic acid [4-(6,7-dimethoxy-quinoline-4-yloxy)-phenyl]-amide(4-fluoro-phenyl)-amide; cyclopropane-1,1-dicarboxylic acid[4-(6,7-dimethoxy-quinoline-4-yloxy)-phenyl]-amide (4-fluoro-phenyl)-amide; N-(4-{[6,7-bis(methyloxy)quinolin-4-yl]oxy}phenyl)-N'-(4 fluorophenyl)cyclopropane-1,1-dicarboxamide; N-(4-{[6,7-bis(methyloxy)quinolin-4-yl]oxy}phenyl)-N'-(4-fluorophenyl)cyclopropane-1,1-dicarboxamide; N-[4-[(6,7-Dimethoxy-4-quinolinyl)oxy]phenyl]-N-(4-fluorophenyl)-1,1-cyclopropanedicarboxamide; N-[4-[(6,7-Dimethoxyquinolin-4-yl)oxy]phenyl]-N inverted exclamation mark -(4-fluorophenyl)cyclopropane-1,1-dicarboxamide
Click to Show/Hide
|
||||
| Indication |
In total 3 Indication(s)
|
||||
| Structure |
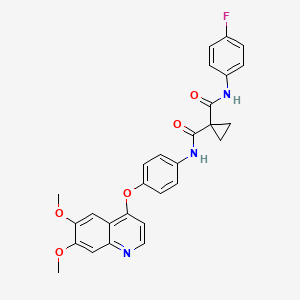
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Clinically Reported Resistance for This Drug
(4 diseases)
[2]
[3]
[4]
[1]
Disease(s) with Resistance Information Discovered by Cell Line Test for This Drug
(2 diseases)
[3]
[5]
|
||||
| Target | Proto-oncogene c-Met (MET) | MET_HUMAN | [2] | ||
| Vascular endothelial growth factor receptor 2 (KDR) | VGFR2_HUMAN | [2] | |||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
C28H24FN3O5
|
||||
| IsoSMILES |
COC1=CC2=C(C=CN=C2C=C1OC)OC3=CC=C(C=C3)NC(=O)C4(CC4)C(=O)NC5=CC=C(C=C5)F
|
||||
| InChI |
1S/C28H24FN3O5/c1-35-24-15-21-22(16-25(24)36-2)30-14-11-23(21)37-20-9-7-19(8-10-20)32-27(34)28(12-13-28)26(33)31-18-5-3-17(29)4-6-18/h3-11,14-16H,12-13H2,1-2H3,(H,31,33)(H,32,34)
|
||||
| InChIKey |
ONIQOQHATWINJY-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
| INTEDE ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-02: Benign/in-situ/malignant neoplasm
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Mast/stem cell growth factor receptor Kit (KIT) | [5] | |||
| Resistant Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Molecule Alteration | Missense mutation | p.D816V (c.2447A>T) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | 32D cells | Bone marrow | Homo sapiens (Human) | CVCL_0118 |
| In Vivo Model | Female Balb/cA-nu/nu mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | The missense mutation p.D816V (c.2447A>T) in gene KIT cause the resistance of Cabozantinib by aberration of the drug's therapeutic target | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | |||||||||||||
|
|
|||||||||||||
| Key Molecule: Hepatocyte growth factor receptor (MET) | [6] | ||||||||||||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | ||||||||||||
| Molecule Alteration | Missense mutation | p.Y1003F (c.3008A>T) |
|||||||||||
| Experimental Note | Revealed Based on the Cell Line Data | ||||||||||||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 | |||||||||
| WEHI-3 cells | Peripheral blood | Mus musculus (Mouse) | CVCL_3622 | ||||||||||
| Hs746T cells | Skeletal muscle | Homo sapiens (Human) | CVCL_0333 | ||||||||||
| Gp2-293 cells | Fetal kidney | Homo sapiens (Human) | CVCL_WI48 | ||||||||||
| Experiment for Molecule Alteration |
Direct sequencing assay | ||||||||||||
| Experiment for Drug Resistance |
CCK-8 assay | ||||||||||||
| Key Molecule: Hepatocyte growth factor receptor (MET) | [6] | ||||||||||||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | ||||||||||||
| Molecule Alteration | Missense mutation | p.D1010Y (c.3028G>T) |
|||||||||||
| Experimental Note | Revealed Based on the Cell Line Data | ||||||||||||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 | |||||||||
| WEHI-3 cells | Peripheral blood | Mus musculus (Mouse) | CVCL_3622 | ||||||||||
| Hs746T cells | Skeletal muscle | Homo sapiens (Human) | CVCL_0333 | ||||||||||
| Gp2-293 cells | Fetal kidney | Homo sapiens (Human) | CVCL_WI48 | ||||||||||
| Experiment for Molecule Alteration |
Direct sequencing assay | ||||||||||||
| Experiment for Drug Resistance |
CCK-8 assay | ||||||||||||
|
|
|||||||||||||
| Key Molecule: Hepatocyte growth factor receptor (MET) | [7] | ||||||||||||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | ||||||||||||
| Molecule Alteration | Missense mutation | p.D1228V (c.3683A>T) |
|||||||||||
| Wild Type Structure | Method: X-ray diffraction | Resolution: 1.71 Å | |||||||||||
| Mutant Type Structure | Method: X-ray diffraction | Resolution: 1.67 Å | |||||||||||
| Download The Information of Sequence | Download The Structure File | ||||||||||||
-
-
G
-
D
1040
|
-
S
-
D
-
I
-
S
-
S
-
P
-
L
-
L
-
Q
N
N
1050
|
T
T
V
V
H
H
I
I
D
D
L
L
S
S
A
A
L
L
N
N
1060
|
P
P
E
E
L
L
V
V
Q
Q
A
A
V
V
Q
Q
H
H
V
V
1070
|
V
V
I
I
G
G
P
P
S
S
S
S
L
L
I
I
V
V
H
H
1080
|
F
F
N
N
E
E
V
V
I
I
G
G
R
R
G
G
H
H
F
F
1090
|
G
G
C
C
V
V
Y
Y
H
H
G
G
T
T
L
L
L
L
D
D
1100
|
N
N
D
D
G
G
K
K
K
K
I
I
H
H
C
C
A
A
V
V
1110
|
K
K
S
S
L
L
N
N
R
R
I
I
T
T
D
D
I
I
G
G
1120
|
E
E
V
V
S
S
Q
Q
F
F
L
L
T
T
E
E
G
G
I
I
1130
|
I
I
M
M
K
K
D
D
F
F
S
S
H
H
P
P
N
N
V
V
1140
|
L
L
S
S
L
L
L
L
G
G
I
I
C
C
L
L
R
R
S
S
1150
|
E
E
G
G
S
S
P
P
L
L
V
V
V
V
L
L
P
P
Y
Y
1160
|
M
M
K
K
H
H
G
G
D
D
L
L
R
R
N
N
F
F
I
I
1170
|
R
R
N
N
E
E
T
T
H
H
N
N
P
P
T
T
V
V
K
K
1180
|
D
D
L
L
I
I
G
G
F
F
G
G
L
L
Q
Q
V
V
A
A
1190
|
K
K
G
G
M
M
K
K
F
Y
L
L
A
A
S
S
K
K
K
K
1200
|
F
F
V
V
H
H
R
R
D
D
L
L
A
A
A
A
R
R
N
N
1210
|
C
C
M
M
L
L
D
D
E
E
K
K
F
F
T
T
V
V
K
K
1220
|
V
V
A
A
D
D
F
F
G
G
L
L
A
A
R
R
D
V
M
M
1230
|
Y
Y
D
D
K
K
E
E
F
Y
D
Y
S
S
V
V
H
H
N
N
1240
|
K
K
T
T
G
G
A
A
K
K
L
L
P
P
V
V
K
K
W
W
1250
|
M
M
A
A
L
L
E
E
S
S
L
L
Q
Q
T
T
Q
Q
K
K
1260
|
F
F
T
T
T
T
K
K
S
S
D
D
V
V
W
W
S
S
F
F
1270
|
G
G
V
V
L
L
L
L
W
W
E
E
L
L
M
M
T
T
R
R
1280
|
G
G
A
A
P
P
P
P
Y
Y
P
P
D
D
V
V
N
N
T
T
1290
|
F
F
D
D
I
I
T
T
V
V
Y
Y
L
L
L
L
Q
Q
G
G
1300
|
R
R
R
R
L
L
L
L
Q
Q
P
P
E
E
Y
Y
C
C
P
P
1310
|
D
D
P
P
L
L
Y
Y
E
E
V
V
M
M
L
L
K
K
C
C
1320
|
W
W
H
H
P
P
K
K
A
A
E
E
M
M
R
R
P
P
S
S
1330
|
F
F
S
S
E
E
L
L
V
V
S
S
R
R
I
I
S
S
A
A
1340
|
I
I
F
F
S
S
T
T
F
F
I
I
G
G
E
-
H
-
Y
-
1350
|
V
-
H
-
V
-
N
-
A
-
T
-
Y
-
V
-
N
-
V
-
1360
|
K
-
|
|||||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| In Vitro Model | PC9 cells | Lung | Homo sapiens (Human) | CVCL_B260 | |||||||||
| 293T cells | Breast | Homo sapiens (Human) | CVCL_0063 | ||||||||||
| Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 | ||||||||||
| Experiment for Molecule Alteration |
Western blot analysis | ||||||||||||
| Experiment for Drug Resistance |
MTS assay | ||||||||||||
| Mechanism Description | There is a patient with metastatic NSCLC with MET-mediated resistance to EGFR TKI who responded to treatment with a type I MET inhibitor, savolitinib, given in combination with a third-generation EGFR inhibitor, osimertinib. The patient then developed acquired resistance mediated by a novel MET kinase domain mutation. | ||||||||||||
| Key Molecule: Hepatocyte growth factor receptor (MET) | [8] | ||||||||||||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | ||||||||||||
| Molecule Alteration | Missense mutation | p.Y1230H (c.3688T>C) |
|||||||||||
| Wild Type Structure | Method: X-ray diffraction | Resolution: 1.71 Å | |||||||||||
| Mutant Type Structure | Method: X-ray diffraction | Resolution: 1.97 Å | |||||||||||
| Download The Information of Sequence | Download The Structure File | ||||||||||||
-
N
-
1050
|
T
-
V
-
H
-
I
-
D
-
L
-
S
-
A
A
L
L
N
N
1060
|
P
P
E
E
L
L
V
V
Q
Q
A
A
V
V
Q
Q
H
H
V
V
1070
|
V
V
I
I
G
G
P
P
S
S
S
S
L
L
I
I
V
V
H
H
1080
|
F
F
N
N
E
E
V
V
I
I
G
G
R
R
G
G
H
H
F
F
1090
|
G
G
C
C
V
V
Y
Y
H
H
G
G
T
T
L
L
L
L
D
D
1100
|
N
N
D
D
G
G
K
K
K
K
I
I
H
H
C
C
A
A
V
V
1110
|
K
K
S
S
L
L
N
N
R
R
I
I
T
T
D
D
I
I
G
G
1120
|
E
E
V
V
S
S
Q
Q
F
F
L
L
T
T
E
E
G
G
I
I
1130
|
I
I
M
M
K
K
D
D
F
F
S
S
H
H
P
P
N
N
V
V
1140
|
L
L
S
S
L
L
L
L
G
G
I
I
C
C
L
L
R
R
S
S
1150
|
E
E
G
G
S
S
P
P
L
L
V
V
V
V
L
L
P
P
Y
Y
1160
|
M
M
K
K
H
H
G
G
D
D
L
L
R
R
N
N
F
F
I
I
1170
|
R
R
N
N
E
E
T
T
H
H
N
N
P
P
T
T
V
V
K
K
1180
|
D
D
L
L
I
I
G
G
F
F
G
G
L
L
Q
Q
V
V
A
A
1190
|
K
K
G
G
M
M
K
K
F
Y
L
L
A
A
S
S
K
K
K
K
1200
|
F
F
V
V
H
H
R
R
D
D
L
L
A
A
A
A
R
R
N
N
1210
|
C
C
M
M
L
L
D
D
E
E
K
K
F
F
T
T
V
V
K
K
1220
|
V
V
A
A
D
D
F
F
G
G
L
L
A
A
R
R
D
D
M
M
1230
|
Y
H
D
D
K
K
E
E
F
Y
D
Y
S
S
V
V
H
H
N
N
1240
|
K
K
T
T
G
G
A
A
K
K
L
L
P
P
V
V
K
K
W
W
1250
|
M
M
A
A
L
L
E
E
S
S
L
L
Q
Q
T
T
Q
Q
K
K
1260
|
F
F
T
T
T
T
K
K
S
S
D
D
V
V
W
W
S
S
F
F
1270
|
G
G
V
V
L
L
L
L
W
W
E
E
L
L
M
M
T
T
R
R
1280
|
G
G
A
A
P
P
P
P
Y
Y
P
P
D
D
V
V
N
N
T
T
1290
|
F
F
D
D
I
I
T
T
V
V
Y
Y
L
L
L
L
Q
Q
G
G
1300
|
R
R
R
R
L
L
L
L
Q
Q
P
P
E
E
Y
Y
C
C
P
P
1310
|
D
D
P
P
L
L
Y
Y
E
E
V
V
M
M
L
L
K
K
C
C
1320
|
W
W
H
H
P
P
K
K
A
A
E
E
M
M
R
R
P
P
S
S
1330
|
F
F
S
S
E
E
L
L
V
V
S
S
R
R
I
I
S
S
A
A
1340
|
I
I
F
F
S
S
T
T
F
F
I
I
G
G
E
E
H
H
Y
Y
1350
|
V
V
H
H
V
V
N
N
A
A
T
T
Y
-
V
-
N
-
V
-
1360
|
K
-
|
|||||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| In Vitro Model | NIH3T3 cells | Embryo | Homo sapiens (Human) | CVCL_0594 | |||||||||
| In Vivo Model | Athymic female mouse PDX model | Mus musculus | |||||||||||
| Experiment for Drug Resistance |
MTS assay | ||||||||||||
| Mechanism Description | MET mutations Y1248H and D1246N are resistance mechanisms for type I MET-TKIs. NIH3T3 cells expressing either mutation showed resistance to both INC280 and crizotinib but not cabozantinib, indicating the potential of sequential use of MET inhibitors may lead to a more durable response. | ||||||||||||
| Key Molecule: Hepatocyte growth factor receptor (MET) | [8] | ||||||||||||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | ||||||||||||
| Molecule Alteration | Missense mutation | p.D1228N (c.3682G>A) |
|||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| In Vitro Model | NIH3T3 cells | Embryo | Homo sapiens (Human) | CVCL_0594 | |||||||||
| In Vivo Model | Athymic female mouse PDX model | Mus musculus | |||||||||||
| Experiment for Drug Resistance |
MTS assay | ||||||||||||
| Mechanism Description | MET mutations Y1248H and D1246N are resistance mechanisms for type I MET-TKIs. NIH3T3 cells expressing either mutation showed resistance to both INC280 and crizotinib but not cabozantinib, indicating the potential of sequential use of MET inhibitors may lead to a more durable response. | ||||||||||||
|
|
|||||||||||||
| Key Molecule: Tyrosine-protein kinase ABL1 (ABL1) | [9] | ||||||||||||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | ||||||||||||
| Molecule Alteration | Missense mutation | p.V299L (c.895G>C) |
|||||||||||
| Experimental Note | Revealed Based on the Cell Line Data | ||||||||||||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 | |||||||||
| Ph+ALL cells | N.A. | N.A. | N.A. | ||||||||||
| Mechanism Description | The missense mutation p.V299L (c.895G>C) in gene ABL1 cause the sensitivity of Cabozantinib by aberration of the drug's therapeutic target | ||||||||||||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Mast/stem cell growth factor receptor Kit (KIT) | [10] | |||
| Sensitive Disease | Gastrointestinal stromal tumor [ICD-11: 2B5B.0] | |||
| Molecule Alteration | Duplication | p.A502_Y503 (c.1504_1509) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Clinical GIST specimens | N.A. | ||
| In Vivo Model | NMRI mouse PDX model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Mechanism Description | Cabozantinib inhibited the KIT signaling pathway in UZLX-GIST4 and -GIST2. In addition, compared with both control and imatinib, cabozantinib significantly reduced microvessel density in all models. Cabozantinib showed antitumor activity in GIST PDX models through inhibition of tumor growth, proliferation, and angiogenesis, in both imatinib-sensitive and imatinib-resistant models. | |||
| Key Molecule: Mast/stem cell growth factor receptor Kit (KIT) | [10] | |||
| Sensitive Disease | Gastrointestinal stromal tumor [ICD-11: 2B5B.0] | |||
| Molecule Alteration | Complex-indel | p.K558_G565delinsR (c.1673_1693del21) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Clinical GIST specimens | N.A. | ||
| In Vivo Model | NMRI mouse PDX model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Mechanism Description | Cabozantinib inhibited the KIT signaling pathway in UZLX-GIST4 and -GIST2. In addition, compared with both control and imatinib, cabozantinib significantly reduced microvessel density in all models. Cabozantinib showed antitumor activity in GIST PDX models through inhibition of tumor growth, proliferation, and angiogenesis, in both imatinib-sensitive and imatinib-resistant models. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: VEGF-2 receptor (KDR) | [11] | |||
| Sensitive Disease | Colorectal cancer [ICD-11: 2B91.1] | |||
| Molecule Alteration | Missense mutation | p.R1032Q (c.3095G>A) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | VEGF signaling pathway | Activation | hsa04370 | |
| In Vitro Model | Colo-320 cells | Colon | Homo sapiens (Human) | CVCL_1989 |
| MDST8 cells | Colon | Homo sapiens (Human) | CVCL_2588 | |
| In Vivo Model | Nude mouse PDX model | Mus musculus | ||
| Experiment for Molecule Alteration |
BEAMing assay; Western blot analysis; immunofluorescence assay | |||
| Experiment for Drug Resistance |
Promega assay | |||
| Mechanism Description | VEGFR2 is somatically mutated across tumor types and that VEGFR2 mutants can be oncogenic and control sensitivity/resistance to antiangiogenic drugs. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Zinc finger and BTB domain-containing protein 7A (ZBTB7A) | [3] | |||
| Metabolic Type | Glucose metabolism | |||
| Resistant Disease | Hepatocellular carcinoma [ICD-11: 2C12.02] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vivo Model | HCC patients | Homo Sapiens | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Cell prognosis assay | |||
| Mechanism Description | In the present work, our results, for the first time, revealed that FBI-1 induced the aerobic glycolysis/Warburg effect of HCC cells by enhancing the expression of HIF-1alpha and its target genes. | |||
| Key Molecule: Zinc finger and BTB domain-containing protein 7A (ZBTB7A) | [3] | |||
| Metabolic Type | Glucose metabolism | |||
| Resistant Disease | Hepatocellular carcinoma [ICD-11: 2C12.02] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | HepG2 cells | Liver | Homo sapiens (Human) | CVCL_0027 |
| MHCC97-H cells | Liver | Homo sapiens (Human) | CVCL_4972 | |
| MHCC97-L cells | Liver | Homo sapiens (Human) | CVCL_4973 | |
| L-02 hepatic non-tumor cells | Liver | Homo sapiens (Human) | CVCL_6926 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | In the present work, our results, for the first time, revealed that FBI-1 induced the aerobic glycolysis/Warburg effect of HCC cells by enhancing the expression of HIF-1alpha and its target genes. | |||
| Key Molecule: Zinc finger and BTB domain-containing protein 7A (ZBTB7A) | [3] | |||
| Metabolic Type | Glucose metabolism | |||
| Resistant Disease | Hepatocellular carcinoma [ICD-11: 2C12.02] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vivo Model | Nude mice, MHCC97-H cells | Mice | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Tumor volume assay | |||
| Mechanism Description | In the present work, our results, for the first time, revealed that FBI-1 induced the aerobic glycolysis/Warburg effect of HCC cells by enhancing the expression of HIF-1alpha and its target genes. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Hepatocyte growth factor receptor (MET) | [4] | |||
| Resistant Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | |||
| Molecule Alteration | Missense mutation | p.D1228N (c.3682G>A) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | |||||||||||||
|
|
|||||||||||||
| Key Molecule: Hepatocyte growth factor receptor (MET) | [12] | ||||||||||||
| Sensitive Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | ||||||||||||
| Molecule Alteration | Missense mutation | p.Y1003N (c.3007T>A) |
|||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| Key Molecule: Hepatocyte growth factor receptor (MET) | [12] | ||||||||||||
| Sensitive Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | ||||||||||||
| Molecule Alteration | Missense mutation | p.Y1003C (c.3008A>G) |
|||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| Key Molecule: Hepatocyte growth factor receptor (MET) | [12] | ||||||||||||
| Sensitive Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | ||||||||||||
| Molecule Alteration | Missense mutation | p.Y1003F (c.3008A>T) |
|||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| Key Molecule: Hepatocyte growth factor receptor (MET) | [12] | ||||||||||||
| Sensitive Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | ||||||||||||
| Molecule Alteration | Missense mutation | p.D1010N (c.3028G>A) |
|||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| Key Molecule: Hepatocyte growth factor receptor (MET) | [12] | ||||||||||||
| Sensitive Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | ||||||||||||
| Molecule Alteration | Missense mutation | p.D1010H (c.3028G>C) |
|||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| Key Molecule: Hepatocyte growth factor receptor (MET) | [12] | ||||||||||||
| Sensitive Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | ||||||||||||
| Molecule Alteration | Missense mutation | p.D1010Y (c.3028G>T) |
|||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| Key Molecule: Proto-oncogene c-Ros (ROS1) | [13] | ||||||||||||
| Sensitive Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | ||||||||||||
| Molecule Alteration | Missense mutation | p.G2032R (c.6094G>A) |
|||||||||||
| Experimental Note | Revealed Based on the Cell Line Data | ||||||||||||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 | |||||||||
| HEK293 FT cells | Kidney | Homo sapiens (Human) | CVCL_6911 | ||||||||||
| Experiment for Molecule Alteration |
Western blot analysis | ||||||||||||
| Experiment for Drug Resistance |
CellTiter-Glo assay | ||||||||||||
| Key Molecule: Proto-oncogene c-Ros (ROS1) | [14] | ||||||||||||
| Sensitive Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | ||||||||||||
| Molecule Alteration | Missense mutation | p.G2032R (c.6094G>A) |
|||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
|
|
|||||||||||||
| Key Molecule: Hepatocyte growth factor receptor (MET) | [7] | ||||||||||||
| Sensitive Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | ||||||||||||
| Molecule Alteration | Missense mutation | p.D1228V (c.3683A>T) |
|||||||||||
| Wild Type Structure | Method: X-ray diffraction | Resolution: 1.71 Å | |||||||||||
| Mutant Type Structure | Method: X-ray diffraction | Resolution: 1.67 Å | |||||||||||
| Download The Information of Sequence | Download The Structure File | ||||||||||||
-
-
G
-
D
1040
|
-
S
-
D
-
I
-
S
-
S
-
P
-
L
-
L
-
Q
N
N
1050
|
T
T
V
V
H
H
I
I
D
D
L
L
S
S
A
A
L
L
N
N
1060
|
P
P
E
E
L
L
V
V
Q
Q
A
A
V
V
Q
Q
H
H
V
V
1070
|
V
V
I
I
G
G
P
P
S
S
S
S
L
L
I
I
V
V
H
H
1080
|
F
F
N
N
E
E
V
V
I
I
G
G
R
R
G
G
H
H
F
F
1090
|
G
G
C
C
V
V
Y
Y
H
H
G
G
T
T
L
L
L
L
D
D
1100
|
N
N
D
D
G
G
K
K
K
K
I
I
H
H
C
C
A
A
V
V
1110
|
K
K
S
S
L
L
N
N
R
R
I
I
T
T
D
D
I
I
G
G
1120
|
E
E
V
V
S
S
Q
Q
F
F
L
L
T
T
E
E
G
G
I
I
1130
|
I
I
M
M
K
K
D
D
F
F
S
S
H
H
P
P
N
N
V
V
1140
|
L
L
S
S
L
L
L
L
G
G
I
I
C
C
L
L
R
R
S
S
1150
|
E
E
G
G
S
S
P
P
L
L
V
V
V
V
L
L
P
P
Y
Y
1160
|
M
M
K
K
H
H
G
G
D
D
L
L
R
R
N
N
F
F
I
I
1170
|
R
R
N
N
E
E
T
T
H
H
N
N
P
P
T
T
V
V
K
K
1180
|
D
D
L
L
I
I
G
G
F
F
G
G
L
L
Q
Q
V
V
A
A
1190
|
K
K
G
G
M
M
K
K
F
Y
L
L
A
A
S
S
K
K
K
K
1200
|
F
F
V
V
H
H
R
R
D
D
L
L
A
A
A
A
R
R
N
N
1210
|
C
C
M
M
L
L
D
D
E
E
K
K
F
F
T
T
V
V
K
K
1220
|
V
V
A
A
D
D
F
F
G
G
L
L
A
A
R
R
D
V
M
M
1230
|
Y
Y
D
D
K
K
E
E
F
Y
D
Y
S
S
V
V
H
H
N
N
1240
|
K
K
T
T
G
G
A
A
K
K
L
L
P
P
V
V
K
K
W
W
1250
|
M
M
A
A
L
L
E
E
S
S
L
L
Q
Q
T
T
Q
Q
K
K
1260
|
F
F
T
T
T
T
K
K
S
S
D
D
V
V
W
W
S
S
F
F
1270
|
G
G
V
V
L
L
L
L
W
W
E
E
L
L
M
M
T
T
R
R
1280
|
G
G
A
A
P
P
P
P
Y
Y
P
P
D
D
V
V
N
N
T
T
1290
|
F
F
D
D
I
I
T
T
V
V
Y
Y
L
L
L
L
Q
Q
G
G
1300
|
R
R
R
R
L
L
L
L
Q
Q
P
P
E
E
Y
Y
C
C
P
P
1310
|
D
D
P
P
L
L
Y
Y
E
E
V
V
M
M
L
L
K
K
C
C
1320
|
W
W
H
H
P
P
K
K
A
A
E
E
M
M
R
R
P
P
S
S
1330
|
F
F
S
S
E
E
L
L
V
V
S
S
R
R
I
I
S
S
A
A
1340
|
I
I
F
F
S
S
T
T
F
F
I
I
G
G
E
-
H
-
Y
-
1350
|
V
-
H
-
V
-
N
-
A
-
T
-
Y
-
V
-
N
-
V
-
1360
|
K
-
|
|||||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| In Vitro Model | PC9 cells | Lung | Homo sapiens (Human) | CVCL_B260 | |||||||||
| 293T cells | Breast | Homo sapiens (Human) | CVCL_0063 | ||||||||||
| Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 | ||||||||||
| Experiment for Molecule Alteration |
Western blot analysis | ||||||||||||
| Experiment for Drug Resistance |
MTS assay | ||||||||||||
| Mechanism Description | There is a patient with metastatic NSCLC with MET-mediated resistance to EGFR TKI who responded to treatment with a type I MET inhibitor, savolitinib, given in combination with a third-generation EGFR inhibitor, osimertinib. The patient then developed acquired resistance mediated by a novel MET kinase domain mutation. | ||||||||||||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Pro-angiogenic factors | [2] | |||
| Resistant Disease | Renal cell carcinoma [ICD-11: 2C90.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | VeroE6/TMPRSS2 cells | Kidney | Chlorocebus sabaeus (Green monkey) (Cercopithecus sabaeus) | CVCL_YQ49 |
| HUVEC cells | Endothelium | Homo sapiens (Human) | N.A. | |
| Experiment for Molecule Alteration |
Secreted protein measurements assay | |||
| Experiment for Drug Resistance |
Flow cytometry | |||
| Mechanism Description | We show that circulating immune cells from patients with ccRCC induce cabozantinib resistance via increased secretion of a set of pro-angiogenic factors. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | |||||||||||||
|
|
|||||||||||||
| Key Molecule: Proto-oncogene tyrosine-protein kinase receptor Ret (RET) | [15] | ||||||||||||
| Sensitive Disease | Thyroid gland cancer [ICD-11: 2D10.0] | ||||||||||||
| Molecule Alteration | Missense mutation | p.M918T (c.2753T>C) |
|||||||||||
| Wild Type Structure | Method: X-ray diffraction | Resolution: 1.64 Å | |||||||||||
| Mutant Type Structure | Method: X-ray diffraction | Resolution: 2.12 Å | |||||||||||
| Download The Information of Sequence | Download The Structure File | ||||||||||||
-
700
|
G
G
P
P
L
L
S
S
L
L
S
S
V
V
D
D
A
A
F
F
710
|
K
K
I
I
L
L
E
E
D
D
P
P
K
K
W
W
E
E
F
F
720
|
P
P
R
R
K
K
N
N
L
L
V
V
L
L
G
G
K
K
T
T
730
|
L
L
G
G
E
E
G
G
E
E
F
F
G
G
K
K
V
V
V
V
740
|
K
K
A
A
T
T
A
A
F
F
H
H
L
L
K
K
G
G
R
R
750
|
A
A
G
G
Y
Y
T
T
T
T
V
V
A
A
V
V
K
K
M
M
760
|
L
L
K
K
E
E
N
N
A
A
S
S
P
P
S
S
E
E
L
L
770
|
R
R
D
D
L
L
L
L
S
S
E
E
F
F
N
N
V
V
L
L
780
|
K
K
Q
Q
V
V
N
N
H
H
P
P
H
H
V
V
I
I
K
K
790
|
L
L
Y
Y
G
G
A
A
C
C
S
S
Q
Q
D
D
G
G
P
P
800
|
L
L
L
L
L
L
I
I
V
V
E
E
Y
Y
A
A
K
K
Y
Y
810
|
G
G
S
S
L
L
R
R
G
G
F
F
L
L
R
R
E
E
S
S
820
|
R
R
K
K
V
V
G
G
P
P
G
G
Y
Y
L
L
G
G
S
S
830
|
G
G
G
G
S
S
R
R
N
N
S
S
S
S
S
S
L
L
D
D
840
|
H
H
P
P
D
D
E
E
R
R
A
A
L
L
T
T
M
M
G
G
850
|
D
D
L
L
I
I
S
S
F
F
A
A
W
W
Q
Q
I
I
S
S
860
|
Q
Q
G
G
M
M
Q
Q
Y
Y
L
L
A
A
E
E
M
M
K
K
870
|
L
L
V
V
H
H
R
R
D
D
L
L
A
A
A
A
R
R
N
N
880
|
I
I
L
L
V
V
A
A
E
E
G
G
R
R
K
K
M
M
K
K
890
|
I
I
S
S
D
D
F
F
G
G
L
L
S
S
R
R
D
D
V
V
900
|
Y
Y
E
E
E
E
D
D
S
S
Y
Y
V
V
K
K
R
R
S
S
910
|
Q
Q
G
G
R
R
I
I
P
P
V
V
K
K
W
W
M
T
A
A
920
|
I
I
E
E
S
S
L
L
F
F
D
D
H
H
I
I
Y
Y
T
T
930
|
T
T
Q
Q
S
S
D
D
V
V
W
W
S
S
F
F
G
G
V
V
940
|
L
L
L
L
W
W
E
E
I
I
V
V
T
T
L
L
G
G
G
G
950
|
N
N
P
P
Y
Y
P
P
G
G
I
I
P
P
P
P
E
E
R
R
960
|
L
L
F
F
N
N
L
L
L
L
K
K
T
T
G
G
H
H
R
R
970
|
M
M
E
E
R
R
P
P
D
D
N
N
C
C
S
S
E
E
E
E
980
|
M
M
Y
Y
R
R
L
L
M
M
L
L
Q
Q
C
C
W
W
K
K
990
|
Q
Q
E
E
P
P
D
D
K
K
R
R
P
P
V
V
F
F
A
A
1000
|
D
D
I
I
S
S
K
K
D
D
L
L
E
E
K
K
M
M
M
M
1010
|
V
V
K
K
R
R
R
R
|
|||||||||||||
| Experimental Note | Revealed Based on the Cell Line Data | ||||||||||||
| In Vitro Model | HEK293 cells | Kidney | Homo sapiens (Human) | CVCL_0045 | |||||||||
| TPC-1 cells | Thyroid | Homo sapiens (Human) | CVCL_6298 | ||||||||||
| MZ-CRC-1 cells | Pleural effusion | Homo sapiens (Human) | CVCL_A656 | ||||||||||
| MTC-TT cells | Thyroid gland | Homo sapiens (Human) | CVCL_1774 | ||||||||||
| Experiment for Molecule Alteration |
Western blot analysis | ||||||||||||
| Experiment for Drug Resistance |
MTT assay | ||||||||||||
| Mechanism Description | The missense mutation p.M918T (c.2753T>C) in gene RET cause the sensitivity of Cabozantinib by aberration of the drug's therapeutic target | ||||||||||||
| Key Molecule: Proto-oncogene tyrosine-protein kinase receptor Ret (RET) | [16] | ||||||||||||
| Sensitive Disease | Thyroid gland cancer [ICD-11: 2D10.0] | ||||||||||||
| Molecule Alteration | Missense mutation | p.M918T (c.2753T>C) |
|||||||||||
| Wild Type Structure | Method: X-ray diffraction | Resolution: 1.64 Å | |||||||||||
| Mutant Type Structure | Method: X-ray diffraction | Resolution: 2.12 Å | |||||||||||
| Download The Information of Sequence | Download The Structure File | ||||||||||||
-
700
|
G
G
P
P
L
L
S
S
L
L
S
S
V
V
D
D
A
A
F
F
710
|
K
K
I
I
L
L
E
E
D
D
P
P
K
K
W
W
E
E
F
F
720
|
P
P
R
R
K
K
N
N
L
L
V
V
L
L
G
G
K
K
T
T
730
|
L
L
G
G
E
E
G
G
E
E
F
F
G
G
K
K
V
V
V
V
740
|
K
K
A
A
T
T
A
A
F
F
H
H
L
L
K
K
G
G
R
R
750
|
A
A
G
G
Y
Y
T
T
T
T
V
V
A
A
V
V
K
K
M
M
760
|
L
L
K
K
E
E
N
N
A
A
S
S
P
P
S
S
E
E
L
L
770
|
R
R
D
D
L
L
L
L
S
S
E
E
F
F
N
N
V
V
L
L
780
|
K
K
Q
Q
V
V
N
N
H
H
P
P
H
H
V
V
I
I
K
K
790
|
L
L
Y
Y
G
G
A
A
C
C
S
S
Q
Q
D
D
G
G
P
P
800
|
L
L
L
L
L
L
I
I
V
V
E
E
Y
Y
A
A
K
K
Y
Y
810
|
G
G
S
S
L
L
R
R
G
G
F
F
L
L
R
R
E
E
S
S
820
|
R
R
K
K
V
V
G
G
P
P
G
G
Y
Y
L
L
G
G
S
S
830
|
G
G
G
G
S
S
R
R
N
N
S
S
S
S
S
S
L
L
D
D
840
|
H
H
P
P
D
D
E
E
R
R
A
A
L
L
T
T
M
M
G
G
850
|
D
D
L
L
I
I
S
S
F
F
A
A
W
W
Q
Q
I
I
S
S
860
|
Q
Q
G
G
M
M
Q
Q
Y
Y
L
L
A
A
E
E
M
M
K
K
870
|
L
L
V
V
H
H
R
R
D
D
L
L
A
A
A
A
R
R
N
N
880
|
I
I
L
L
V
V
A
A
E
E
G
G
R
R
K
K
M
M
K
K
890
|
I
I
S
S
D
D
F
F
G
G
L
L
S
S
R
R
D
D
V
V
900
|
Y
Y
E
E
E
E
D
D
S
S
Y
Y
V
V
K
K
R
R
S
S
910
|
Q
Q
G
G
R
R
I
I
P
P
V
V
K
K
W
W
M
T
A
A
920
|
I
I
E
E
S
S
L
L
F
F
D
D
H
H
I
I
Y
Y
T
T
930
|
T
T
Q
Q
S
S
D
D
V
V
W
W
S
S
F
F
G
G
V
V
940
|
L
L
L
L
W
W
E
E
I
I
V
V
T
T
L
L
G
G
G
G
950
|
N
N
P
P
Y
Y
P
P
G
G
I
I
P
P
P
P
E
E
R
R
960
|
L
L
F
F
N
N
L
L
L
L
K
K
T
T
G
G
H
H
R
R
970
|
M
M
E
E
R
R
P
P
D
D
N
N
C
C
S
S
E
E
E
E
980
|
M
M
Y
Y
R
R
L
L
M
M
L
L
Q
Q
C
C
W
W
K
K
990
|
Q
Q
E
E
P
P
D
D
K
K
R
R
P
P
V
V
F
F
A
A
1000
|
D
D
I
I
S
S
K
K
D
D
L
L
E
E
K
K
M
M
M
M
1010
|
V
V
K
K
R
R
R
R
|
|||||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| Key Molecule: Proto-oncogene tyrosine-protein kinase receptor Ret (RET) | [15] | ||||||||||||
| Sensitive Disease | Thyroid gland cancer [ICD-11: 2D10.0] | ||||||||||||
| Molecule Alteration | Missense mutation | p.C634W (c.1902C>G) |
|||||||||||
| Experimental Note | Revealed Based on the Cell Line Data | ||||||||||||
| In Vitro Model | HEK293 cells | Kidney | Homo sapiens (Human) | CVCL_0045 | |||||||||
| TPC-1 cells | Thyroid | Homo sapiens (Human) | CVCL_6298 | ||||||||||
| MZ-CRC-1 cells | Pleural effusion | Homo sapiens (Human) | CVCL_A656 | ||||||||||
| MTC-TT cells | Thyroid gland | Homo sapiens (Human) | CVCL_1774 | ||||||||||
| Experiment for Molecule Alteration |
Western blot analysis | ||||||||||||
| Experiment for Drug Resistance |
MTT assay | ||||||||||||
| Mechanism Description | The missense mutation p.C634W (c.1902C>G) in gene RET cause the sensitivity of Cabozantinib by aberration of the drug's therapeutic target | ||||||||||||
| Key Molecule: Proto-oncogene tyrosine-protein kinase receptor Ret (RET) | [17] | ||||||||||||
| Sensitive Disease | Thyroid gland cancer [ICD-11: 2D10.0] | ||||||||||||
| Molecule Alteration | Missense mutation | p.C634W (c.1902C>G) |
|||||||||||
| Experimental Note | Revealed Based on the Cell Line Data | ||||||||||||
| In Vitro Model | TT cells | Thyroid gland | Homo sapiens (Human) | CVCL_1774 | |||||||||
| In Vivo Model | Female nu/nu mouse xenograft model | Mus musculus | |||||||||||
| Experiment for Molecule Alteration |
Kinase inhibition assay | ||||||||||||
| Mechanism Description | The missense mutation p.C634W (c.1902C>G) in gene RET cause the sensitivity of Cabozantinib by unusual activation of pro-survival pathway | ||||||||||||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | |||||||||||||
|
|
|||||||||||||
| Key Molecule: Proto-oncogene tyrosine-protein kinase receptor Ret (RET) | [1] | ||||||||||||
| Resistant Disease | Multiple endocrine neoplasia [ICD-11: 2F7A.0] | ||||||||||||
| Molecule Alteration | Missense mutation | p.M918T |
|||||||||||
| Wild Type Structure | Method: X-ray diffraction | Resolution: 1.64 Å | |||||||||||
| Mutant Type Structure | Method: X-ray diffraction | Resolution: 2.12 Å | |||||||||||
| Download The Information of Sequence | Download The Structure File | ||||||||||||
-
700
|
G
G
P
P
L
L
S
S
L
L
S
S
V
V
D
D
A
A
F
F
710
|
K
K
I
I
L
L
E
E
D
D
P
P
K
K
W
W
E
E
F
F
720
|
P
P
R
R
K
K
N
N
L
L
V
V
L
L
G
G
K
K
T
T
730
|
L
L
G
G
E
E
G
G
E
E
F
F
G
G
K
K
V
V
V
V
740
|
K
K
A
A
T
T
A
A
F
F
H
H
L
L
K
K
G
G
R
R
750
|
A
A
G
G
Y
Y
T
T
T
T
V
V
A
A
V
V
K
K
M
M
760
|
L
L
K
K
E
E
N
N
A
A
S
S
P
P
S
S
E
E
L
L
770
|
R
R
D
D
L
L
L
L
S
S
E
E
F
F
N
N
V
V
L
L
780
|
K
K
Q
Q
V
V
N
N
H
H
P
P
H
H
V
V
I
I
K
K
790
|
L
L
Y
Y
G
G
A
A
C
C
S
S
Q
Q
D
D
G
G
P
P
800
|
L
L
L
L
L
L
I
I
V
V
E
E
Y
Y
A
A
K
K
Y
Y
810
|
G
G
S
S
L
L
R
R
G
G
F
F
L
L
R
R
E
E
S
S
820
|
R
R
K
K
V
V
G
G
P
P
G
G
Y
Y
L
L
G
G
S
S
830
|
G
G
G
G
S
S
R
R
N
N
S
S
S
S
S
S
L
L
D
D
840
|
H
H
P
P
D
D
E
E
R
R
A
A
L
L
T
T
M
M
G
G
850
|
D
D
L
L
I
I
S
S
F
F
A
A
W
W
Q
Q
I
I
S
S
860
|
Q
Q
G
G
M
M
Q
Q
Y
Y
L
L
A
A
E
E
M
M
K
K
870
|
L
L
V
V
H
H
R
R
D
D
L
L
A
A
A
A
R
R
N
N
880
|
I
I
L
L
V
V
A
A
E
E
G
G
R
R
K
K
M
M
K
K
890
|
I
I
S
S
D
D
F
F
G
G
L
L
S
S
R
R
D
D
V
V
900
|
Y
Y
E
E
E
E
D
D
S
S
Y
Y
V
V
K
K
R
R
S
S
910
|
Q
Q
G
G
R
R
I
I
P
P
V
V
K
K
W
W
M
T
A
A
920
|
I
I
E
E
S
S
L
L
F
F
D
D
H
H
I
I
Y
Y
T
T
930
|
T
T
Q
Q
S
S
D
D
V
V
W
W
S
S
F
F
G
G
V
V
940
|
L
L
L
L
W
W
E
E
I
I
V
V
T
T
L
L
G
G
G
G
950
|
N
N
P
P
Y
Y
P
P
G
G
I
I
P
P
P
P
E
E
R
R
960
|
L
L
F
F
N
N
L
L
L
L
K
K
T
T
G
G
H
H
R
R
970
|
M
M
E
E
R
R
P
P
D
D
N
N
C
C
S
S
E
E
E
E
980
|
M
M
Y
Y
R
R
L
L
M
M
L
L
Q
Q
C
C
W
W
K
K
990
|
Q
Q
E
E
P
P
D
D
K
K
R
R
P
P
V
V
F
F
A
A
1000
|
D
D
I
I
S
S
K
K
D
D
L
L
E
E
K
K
M
M
M
M
1010
|
V
V
K
K
R
R
R
R
|
|||||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| In Vitro Model | BaF3 cells | Bone | Mus musculus (Mouse) | CVCL_0161 | |||||||||
| Experiment for Molecule Alteration |
qRT-PCR | ||||||||||||
| Experiment for Drug Resistance |
LC50 assay | ||||||||||||
| Mechanism Description | M918T is a RET mutation prevalent in aggressive multiple endocrine neoplasia type 2B. M918T mutation is located at distant sites away from the TKI binding pocket. IC50s of cabozantinib, lenvatinib, vandetanib and nintedanib in BaF3/KR (M918T) cells were 6.5-fold, 7.5-fold, 4.3-fold and 1.7-fold, respectively, higher than in BaF3/KR cells. | ||||||||||||
| Key Molecule: Proto-oncogene tyrosine-protein kinase receptor Ret (RET) | [1] | ||||||||||||
| Resistant Disease | Multiple endocrine neoplasia [ICD-11: 2F7A.0] | ||||||||||||
| Molecule Alteration | Missense mutation | p.M918T |
|||||||||||
| Wild Type Structure | Method: X-ray diffraction | Resolution: 1.64 Å | |||||||||||
| Mutant Type Structure | Method: X-ray diffraction | Resolution: 2.12 Å | |||||||||||
| Download The Information of Sequence | Download The Structure File | ||||||||||||
-
700
|
G
G
P
P
L
L
S
S
L
L
S
S
V
V
D
D
A
A
F
F
710
|
K
K
I
I
L
L
E
E
D
D
P
P
K
K
W
W
E
E
F
F
720
|
P
P
R
R
K
K
N
N
L
L
V
V
L
L
G
G
K
K
T
T
730
|
L
L
G
G
E
E
G
G
E
E
F
F
G
G
K
K
V
V
V
V
740
|
K
K
A
A
T
T
A
A
F
F
H
H
L
L
K
K
G
G
R
R
750
|
A
A
G
G
Y
Y
T
T
T
T
V
V
A
A
V
V
K
K
M
M
760
|
L
L
K
K
E
E
N
N
A
A
S
S
P
P
S
S
E
E
L
L
770
|
R
R
D
D
L
L
L
L
S
S
E
E
F
F
N
N
V
V
L
L
780
|
K
K
Q
Q
V
V
N
N
H
H
P
P
H
H
V
V
I
I
K
K
790
|
L
L
Y
Y
G
G
A
A
C
C
S
S
Q
Q
D
D
G
G
P
P
800
|
L
L
L
L
L
L
I
I
V
V
E
E
Y
Y
A
A
K
K
Y
Y
810
|
G
G
S
S
L
L
R
R
G
G
F
F
L
L
R
R
E
E
S
S
820
|
R
R
K
K
V
V
G
G
P
P
G
G
Y
Y
L
L
G
G
S
S
830
|
G
G
G
G
S
S
R
R
N
N
S
S
S
S
S
S
L
L
D
D
840
|
H
H
P
P
D
D
E
E
R
R
A
A
L
L
T
T
M
M
G
G
850
|
D
D
L
L
I
I
S
S
F
F
A
A
W
W
Q
Q
I
I
S
S
860
|
Q
Q
G
G
M
M
Q
Q
Y
Y
L
L
A
A
E
E
M
M
K
K
870
|
L
L
V
V
H
H
R
R
D
D
L
L
A
A
A
A
R
R
N
N
880
|
I
I
L
L
V
V
A
A
E
E
G
G
R
R
K
K
M
M
K
K
890
|
I
I
S
S
D
D
F
F
G
G
L
L
S
S
R
R
D
D
V
V
900
|
Y
Y
E
E
E
E
D
D
S
S
Y
Y
V
V
K
K
R
R
S
S
910
|
Q
Q
G
G
R
R
I
I
P
P
V
V
K
K
W
W
M
T
A
A
920
|
I
I
E
E
S
S
L
L
F
F
D
D
H
H
I
I
Y
Y
T
T
930
|
T
T
Q
Q
S
S
D
D
V
V
W
W
S
S
F
F
G
G
V
V
940
|
L
L
L
L
W
W
E
E
I
I
V
V
T
T
L
L
G
G
G
G
950
|
N
N
P
P
Y
Y
P
P
G
G
I
I
P
P
P
P
E
E
R
R
960
|
L
L
F
F
N
N
L
L
L
L
K
K
T
T
G
G
H
H
R
R
970
|
M
M
E
E
R
R
P
P
D
D
N
N
C
C
S
S
E
E
E
E
980
|
M
M
Y
Y
R
R
L
L
M
M
L
L
Q
Q
C
C
W
W
K
K
990
|
Q
Q
E
E
P
P
D
D
K
K
R
R
P
P
V
V
F
F
A
A
1000
|
D
D
I
I
S
S
K
K
D
D
L
L
E
E
K
K
M
M
M
M
1010
|
V
V
K
K
R
R
R
R
|
|||||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| In Vitro Model | BaF3 cells | Bone | Mus musculus (Mouse) | CVCL_0161 | |||||||||
| Experiment for Molecule Alteration |
qRT-PCR | ||||||||||||
| Experiment for Drug Resistance |
LC50 assay | ||||||||||||
| Mechanism Description | M918T is a RET mutation prevalent in aggressive multiple endocrine neoplasia type 2B. M918T mutation is located at distant sites away from the TKI binding pocket. IC50s of cabozantinib, lenvatinib, vandetanib and nintedanib in BaF3/KR (M918T) cells were 6.5-fold, 7.5-fold, 4.3-fold and 1.7-fold, respectively, higher than in BaF3/KR cells. | ||||||||||||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
