Drug Information
Drug (ID: DG00230) and It's Reported Resistant Information
| Name |
Bosutinib
|
||||
|---|---|---|---|---|---|
| Synonyms |
SKI 606; SKI606; Bosutinib (USAN); PF-5208763; SKI-606; Xy]-3-quinolinecarbonitrile; 4-((2,4-dichloro-5-methoxyphenyl)amino)-6-methoxy-7-(3-(4-methyl-1-piperazinyl)propoxy)-3-quinolinecarbonitrile; 4-((2,4-dichloro-5-methoxyphenyl)amino)-6-methoxy-7-(3-(4-methylpiperazin-1-yl)propoxy)quinoline-3-carbonitrile; 4-(2,4-dichloro-5-methoxyanilino)-6-methoxy-7-[3-(4-methylpiperazin-1-yl)propoxy]quinoline-3-carbonitrile; 4-[(2,4-Dichloro-5-methoxyphenyl)amino]-6-methoxy-7-[3-(4-methyl-1-piperazinyl)propo; 4-[(2,4-dichloro-5-methoxyphenyl)amino]-6-methoxy-7-[3-(4-methylpiperazin-1-yl)propoxy]quinoline-3-carbonitrile; Bosutinib (BCR-ABL inhibitor 3rd gen)
Click to Show/Hide
|
||||
| Indication |
In total 1 Indication(s)
|
||||
| Structure |
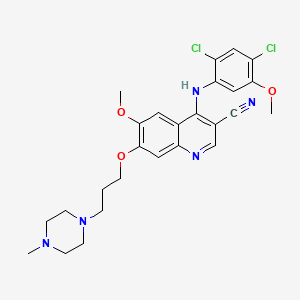
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Clinically Reported Resistance for This Drug
(1 diseases)
[3]
|
||||
| Target | Proto-oncogene c-Src (SRC) | SRC_HUMAN | [1] | ||
| Tyrosine-protein kinase ABL1 (ABL) | ABL1_HUMAN | [2] | |||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
C26H29Cl2N5O3
|
||||
| IsoSMILES |
CN1CCN(CC1)CCCOC2=C(C=C3C(=C2)N=CC(=C3NC4=CC(=C(C=C4Cl)Cl)OC)C#N)OC
|
||||
| InChI |
1S/C26H29Cl2N5O3/c1-32-6-8-33(9-7-32)5-4-10-36-25-13-21-18(11-24(25)35-3)26(17(15-29)16-30-21)31-22-14-23(34-2)20(28)12-19(22)27/h11-14,16H,4-10H2,1-3H3,(H,30,31)
|
||||
| InChIKey |
UBPYILGKFZZVDX-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
| VARIDT ID | |||||
| INTEDE ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-02: Benign/in-situ/malignant neoplasm
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | |||||||||||||
|
|
|||||||||||||
| Key Molecule: Tyrosine-protein kinase ABL1 (ABL1) | [3] | ||||||||||||
| Resistant Disease | Chronic myeloid leukemia [ICD-11: 2A20.0] | ||||||||||||
| Molecule Alteration | Missense mutation | p.F359V |
|||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| In Vivo Model | A retrospective survey in conducting clinical studies | Homo sapiens | |||||||||||
| Experiment for Molecule Alteration |
Direct sequencing assay | ||||||||||||
| Experiment for Drug Resistance |
Overall survival assay; Event-free survival (EFS) assay | ||||||||||||
| Mechanism Description | Patients with more than one BCR-ABL1 mutation fare worse than those with no or one mutation. | ||||||||||||
| Key Molecule: Tyrosine-protein kinase ABL1 (ABL1) | [3] | ||||||||||||
| Resistant Disease | Chronic myeloid leukemia [ICD-11: 2A20.0] | ||||||||||||
| Molecule Alteration | Missense mutation | p.M351T |
|||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| In Vivo Model | A retrospective survey in conducting clinical studies | Homo sapiens | |||||||||||
| Experiment for Molecule Alteration |
Direct sequencing assay | ||||||||||||
| Experiment for Drug Resistance |
Overall survival assay; Event-free survival (EFS) assay | ||||||||||||
| Mechanism Description | Patients with more than one BCR-ABL1 mutation fare worse than those with no or one mutation. | ||||||||||||
| Key Molecule: Tyrosine-protein kinase ABL1 (ABL1) | [4] | ||||||||||||
| Resistant Disease | Chronic myeloid leukemia [ICD-11: 2A20.0] | ||||||||||||
| Molecule Alteration | Missense mutation | p.F359I |
|||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| Experiment for Molecule Alteration |
Direct sequencing assay | ||||||||||||
| Experiment for Drug Resistance |
Tritiated thymidine incorporation assay | ||||||||||||
| Mechanism Description | L248R was identified in a patient with lymphoid Blast Crisis (BC) CML (Patient no. 1), in cis with a pre-existing mutation. The patient initially presented with an imatinib-resistant F359I mutation. | ||||||||||||
| Key Molecule: Tyrosine-protein kinase ABL1 (ABL1) | [4] | ||||||||||||
| Resistant Disease | Chronic myeloid leukemia [ICD-11: 2A20.0] | ||||||||||||
| Molecule Alteration | Missense mutation | p.L248R |
|||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| Experiment for Molecule Alteration |
Direct sequencing assay | ||||||||||||
| Experiment for Drug Resistance |
Tritiated thymidine incorporation assay | ||||||||||||
| Mechanism Description | Mutations may impair TkI activity by directly or indirectly impairing the drug binding to the protein. We report the discovery of three new BCR/ABL mutations, L248R, T315V, and F317R identified in two patients with CML (L248R and T315V) and in one patient with Ph+ acute lymphoblastic leukemia (ALL) (F317R). | ||||||||||||
|
|
|||||||||||||
| Key Molecule: GTPase Nras (NRAS) | [1], [2] | ||||||||||||
| Resistant Disease | Chronic myeloid leukemia [ICD-11: 2A20.0] | ||||||||||||
| Molecule Alteration | Missense mutation | p.G12V |
|||||||||||
| Wild Type Structure | Method: X-ray diffraction | Resolution: 1.98 Å | |||||||||||
| Mutant Type Structure | Method: X-ray diffraction | Resolution: 1.96 Å | |||||||||||
| Download The Information of Sequence | Download The Structure File | ||||||||||||
-
G
-
0
|
S
-
M
M
T
T
E
E
Y
Y
K
K
L
L
V
V
V
V
V
V
10
|
G
G
A
A
G
V
G
G
V
V
G
G
K
K
S
S
A
A
L
L
20
|
T
T
I
I
Q
Q
L
L
I
I
Q
Q
N
N
H
H
F
F
V
V
30
|
D
D
E
E
Y
Y
D
D
P
P
T
T
I
I
E
E
D
D
S
S
40
|
Y
Y
R
R
K
K
Q
Q
V
V
V
V
I
I
D
D
G
G
E
E
50
|
T
T
C
C
L
L
L
L
D
D
I
I
L
L
D
D
T
T
A
A
60
|
G
G
Q
Q
E
E
E
E
Y
Y
S
S
A
A
M
M
R
R
D
D
70
|
Q
Q
Y
Y
M
M
R
R
T
T
G
G
E
E
G
G
F
F
L
L
80
|
C
C
V
V
F
F
A
A
I
I
N
N
N
N
T
T
K
K
S
S
90
|
F
F
E
E
D
D
I
I
H
H
H
H
Y
Y
R
R
E
E
Q
Q
100
|
I
I
K
K
R
R
V
V
K
K
D
D
S
S
E
E
D
D
V
V
110
|
P
P
M
M
V
V
L
L
V
V
G
G
N
N
K
K
S
S
D
D
120
|
L
L
P
P
S
S
R
R
T
T
V
V
D
D
T
T
K
K
Q
Q
130
|
A
A
Q
Q
D
D
L
L
A
A
R
R
S
S
Y
Y
G
G
I
I
140
|
P
P
F
F
I
I
E
E
T
T
S
S
A
A
K
K
T
T
R
R
150
|
Q
Q
G
G
V
V
D
D
D
D
A
A
F
F
Y
Y
T
T
L
L
160
|
V
V
R
R
E
E
I
I
R
R
K
K
H
H
K
K
E
E
K
K
170
|
M
M
S
S
K
K
D
D
G
G
K
K
K
K
K
K
K
K
K
K
180
|
K
K
S
S
K
K
T
T
K
K
C
C
V
V
I
I
M
M
|
|||||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| Cell Pathway Regulation | JAKT2/STAT signaling pathway | Activation | hsa04030 | ||||||||||
| RAF/KRAS/MEK signaling pathway | Activation | hsa04010 | |||||||||||
| In Vitro Model | HL60 cells | Peripheral blood | Homo sapiens (Human) | CVCL_0002 | |||||||||
| U937 cells | Blood | Homo sapiens (Human) | CVCL_0007 | ||||||||||
| K562 cells | Blood | Homo sapiens (Human) | CVCL_0004 | ||||||||||
| KCL-22 cells | Bone marrow | Homo sapiens (Human) | CVCL_2091 | ||||||||||
| Sup-B15 cells | Bone marrow | Homo sapiens (Human) | CVCL_0103 | ||||||||||
| HEL cells | Blood | Homo sapiens (Human) | CVCL_0001 | ||||||||||
| HMC-1.2 cells | Blood | Homo sapiens (Human) | CVCL_H205 | ||||||||||
| In Vivo Model | A retrospective survey in conducting clinical studies | Homo sapiens | |||||||||||
| Experiment for Molecule Alteration |
Next-generation sequencing assay; Sanger Sequencing assay | ||||||||||||
| Mechanism Description | This mutation is well known for its effects on proliferation and its association with AML and MPN, suggesting that this variant might have been involved in the TkI resistance of this patient. | ||||||||||||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
