Drug Information
Drug (ID: DG02036) and It's Reported Resistant Information
| Name |
Arenobufagin
|
||||
|---|---|---|---|---|---|
| Synonyms |
Arenobufagin|464-74-4|5-[(3S,5R,8R,9S,10S,11S,13R,14S,17R)-3,11,14-trihydroxy-10,13-dimethyl-12-oxo-2,3,4,5,6,7,8,9,11,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-17-yl]pyran-2-one|12-Oxo-3-beta,11-alpha,14-trihydroxy-5-beta-bufa-20,22-dienolide|27R42QLM25|3,11,14-trihydroxy-12-oxo-bufa-20,22-dienolide|5beta-Bufa-20,22-dienolide, 3beta,11alpha,14-trihydroxy-12-oxo-|5-beta-BUFA-20,22-DIENOLIDE, 12-OXO-3-beta,11-alpha,14-TRIHYDROXY-|Bufa-20,22-dienolide, 3,11,14-trihydroxy-12-oxo-, (3beta,5beta,11alpha)-|5-((3S,5R,8R,9S,10S,11S,13R,14S,17R)-3,11,14-trihydroxy-10,13-dimethyl-12-oxohexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)-2H-pyran-2-one|5-((3S,5R,10S,11S,13R,14S,17R)-3,11,14-trihydroxy-10,13-dimethyl-12-oxo-2,3,4,5,6,7,8,9,11,15,16,17-dodecahydro-1H-cyclopenta(a)phenanthren-17-yl)pyran-2-one|5-((3S,5R,8R,9S,10S,11S,13R,14S,17R)-3,11,14-trihydroxy-10,13-dimethyl-12-oxo-2,3,4,5,6,7,8,9,11,15,16,17-dodecahydro-1H-cyclopenta(a)phenanthren-17-yl)pyran-2-one|5-(5,11,17-trihydroxy-2,15-dimethyl-16-oxotetracyclo(8.7.0.02,7.011,15)heptadecan-14-yl)-2H-pyran-2-one|5-[(3S,5R,10S,11S,13R,14S,17R)-3,11,14-trihydroxy-10,13-dimethyl-12-oxo-2,3,4,5,6,7,8,9,11,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-17-yl]pyran-2-one|5-{5,11,17-trihydroxy-2,15-dimethyl-16-oxotetracyclo[8.7.0.02,7.011,15]heptadecan-14-yl}-2H-pyran-2-one|UNII-27R42QLM25|12-Oxo-3-.beta.,11-.alpha.,14-trihydroxy-5-.beta.-bufa-20,22-dienolide|5.beta.-Bufa-20,22-dienolide, 3.beta.,11.alpha.,14-trihydroxy-12-oxo-|CHEMBL4086974|SCHEMBL21578402|DTXSID00963565|5-.beta.-Bufa-20,22-dienolide, 12-oxo-3-.beta.,11-.alpha.,14-trihydroxy-|Bufa-20,22-dienolide, 3,11,14-trihydroxy-12-oxo-, (3.beta.,5.beta.,11.alpha.)-|CHEBI:197067|GLXC-13270|HY-N0876|AKOS030526814|CS-3693|FA74189|NCGC00485924-01|AC-34734|AS-76734|DA-50681|C20035|(3beta,5beta,11alpha)-3,11,14-Trihydroxy-12-oxobufa-20,22-dienolide|Bufa-20,22-dienolide, 3,11,14-trihydroxy-12-oxo- (3-beta,5-beta,11-alpha)-|Bufa-20,22-dienolide, 3,11,14-trihydroxy-12-oxo-(3-beta,5-beta,11-alpha)-|5-((3S,5R,10S,11S,13R,14S,17R)-3,11,14-trihydroxy-10,13-dimethyl-12-oxohexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)-2H-pyran-2-one
Click to Show/Hide
|
||||
| Indication |
In total 1 Indication(s)
.
|
||||
| Structure |
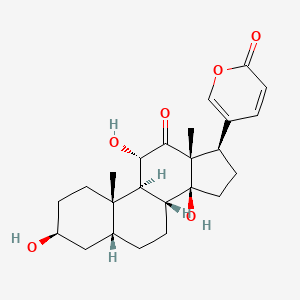
|
||||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
C24H32O6
|
||||
| IsoSMILES |
C[C@]12CC[C@@H](C[C@H]1CC[C@@H]3[C@@H]2[C@@H](C(=O)[C@]4([C@@]3(CC[C@@H]4C5=COC(=O)C=C5)O)C)O)O
|
||||
| InChI |
InChI=1S/C24H32O6/c1-22-9-7-15(25)11-14(22)4-5-17-19(22)20(27)21(28)23(2)16(8-10-24(17,23)29)13-3-6-18(26)30-12-13/h3,6,12,14-17,19-20,25,27,29H,4-5,7-11H2,1-2H3/t14-,15+,16-,17-,19-,20+,22+,23+,24+/m1/s1
|
||||
| InChIKey |
JGDCRWYOMWSTFC-AZGSIFHYSA-N
|
||||
| PubChem CID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-02: Benign/in-situ/malignant neoplasm
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Proprotein convertase subtilisin/kexin type 9 (PCSK9) | [1] | |||
| Metabolic Type | Lipid metabolism | |||
| Sensitive Disease | Hepatocellular carcinoma [ICD-11: 2C12.02] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Liver cancer [ICD-11: 2C12] | |||
| The Specified Disease | Hepatocellular carcinoma | |||
| The Studied Tissue | Liver tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 5.96E-09 Fold-change: 4.92E-01 Z-score: 5.99E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cholesterol metabolism | Activation | hsa04979 | |
| In Vivo Model | Hepa1-6 hepatocellular carcinoma transplanted tumor model mice | Mice | ||
| Experiment for Molecule Alteration |
Western blot analysis and immunohistochemical assays | |||
| Experiment for Drug Resistance |
Tumor volume assay | |||
| Mechanism Description | ARBU significantly inhibited the proliferation of Hepa1-6 in vivo and in vitro, regulated cholesterol metabolism, and promoted the M1-type polarization of macrophages in the tumor microenvironment. ARBU inhibits cholesterol synthesis in the TME through the PCSK9/LDL-R signaling pathway, thereby blocking macrophage M2 polarization, promoting apoptosis of the tumor cells, and inhibiting their proliferation and migration. | |||
| Key Molecule: Proprotein convertase subtilisin/kexin type 9 (PCSK9) | [1] | |||
| Metabolic Type | Lipid metabolism | |||
| Sensitive Disease | Hepatocellular carcinoma [ICD-11: 2C12.02] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Liver cancer [ICD-11: 2C12] | |||
| The Specified Disease | Hepatocellular carcinoma | |||
| The Studied Tissue | Liver tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 5.96E-09 Fold-change: 4.92E-01 Z-score: 5.99E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cholesterol metabolism | Activation | hsa04979 | |
| In Vitro Model | Hepa1-6 cells | Liver | Mus musculus (Mouse) | CVCL_0327 |
| Experiment for Molecule Alteration |
Western blot analysis and immunohistochemical assays | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | ARBU significantly inhibited the proliferation of Hepa1-6 in vivo and in vitro, regulated cholesterol metabolism, and promoted the M1-type polarization of macrophages in the tumor microenvironment. ARBU inhibits cholesterol synthesis in the TME through the PCSK9/LDL-R signaling pathway, thereby blocking macrophage M2 polarization, promoting apoptosis of the tumor cells, and inhibiting their proliferation and migration. | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
