Drug Information
Drug (ID: DG02026) and It's Reported Resistant Information
| Name |
PD-166866
|
||||
|---|---|---|---|---|---|
| Synonyms |
192705-79-6|PD-166866|PD166866|PD 166866|1-[2-amino-6-(3,5-dimethoxyphenyl)pyrido[2,3-d]pyrimidin-7-yl]-3-tert-butylurea|Urea, N-[2-amino-6-(3,5-dimethoxyphenyl)pyrido[2,3-d]pyrimidin-7-yl]-N'-(1,1-dimethylethyl)-|NA856793UT|1-(2-Amino-6-(3,5-dimethoxyphenyl)-pyrido(2,3-d)pyrimidin-7-yl)-3-tert-butyl urea|1-(2-Amino-6-(3,5-dimethoxyphenyl)pyrido[2,3-d]pyrimidin-7-yl)-3-(tert-butyl)urea|CHEMBL299763|CHEBI:156259|DTXSID20416144|1-[2-Amino-6-(3,5-dimethoxyphenyl)-pyrido[2,3-d]pyrimidin-7-yl]-3-tert-butyl urea|Urea, N-(2-amino-6-(3,5-dimethoxyphenyl)pyrido(2,3-d)pyrimidin-7-yl)-N'-(1,1-dimethylethyl)-|1-(2-amino-6-(3,5-dimethoxy-phenyl)-pyrido(2,3-d)pyrimidin-7-yl)-3-tert-butyl-urea|1-(2-amino-6-(3,5-dimethoxyphenyl)pyrido[2,3-d]pyrimidin-7-yl)-3-(tert-butyl)urea.|N-[2-amino-6-(3,5-dimethoxyphenyl)pyrido[2,3-d]pyrimidin-7-yl]-N'-(1,1-dimethylethyl)-urea|UNII-NA856793UT|N-(2-amino-6-(3,5-dimethoxyphenyl)pyrido(2,3-d)pyrimidin-7-yl)-N'-(1,1-dimethylethyl)-urea|MFCD12922514|6-arylpyrido[2,3-d]pyrimidine deriv. 25|PFE-PKIS 3|Urea,N-[2-amino-6-(3,5-dimethoxyphenyl)pyrido[2,3-d]pyrimidin-7-yl]-N'-(1,1-dimethylethyl)-|FGF Receptor Tyrosine Kinase|BDBM3443|SCHEMBL1248489|DTXCID20366993|HMS3263L17|BCP20054|EX-A1324|Tox21_501178|PDSP1_000683|PDSP2_000673|s8493|AKOS025286334|CCG-222482|CS-6407|FP26777|NCGC00261863-01|AC-35802|AS-74459|HY-101296|PD166866?|C73670|PD-166866, >=98% (HPLC)|PD-166866 (PD166866)|Q27284760|FGF Receptor Tyrosine Kinase Inhibitor - CAS 192705-79-6|N-[2-Amino-6-(3,5-dimethoxyphenyl)pyrido[2,3-d]pyrimidin-7-yl]-N'-(1,1-dimethylethyl)|663-082-2
Click to Show/Hide
|
||||
| Indication |
In total 1 Indication(s)
Phase 4
|
||||
| Structure |
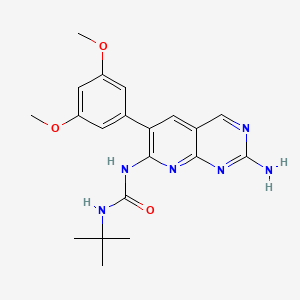
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Resistance Information Discovered by Cell Line Test for This Drug
(1 diseases)
[1]
|
||||
| Target | Fibroblast growth factor receptor (FGFR) | NOUNIPROTAC | |||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
C20H24N6O3
|
||||
| IsoSMILES |
CC(C)(C)NC(=O)NC1=C(C=C2C=NC(=NC2=N1)N)C3=CC(=CC(=C3)OC)OC
|
||||
| InChI |
InChI=1S/C20H24N6O3/c1-20(2,3)26-19(27)25-17-15(8-12-10-22-18(21)24-16(12)23-17)11-6-13(28-4)9-14(7-11)29-5/h6-10H,1-5H3,(H4,21,22,23,24,25,26,27)
|
||||
| InChIKey |
NHJSWORVNIOXIT-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-02: Benign/in-situ/malignant neoplasm
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Activating transcription factor 4 (ATF4) | [1] | |||
| Metabolic Type | Glucose metabolism | |||
| Resistant Disease | T-cell acute lymphoblastic leukemia [ICD-11: 2A90.5] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vivo Model | The 6 to 8-week-old NCG mice, with Jurkat-luciferase cell | Mice | ||
| Experiment for Molecule Alteration |
qRT-PCR; Western blot analysis | |||
| Experiment for Drug Resistance |
Cd7 antibody assay | |||
| Mechanism Description | Mechanistically, we found that FGFR1 inhibitors markedly increased the expression of ATF4, which was a major initiator for T-ALL resistance to FGFR1 inhibitors. We further revealed that FGFR1 inhibitors induced expression of ATF4 through enhancing chromatin accessibility combined with translational activation via the GCN2-eIF2alpha pathway. Subsequently, ATF4 remodeled the amino acid metabolism by stimulating the expression of multiple metabolic genes ASNS, ASS1, PHGDH and SLC1A5, maintaining the activation of mTORC1, which contributed to the drug resistance in T-ALL cells. | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
