| Synonyms |
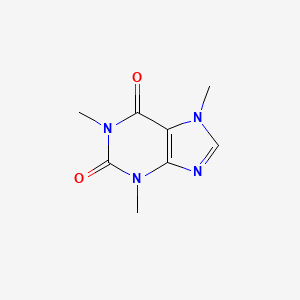
caffeine|58-08-2|Guaranine|1,3,7-Trimethylxanthine|Methyltheobromine|Theine|Thein|Cafeina|Koffein|Mateina|Alert-pep|Caffein|Cafipel|Coffeine|Refresh'n|Stim|Anhydrous caffeine|Cafamil|Cafecon|Caffedrine|Caffine|Vivarin|Nodaca|No-Doz|Eldiatric C|Hycomine|Coffein|Dexitac|Organex|7-Methyltheophylline|Nix Nap|Methyltheobromide|Coffeinum|Durvitan|Caffeine, synthetic|3,7-Dihydro-1,3,7-trimethyl-1H-purine-2,6-dione|Phensal|Tirend|1,3,7-Trimethyl-2,6-dioxopurine|1,3,7-Trimethylpurine-2,6-dione|Quick-Pep|Theophylline, 7-methyl|Tri-Aqua|Synalgos|Kofein|Miudol|Caffeine, anhydrous|DHCplus|Theobromine, 1-methyl-|1-methyltheobromine|1H-Purine-2,6-dione, 3,7-dihydro-1,3,7-trimethyl-|Caffeine (natural)|Xanthine, 1,3,7-trimethyl|Propoxyphene Compound 65|Methylxanthine theophylline|Kofein [Czech]|Coffein [German]|Koffein [German]|caffenium|teina|Caffeina|Caffeina [Italian]|NCI-C02733|Theophylline Me|Theobromine Me|1,3,7-Trimethyl-3,7-dihydro-1H-purine-2,6-dione|CAFFEINE ANHYDROUS|FEMA No. 2224|1,3,7-trimethyl-2,3,6,7-tetrahydro-1H-purine-2,6-dione|cafeine|SK-65 Compound|Caffeinum|Anacin|HSDB 36|Anacin Maximum Strength|P-A-C Analgesic Tablets|BRN 0017705|NSC 5036|CCRIS 1314|1,3,7-trimethyl-1H-purine-2,6(3H,7H)-dione|AI3-20154|NSC-5036|EINECS 200-362-1|A.S.A. and Codeine Compound|UNII-3G6A5W338E|Caffeine melting point standard|DTXSID0020232|CHEBI:27732|3G6A5W338E|MFCD00005758|CHEMBL113|Caffeine [USP:BAN:JAN]|DTXCID40232|ESGIC COMPONENT CAFFEINE|TRIAD COMPONENT CAFFEINE|CFF|DIMENHYDRINATE IMPURITY C|FEMCET COMPONENT CAFFEINE|TREZIX COMPONENT CAFFEINE|ANOQUAN COMPONENT CAFFEINE|NSC5036|CAFERGOT COMPONENT CAFFEINE|EC 200-362-1|EXCEDRIN COMPONENT CAFFEINE|FIORICET COMPONENT CAFFEINE|FIORINAL COMPONENT CAFFEINE|MIGERGOT COMPONENT CAFFEINE|NORGESIC COMPONENT CAFFEINE|WIGRAINE COMPONENT CAFFEINE|DHC PLUS COMPONENT CAFFEINE|INVAGESIC COMPONENT CAFFEINE|LANORINAL COMPONENT CAFFEINE|5-26-13-00558 (Beilstein Handbook Reference)|ORPHENGESIC COMPONENT CAFFEINE|SYNALGOS-DC COMPONENT CAFFEINE|Caffeine-13C|1,3,7-trimethyl-2,6-dioxo-1,2,3,6-tetrahydropurine|MEDIGESIC PLUS COMPONENT CAFFEINE|SYNALGOS-DC-A COMPONENT CAFFEINE|Caffeine-13C3|SK 65 Compound|DARVON COMPOUND COMPONENT CAFFEINE|INVAGESIC FORTE COMPONENT CAFFEINE|CAFFEINE (IARC)|CAFFEINE [IARC]|CAFFEINE (II)|CAFFEINE [II]|ORPHENGESIC FORTE COMPONENT CAFFEINE|Caffeine [BAN:JAN]|CAFFEINE (MART.)|CAFFEINE [MART.]|CAFFEINE (USP-RS)|CAFFEINE [USP-RS]|Caffeine (USP:BAN:JAN)|3,7-Dihydro-1,3,7-trimethyl-1H-purin-2,6-dion|CAFFEINE (EP MONOGRAPH)|CAFFEINE [EP MONOGRAPH]|CAFFEINE (USP MONOGRAPH)|CAFFEINE [USP MONOGRAPH]|CAS-58-08-2|Caffeine (USP)|SMR000326667|THEOPHYLLINE IMPURITY A (EP IMPURITY)|THEOPHYLLINE IMPURITY A [EP IMPURITY]|DIMENHYDRINATE IMPURITY C (EP IMPURITY)|DIMENHYDRINATE IMPURITY C [EP IMPURITY]|PENTOXIFYLLINE IMPURITY F (EP IMPURITY)|PENTOXIFYLLINE IMPURITY F [EP IMPURITY]|SR-01000075187|Anhydrous caffeine (TN)|Monomethyl derivative of Theophylline|Anhydrous caffeine (JP15)|metilteobromina|DiurexUltimate|Kafeina|DiurexUltra|Respia|trimetilksantina|Awake|NoSnooze|Allertness Aid|Uplift Chewable|Alertness Aid|Conrx Alert|Sprayable Energy|Stay Awake|Alert Aid|Methyltheophylline|NoSnoozeCircle K|1gfz|careone stay awake|Caffeine, BioXtra|Caffeine 200mg|Diurex Water Pills|Topcare Stay Awake|TNP00310|Monohydrate Caffeine|Caffeine1508|NoDoz Alertness Aid|Respia (TN)|AwakeMaximum Strength|Sohmed Alertness Aid|1-methyl-Theobromine|357 Super Magnum|7-methyl Theophylline|Cafergot (Salt/Mix)|1,7-Trimethylxanthine|Diurex Water PillsXPL|Spectrum_001301|1l5q|1l7x|2a3b|3g6m|3,7-dihydro-1,3,7-trimethyl-1H-purine|CAFFEINE [FHFI]|CAFFEINE [HSDB]|CAFFEINE [FCC]|Xanthine,3,7-trimethyl|CAFFEINE [MI]|1 3 7-trimetilksantina|CAFFEINE [VANDF]|CAFFEINUM [HPUS]|1,3,7-Trimethylxantine|Alertness Aid Stay Awake|Spectrum2_001261|Spectrum3_000321|Spectrum4_001782|Spectrum5_000423|Lopac-C-0750|1 3 7-Trimethylxanthine|Caffeine 200 mg Tablets|Jet Alert Double Strength|Stay AwakeMaximum Strength|bmse000206|CAFFEINE [WHO-DD]|CAFFEINE [WHO-IP]|Jet Alert Regular Strength|MolMap_000054|Probes1_000150|Probes2_000128|C 0750|SCHEMBL5671|Caffeine Tablets, 200 mg|Anhydrous caffeine (JP18)|NCIOpen2_008255|BIDD:PXR0172|Caffeine tablet, film-coated|Lopac0_000228|BSPBio_001921|GTPL407|KBioGR_002325|KBioSS_001781|1,3,7-trimethyl-1,3,7-trihydropurine-2,6-dione|MLS001055341|MLS001056714|MLS001066409|BIDD:ER0554|BIDD:GT0632|Caffeine, 1mg/ml in methanol|DivK1c_000730|SPECTRUM1500155|CU-01000012617-3|SPBio_001222|CAFFEINE [ORANGE BOOK]|829 - Tea quality parameters|MEGxp0_001350|ANHYDROUS CAFFEINE [JAN]|component of Dilone (Salt/Mix)|1,7-Trimethyl-2,6-dioxopurine|ACon1_000085|BDBM10849|HMS502E12|KBio1_000730|KBio2_001781|KBio2_004349|KBio2_006917|KBio3_001141|Caffeine 1.0 mg/ml in Methanol|CAFFEINE(3-METHYL-13C)|COFFEINUM [WHO-IP LATIN]|Caffeine, powder, ReagentPlus(R)|component of Percodan (Salt/Mix)|NINDS_000730|Bio1_000473|Bio1_000962|Bio1_001451|CAFFEINE COMPONENT OF ESGIC|CAFFEINE COMPONENT OF TRIAD|HMS1920I09|HMS2091O11|HMS2232M13|HMS3260N17|HMS3372J18|HMS3435F10|HMS3715D13|Pharmakon1600-01500155|Topcare Stay AwakeMaximum Strength|NoDoz Caplets and Chewable Tablets|Caffeine 10 microg/mL in Methanol|CAFFEINE ANHYDROUS [WHO-IP]|CAFFEINE COMPONENT OF FEMCET|CIA28298|CS-M0795|DDA07266|NoDoz Alertness AidMaximum Strength|XCA23885|CAFFEINE COMPONENT OF ANOQUAN|Tox21_201685|Tox21_300010|Tox21_500228|caffeine (1,3,7-trimethylxanthine)|Caffeine 100 microg/mL in Methanol|Carolina Cannabis Uplifting Chewables|CCG-38825|HB2846|NSC755917|PDSP1_001016|PDSP1_001235|PDSP2_001000|PDSP2_001219|STK177283|Valumeds Maximum Strength Stay Awake|CAFFEINE COMPONENT OF CAFERGOT|CAFFEINE COMPONENT OF DHC PLUS|CAFFEINE COMPONENT OF EXCEDRIN|CAFFEINE COMPONENT OF FIORICET|CAFFEINE COMPONENT OF FIORINAL|CAFFEINE COMPONENT OF MIGERGOT|CAFFEINE COMPONENT OF NORGESIC|CAFFEINE COMPONENT OF WIGRAINE|Propoxyphene Compound 65 (Salt/Mix)|AKOS000121334|CAFFEINE COMPONENT OF INVAGESIC|CAFFEINE COMPONENT OF LANORINAL|5-26-13-00558 (Beilstein)|Bayer Select Headache Pain (Salt/Mix)|Caffeine, anhydrous, 99%, FCC, FG|DB00201|FT05891|LP00228|NSC-755917|SDCCGMLS-0064595.P001|SDCCGMLS-0064595.P002|SDCCGSBI-0050216.P005|CAFFEINE COMPONENT OF ORPHENGESIC|IDI1_000730|USEPA/OPP Pesticide Code: 000660|3,3,7-trimethyl-1H-purine-2,6-dione|CAFFEINE COMPONENT OF SYNALGOS-DC|NCGC00015208-01|NCGC00015208-02|NCGC00015208-03|NCGC00015208-04|NCGC00015208-05|NCGC00015208-06|NCGC00015208-07|NCGC00015208-08|NCGC00015208-10|NCGC00015208-11|NCGC00015208-12|NCGC00015208-13|NCGC00015208-14|NCGC00015208-15|NCGC00015208-16|NCGC00015208-17|NCGC00015208-18|NCGC00015208-20|NCGC00015208-27|NCGC00015208-29|NCGC00090699-01|NCGC00090699-02|NCGC00090699-03|NCGC00090699-04|NCGC00090699-05|NCGC00090699-06|NCGC00090699-07|NCGC00090699-08|NCGC00090699-09|NCGC00168808-01|NCGC00168808-02|NCGC00254057-01|NCGC00259234-01|NCGC00260913-01|95789-13-2|AC-12774|AS-15340|Caffeine, SAJ special grade, >=98.5%|component of P-A-C Compound (Salt/Mix)|CAFFEINE COMPONENT OF SYNALGOS-DC-A|component of A.S.A. Compound (Salt/Mix)|SBI-0050216.P004|CAFFEINE COMPONENT OF MEDIGESIC PLUS|DB-023002|WLN: T56 BN DN FNVNVJ B1 F1 H1|C2042|CAFFEINE COMPONENT OF DARVON COMPOUND|CAFFEINE COMPONENT OF INVAGESIC FORTE|EU-0100228|NS00000273|526 - Nutritional Analysis of Energy Drinks|CAFFEINE COMPONENT OF ORPHENGESIC FORTE|EN300-21663|BIM-0050216.0001|C07481|D00528|Q60235|1,3,7-trimethyl-3,7-dihydropurine-2,6-dione|1H-Purine-2, 3,7-dihydro-1,3,7-trimethyl-|AB00051930-09|AB00051930_10|Caffeine, purum, anhydrous, >=99.0% (HPLC)|3,7-dihydro-1,3,7-trimethyl-1H-purine (9CI)|Caffeine, anhydrous, tested according to Ph.Eur.|L000155|Caffeine, Sigma Reference Standard, vial of 250 mg|SR-01000075187-1|SR-01000075187-4|SR-01000075187-7|SR-01000075187-8|BRD-K02404261-001-02-7|BRD-K02404261-001-03-5|BRD-K02404261-001-07-6|BRD-K02404261-001-16-7|Caffeine, certified reference material, TraceCERT(R)|Caffeine, meets USP testing specifications, anhydrous|Melting point standard 235-237C, analytical standard|1,3,7-Trimethyl-3,7-dihydro-1H-purine-2,6-dione #|Caffeine, British Pharmacopoeia (BP) Reference Standard|Caffeine, European Pharmacopoeia (EP) Reference Standard|F3371-0262|THEOPHYLLINE MONOHYDRATE IMPURITY A [EP IMPURITY]|Z104507896|07E4FB58-FD79-4175-8E3D-05BF96954522|3,7-Dihydro-1,3,7-trimethyl-1H-purin-2,6-dion (coffein)|Caffeine, United States Pharmacopeia (USP) Reference Standard|Caffeine, Pharmaceutical Secondary Standard; Certified Reference Material|Caffeine anhydrous;3,7-Dihydro-1,3,7-trimethyl-1H-purine-2,6-dione;7-Methyltheophylline|Caffeine for system suitability, European Pharmacopoeia (EP) Reference Standard|Caffeine melting point standard, United States Pharmacopeia (USP) Reference Standard|InChI=1/C8H10N4O2/c1-10-4-9-6-5(10)7(13)12(3)8(14)11(6)2/h4H,1-3H|114303-55-8|200-362-1|Caffeine Melting Point Standard, Pharmaceutical Secondary Standard; Certified Reference Material|Mettler-Toledo Calibration substance ME 18872, Caffeine, analytical standard, for the calibration of the thermosystem 900, traceable to primary standards (LGC)
Click to Show/Hide
|