Drug Information
Drug (ID: DG01541) and It's Reported Resistant Information
| Name |
BMS-777607
|
||||
|---|---|---|---|---|---|
| Synonyms |
BMS-777607; 1025720-94-8; BMS 777607; 1196681-44-3; BMS777607; ASLAN-002; UNII-A3MMS6HDO1; ASLAN002; N-(4-(2-Amino-3-chloropyridin-4-yloxy)-3-fluorophenyl)-4-ethoxy-1-(4-fluorophenyl)-2-oxo-1,2-dihydropyridine-3-carboxamide; A3MMS6HDO1; N-[4-(2-amino-3-chloropyridin-4-yl)oxy-3-fluorophenyl]-4-ethoxy-1-(4-fluorophenyl)-2-oxopyridine-3-carboxamide; BMS 817378; 3-Pyridinecarboxamide, N-[4-[(2-amino-3-chloro-4-pyridinyl)oxy]-3-fluorophenyl]-4-ethoxy-1-(4-fluorophenyl)-1,2-dihydro-2-oxo-; N-(4-((2-amino-3-chloropyridin-4-yl)oxy)-3-fluorophenyl)-4-ethoxy-1-(4-fluorophenyl)-2-oxo-1,2-dihydropyridine-3-carboxamide; N-[4-[(2-Amino-3-chloropyridin-4-yl)oxy]-3-fluorophenyl]-4-ethoxy-1-(4-fluorophenyl)-2-oxo-1,2-dihydropyridine-3-carboxamide; N-{4-[(2-amino-3-chloropyridin-4-yl)oxy]-3-fluorophenyl}-4-ethoxy-1-(4-fluorophenyl)-2-oxo-1,2-dihydropyridine-3-carboxamide; 3-Pyridinecarboxamide, N-[4-[(2-amino-3-chloro-4-pyridinyl)oxy]-3-fluorophenyl]-4-ethoxy-1-(4-fluorophenyl)-1,2-dihydro-2-oxo-;3-Pyridinecarboxamide, N-[4-[(2-amino-3-chloro-4-pyridinyl)oxy]-3-fluorophenyl]-4-ethoxy-1-(4-fluorophenyl)-1,2-dihydro-2-oxo-; N-(4-((2-amino-3-chloropyridin-4-yl)oxy)-3-fluorophenyl)-4-ethoxy-1-(4-fluorophenyl)-2-oxo-1,2-dihydropyridine-3-carboxamide.; MLS006010958; C25H19ClF2N4O4; CHEMBL460702; GTPL7953; SCHEMBL2588311; BDBM28031; CHEBI:91409; DTXSID50145278; EX-A004; 3f82; HMS3295I07; HMS3655C05; AMY24190; AOB87395; BCP02303; BCP13190; BMS777606; MFCD16495773; NSC764090; NSC799365; s1561; ZINC39716080; AKOS026750587; BCP9000432; CCG-264913; CS-0227; DB12064; EX-7213; NSC-764090; NSC-799365; QC-7244; SB16607; NCGC00263157-01; NCGC00263157-09; AC-30912; AC-31421; AS-17034; DA-35016; HY-12076; N-[4-[(2-Amino-3-chloro-4-pyridinyl)oxy]-3-fluorophenyl]-4-ethoxy-1-(4-fluorophenyl)-1,2-dihydro-2-oxo-3-pyridinecarboxamide; N-{4-[(2-Amino-3-chloro-4-pyridinyl)oxy]-3-fluorophenyl}-4-ethoxy -1-(4-fluorophenyl)-2-oxo-1,2-dihydro-3-pyridinecarboxamide; SMR004702762; BMS-777607;BMS777607; FT-0701277; FT-0722977; SW219298-1; X7495; A25030; BMS-777607, >=98% (HPLC); J-004169; J-523337; BRD-K40738845-001-01-9; Q27075371; N-(4-(2-amino-3-chloropyridin-4-yloxy )-3-fluorophenyl)-4-ethoxy-1-(4-fluorophenyl)-2-oxo-1,2-dihydropyridine-3-carboxamide; N-[4-[(2-Amino-3-chloro-4-pyridinyl)oxy]-3-fluorophenyl]-4-ethoxy-1-(4-fluorophenyl)-1,2-dihydro-2-oxo-3-pyridinecarboxamide,; N-[4-[(2-Amino-3-chloropyridin-4-yl)oxy]-3-fluorophenyl]-4-ethoxy-1-(4-fluorophenyl)-2-oxo-1,2-dihydropyridine-3-carboxamide;BMS-817378;BMS-777607
Click to Show/Hide
|
||||
| Indication |
In total 6 Indication(s)
|
||||
| Structure |
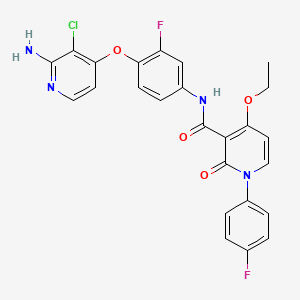
|
||||
| Target | RAC-alpha serine/threonine-protein kinase (AKT1) | AKT1_HUMAN | [1] | ||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
7
|
||||
| IsoSMILES |
CCOC1=C(C(=O)N(C=C1)C2=CC=C(C=C2)F)C(=O)NC3=CC(=C(C=C3)OC4=C(C(=NC=C4)N)Cl)F
|
||||
| InChI |
InChI=1S/C25H19ClF2N4O4/c1-2-35-19-10-12-32(16-6-3-14(27)4-7-16)25(34)21(19)24(33)31-15-5-8-18(17(28)13-15)36-20-9-11-30-23(29)22(20)26/h3-13H,2H2,1H3,(H2,29,30)(H,31,33)
|
||||
| InChIKey |
VNBRGSXVFBYQNN-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-02: Benign/in-situ/malignant neoplasm
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | |||||||||||||
|
|
|||||||||||||
| Key Molecule: Hepatocyte growth factor receptor (MET) | [1] | ||||||||||||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | ||||||||||||
| Molecule Alteration | Missense mutation | p.Y1230H (c.3688T>C) |
|||||||||||
| Wild Type Structure | Method: X-ray diffraction | Resolution: 1.71 Å | |||||||||||
| Mutant Type Structure | Method: X-ray diffraction | Resolution: 1.97 Å | |||||||||||
| Download The Information of Sequence | Download The Structure File | ||||||||||||
-
N
-
1050
|
T
-
V
-
H
-
I
-
D
-
L
-
S
-
A
A
L
L
N
N
1060
|
P
P
E
E
L
L
V
V
Q
Q
A
A
V
V
Q
Q
H
H
V
V
1070
|
V
V
I
I
G
G
P
P
S
S
S
S
L
L
I
I
V
V
H
H
1080
|
F
F
N
N
E
E
V
V
I
I
G
G
R
R
G
G
H
H
F
F
1090
|
G
G
C
C
V
V
Y
Y
H
H
G
G
T
T
L
L
L
L
D
D
1100
|
N
N
D
D
G
G
K
K
K
K
I
I
H
H
C
C
A
A
V
V
1110
|
K
K
S
S
L
L
N
N
R
R
I
I
T
T
D
D
I
I
G
G
1120
|
E
E
V
V
S
S
Q
Q
F
F
L
L
T
T
E
E
G
G
I
I
1130
|
I
I
M
M
K
K
D
D
F
F
S
S
H
H
P
P
N
N
V
V
1140
|
L
L
S
S
L
L
L
L
G
G
I
I
C
C
L
L
R
R
S
S
1150
|
E
E
G
G
S
S
P
P
L
L
V
V
V
V
L
L
P
P
Y
Y
1160
|
M
M
K
K
H
H
G
G
D
D
L
L
R
R
N
N
F
F
I
I
1170
|
R
R
N
N
E
E
T
T
H
H
N
N
P
P
T
T
V
V
K
K
1180
|
D
D
L
L
I
I
G
G
F
F
G
G
L
L
Q
Q
V
V
A
A
1190
|
K
K
G
G
M
M
K
K
F
Y
L
L
A
A
S
S
K
K
K
K
1200
|
F
F
V
V
H
H
R
R
D
D
L
L
A
A
A
A
R
R
N
N
1210
|
C
C
M
M
L
L
D
D
E
E
K
K
F
F
T
T
V
V
K
K
1220
|
V
V
A
A
D
D
F
F
G
G
L
L
A
A
R
R
D
D
M
M
1230
|
Y
H
D
D
K
K
E
E
F
Y
D
Y
S
S
V
V
H
H
N
N
1240
|
K
K
T
T
G
G
A
A
K
K
L
L
P
P
V
V
K
K
W
W
1250
|
M
M
A
A
L
L
E
E
S
S
L
L
Q
Q
T
T
Q
Q
K
K
1260
|
F
F
T
T
T
T
K
K
S
S
D
D
V
V
W
W
S
S
F
F
1270
|
G
G
V
V
L
L
L
L
W
W
E
E
L
L
M
M
T
T
R
R
1280
|
G
G
A
A
P
P
P
P
Y
Y
P
P
D
D
V
V
N
N
T
T
1290
|
F
F
D
D
I
I
T
T
V
V
Y
Y
L
L
L
L
Q
Q
G
G
1300
|
R
R
R
R
L
L
L
L
Q
Q
P
P
E
E
Y
Y
C
C
P
P
1310
|
D
D
P
P
L
L
Y
Y
E
E
V
V
M
M
L
L
K
K
C
C
1320
|
W
W
H
H
P
P
K
K
A
A
E
E
M
M
R
R
P
P
S
S
1330
|
F
F
S
S
E
E
L
L
V
V
S
S
R
R
I
I
S
S
A
A
1340
|
I
I
F
F
S
S
T
T
F
F
I
I
G
G
E
E
H
H
Y
Y
1350
|
V
V
H
H
V
V
N
N
A
A
T
T
Y
-
V
-
N
-
V
-
1360
|
K
-
|
|||||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| In Vitro Model | NIH3T3 cells | Embryo | Homo sapiens (Human) | CVCL_0594 | |||||||||
| In Vivo Model | Athymic female mouse PDX model | Mus musculus | |||||||||||
| Experiment for Drug Resistance |
MTS assay | ||||||||||||
| Mechanism Description | MET mutations Y1248H and D1246N are resistance mechanisms for type I MET-TKIs. NIH3T3 cells expressing either mutation showed resistance to both INC280 and crizotinib but not cabozantinib, indicating the potential of sequential use of MET inhibitors may lead to a more durable response. | ||||||||||||
| Key Molecule: Hepatocyte growth factor receptor (MET) | [1] | ||||||||||||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | ||||||||||||
| Molecule Alteration | Missense mutation | p.D1228N (c.3682G>A) |
|||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| In Vitro Model | NIH3T3 cells | Embryo | Homo sapiens (Human) | CVCL_0594 | |||||||||
| In Vivo Model | Athymic female mouse PDX model | Mus musculus | |||||||||||
| Experiment for Drug Resistance |
MTS assay | ||||||||||||
| Mechanism Description | MET mutations Y1248H and D1246N are resistance mechanisms for type I MET-TKIs. NIH3T3 cells expressing either mutation showed resistance to both INC280 and crizotinib but not cabozantinib, indicating the potential of sequential use of MET inhibitors may lead to a more durable response. | ||||||||||||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
