Drug Information
Drug (ID: DG01531) and It's Reported Resistant Information
| Name |
Cobimetinib
|
||||
|---|---|---|---|---|---|
| Synonyms |
Cobimetinib; 934660-93-2; GDC-0973; XL518; Cotellic; Xl-518; GDC 0973; RG7420; XL 518; RG 7420; UNII-ER29L26N1X; ER29L26N1X; C21H21F3IN3O2; CHEMBL2146883; XL518 (GDC-0973); Cobimetinib (GDC-0973, RG7420); (S)-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)phenyl)(3-hydroxy-3-(piperidin-2-yl)azetidin-1-yl)methanone; [3,4-Bis(Fluoranyl)-2-[(2-Fluoranyl-4-Iodanyl-Phenyl)amino]phenyl]-[3-Oxidanyl-3-[(2s)-Piperidin-2-Yl]azetidin-1-Yl]methanone; [3,4-difluoro-2-(2-fluoro-4-iodoanilino)phenyl]-[3-hydroxy-3-[(2S)-piperidin-2-yl]azetidin-1-yl]methanone; [3,4-difluoro-2-(2-fluoro-4-iodo-phenylamino)-phenyl]-((s)-3-hydroxy-3-piperidin-2-yl-azetidin-1-yl)-methanone; EUI; Cobimetinib Butyrate; Cobimetinib [USAN:INN]; cometinib; Cobimetinib (USAN/INN); SCHEMBL189565; GTPL7626; QCR-87; XL518;RG7420;Cobimetinib; CHEBI:90851; DTXSID60239435; EX-A673; GDC0973; GDC0973; XL518; Cobimetinib; 3556AH; BDBM50391802; MFCD22124461; NSC768068; NSC781257; NSC800075; s8041; ZINC60325170; BCP9000716; CCG-264727; CS-0521; DB05239; NSC-768068; NSC-781257; NSC-800075; VS-0129; NCGC00346455-03; NCGC00346455-05; (3,4-difluoro-2-(2-fluoro-4-iodophenylamino)phenyl)(3-hydroxy-3-(piperidin-2-yl)azetidin-1-yl)methanone; HY-13064; Methanone, (3,4-difluoro-2-((2-fluoro-4-iodophenyl)amino)phenyl)(3-hydroxy-3-((2S)-2-piperidinyl)-1-azetidinyl)-; RO-5514041; D10405; J-525162; Q15708292; (3,4-difluoro-2-(2-fluoro-4-iodophenylamino)phenyl)(3-hydroxy-3-((S)-piperidin-2-yl)cyclobutyl)methanone; [3,4-difluoro-2-(2-fluoro-4-iodoanilino)phenyl]-[3-hydroxy-3-[(2S)-piperidin-2-yl]azetidin; [3,4-difluoro-2-(2-fluoro-4-iodoanilino)phenyl]{3-hydroxy-3-[(2S)-piperidin-2-yl]azetidin-1-yl}methanone; [3,4-difluoro-2-[(2-fluoro-4-iodophenyl)amino]phenyl]-[3-hydroxy-3-[(2S)-piperidin-2-yl]azetidin-1-yl]methanone; {3,4-difluoro-2-[(2-fluoro-4-iodophenyl)amino]phenyl}{3-hydroxy-3-[(2S)-piperidin-2-yl]azetidin-1-yl}methanone
Click to Show/Hide
|
||||
| Indication |
In total 1 Indication(s)
|
||||
| Structure |
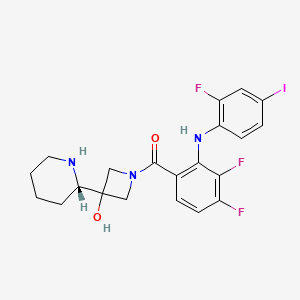
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Clinically Reported Resistance for This Drug
(1 diseases)
[2]
|
||||
| Target | cAMP-dependent chloride channel (CFTR) | CFTR_HUMAN | [1] | ||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
4
|
||||
| IsoSMILES |
C1CCN[C@@H](C1)C2(CN(C2)C(=O)C3=C(C(=C(C=C3)F)F)NC4=C(C=C(C=C4)I)F)O
|
||||
| InChI |
InChI=1S/C21H21F3IN3O2/c22-14-6-5-13(19(18(14)24)27-16-7-4-12(25)9-15(16)23)20(29)28-10-21(30,11-28)17-3-1-2-8-26-17/h4-7,9,17,26-27,30H,1-3,8,10-11H2/t17-/m0/s1
|
||||
| InChIKey |
BSMCAPRUBJMWDF-KRWDZBQOSA-N
|
||||
| PubChem CID | |||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-02: Benign/in-situ/malignant neoplasm
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: MAPK/ERK kinase 1 (MEK1) | [2] | |||
| Resistant Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Molecule Alteration | Missense mutation | p.V211D (c.632T>A) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | NIH-3T3 cells | Embryo | Mus musculus (Mouse) | CVCL_0594 |
| Phoenix AMPHO cells | Fetal kidney | Homo sapiens (Human) | CVCL_H716 | |
| In Vivo Model | NOD scid gamma xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Single cell sequencing assay | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | |||||||||||||
|
|
|||||||||||||
| Key Molecule: Serine/threonine-protein kinase B-raf (BRAF) | [3] | ||||||||||||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | ||||||||||||
| Molecule Alteration | Missense mutation | p.V600E (c.1799T>A) |
|||||||||||
| Wild Type Structure | Method: X-ray diffraction | Resolution: 2.55 Å | |||||||||||
| Mutant Type Structure | Method: X-ray diffraction | Resolution: 3.20 Å | |||||||||||
| Download The Information of Sequence | Download The Structure File | ||||||||||||
-
420
|
M
M
D
D
R
R
G
G
S
S
H
H
H
H
H
H
H
H
H
H
430
|
H
H
G
G
S
S
E
E
D
D
R
R
N
N
R
R
M
M
K
K
440
|
T
T
L
L
G
G
R
R
R
R
D
D
S
S
S
S
D
D
D
D
450
|
W
W
E
E
I
I
P
P
D
D
G
G
Q
Q
I
I
T
T
V
V
460
|
G
G
Q
Q
R
R
I
I
G
G
S
S
G
G
S
S
F
F
G
G
470
|
T
T
V
V
Y
Y
K
K
G
G
K
K
W
W
H
H
G
G
D
D
480
|
V
V
A
A
V
V
K
K
M
M
L
L
N
N
V
V
T
T
A
A
490
|
P
P
T
T
P
P
Q
Q
Q
Q
L
L
Q
Q
A
A
F
F
K
K
500
|
N
N
E
E
V
V
G
G
V
V
L
L
R
R
K
K
T
T
R
R
510
|
H
H
V
V
N
N
I
I
L
L
L
L
F
F
M
M
G
G
Y
Y
520
|
S
S
T
T
K
K
P
P
Q
Q
L
L
A
A
I
I
V
V
T
T
530
|
Q
Q
W
W
C
C
E
E
G
G
S
S
S
S
L
L
Y
Y
H
H
540
|
H
H
L
L
H
H
I
I
I
I
E
E
T
T
K
K
F
F
E
E
550
|
M
M
I
I
K
K
L
L
I
I
D
D
I
I
A
A
R
R
Q
Q
560
|
T
T
A
A
Q
Q
G
G
M
M
D
D
Y
Y
L
L
H
H
A
A
570
|
K
K
S
S
I
I
I
I
H
H
R
R
D
D
L
L
K
K
S
S
580
|
N
N
N
N
I
I
F
F
L
L
H
H
E
E
D
D
L
L
T
T
590
|
V
V
K
K
I
I
G
G
D
D
F
F
G
G
L
L
A
A
T
T
600
|
V
E
K
K
S
S
R
R
W
W
S
S
G
G
S
S
H
H
Q
Q
610
|
F
F
E
E
Q
Q
L
L
S
S
G
G
S
S
I
I
L
L
W
W
620
|
M
M
A
A
P
P
E
E
V
V
I
I
R
R
M
M
Q
Q
D
D
630
|
K
K
N
N
P
P
Y
Y
S
S
F
F
Q
Q
S
S
D
D
V
V
640
|
Y
Y
A
A
F
F
G
G
I
I
V
V
L
L
Y
Y
E
E
L
L
650
|
M
M
T
T
G
G
Q
Q
L
L
P
P
Y
Y
S
S
N
N
I
I
660
|
N
N
N
N
R
R
D
D
Q
Q
I
I
I
I
F
F
M
M
V
V
670
|
G
G
R
R
G
G
Y
Y
L
L
S
S
P
P
D
D
L
L
S
S
680
|
K
K
V
V
R
R
S
S
N
N
C
C
P
P
K
K
A
A
M
M
690
|
K
K
R
R
L
L
M
M
A
A
E
E
C
C
L
L
K
K
K
K
700
|
K
K
R
R
D
D
E
E
R
R
P
P
L
L
F
F
P
P
Q
Q
710
|
I
I
L
L
A
A
S
S
I
I
E
E
L
L
L
L
A
A
R
R
720
|
S
S
L
L
P
P
K
K
I
I
H
H
R
R
|
|||||||||||||
| Experimental Note | Revealed Based on the Cell Line Data | ||||||||||||
| Mechanism Description | The missense mutation p.V600E (c.1799T>A) in gene BRAF cause the sensitivity of Cobimetinib by unusual activation of pro-survival pathway | ||||||||||||
| Key Molecule: Serine/threonine-protein kinase B-raf (BRAF) | [1] | ||||||||||||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | ||||||||||||
| Molecule Alteration | Complex-indel | p.N486_T491delinsK (c.1458_1472del15) |
|||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 | |||||||||
| Experiment for Molecule Alteration |
Whole exome sequencing; Targeted Exon Sequencing | ||||||||||||
| Experiment for Drug Resistance |
CellTiter-Glo assay; IC50 assay | ||||||||||||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Serine/threonine-protein kinase B-raf (BRAF) | [4] | |||
| Sensitive Disease | Melanoma [ICD-11: 2C30.0] | |||
| Molecule Alteration | Missense mutation | p.L597S (c.1789_1790delCTinsTC) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | MAPK signaling pathway | Inhibition | hsa04010 | |
| In Vitro Model | Skin sample | N.A. | ||
| In Vivo Model | Mouse PDX model | Mus musculus | ||
| Experiment for Drug Resistance |
Crystal violet staining assay | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Neurofibromin 1 (NF1) | [5] | |||
| Metabolic Type | Lipid metabolism | |||
| Sensitive Disease | ER+ breast adenocarcinoma [ICD-11: 2C61.1] | |||
| Molecule Alteration | Mutation | . |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vivo Model | Rat, with ER + MCF7 cell lines | Rats | ||
| Experiment for Molecule Alteration |
LC-MS | |||
| Experiment for Drug Resistance |
Incucyte proliferation assay | |||
| Mechanism Description | Lastly,NF1deficiency alters the synergy between metabolic inhibitors and traditional targeted inhibitors. This includes increased synergy with inhibitors targeting glycolysis, glutamine metabolism, mitochondrial fatty acid transport, and TG synthesis. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | |||||||||||||
|
|
|||||||||||||
| Key Molecule: MAPK/ERK kinase 1 (MEK1) | [1] | ||||||||||||
| Sensitive Disease | Lymphatic system cancer [ICD-11: 2E81.1] | ||||||||||||
| Molecule Alteration | Missense mutation | p.P124L (c.371C>T) |
|||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 | |||||||||
| Experiment for Molecule Alteration |
Whole exome sequencing; Targeted Exon Sequencing | ||||||||||||
| Experiment for Drug Resistance |
CellTiter-Glo assay; IC50 assay | ||||||||||||
| Key Molecule: MAPK/ERK kinase 1 (MEK1) | [6] | ||||||||||||
| Sensitive Disease | Lymphatic system cancer [ICD-11: 2E81.1] | ||||||||||||
| Molecule Alteration | Missense mutation | p.Q56P (c.167A>C) |
|||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| Key Molecule: MAPK/ERK kinase 1 (MEK1) | [1] | ||||||||||||
| Sensitive Disease | Lymphatic system cancer [ICD-11: 2E81.1] | ||||||||||||
| Molecule Alteration | IF-deletion | p.P105_I107delPAI (c.314_322delCCGCAATCC) |
|||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 | |||||||||
| Experiment for Molecule Alteration |
Whole exome sequencing; Targeted Exon Sequencing | ||||||||||||
| Experiment for Drug Resistance |
CellTiter-Glo assay; IC50 assay | ||||||||||||
| Key Molecule: MAPK/ERK kinase 1 (MEK1) | [1] | ||||||||||||
| Sensitive Disease | Lymphatic system cancer [ICD-11: 2E81.1] | ||||||||||||
| Molecule Alteration | Missense mutation | p.P124Q (c.371C>A) |
|||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 | |||||||||
| Experiment for Molecule Alteration |
Whole exome sequencing; Targeted Exon Sequencing | ||||||||||||
| Experiment for Drug Resistance |
CellTiter-Glo assay; IC50 assay | ||||||||||||
|
|
|||||||||||||
| Key Molecule: Serine/threonine-protein kinase B-raf (BRAF) | [1] | ||||||||||||
| Sensitive Disease | Lymphatic system cancer [ICD-11: 2E81.1] | ||||||||||||
| Molecule Alteration | Missense mutation | p.V600E (c.1799T>A) |
|||||||||||
| Wild Type Structure | Method: X-ray diffraction | Resolution: 2.55 Å | |||||||||||
| Mutant Type Structure | Method: X-ray diffraction | Resolution: 3.20 Å | |||||||||||
| Download The Information of Sequence | Download The Structure File | ||||||||||||
-
420
|
M
M
D
D
R
R
G
G
S
S
H
H
H
H
H
H
H
H
H
H
430
|
H
H
G
G
S
S
E
E
D
D
R
R
N
N
R
R
M
M
K
K
440
|
T
T
L
L
G
G
R
R
R
R
D
D
S
S
S
S
D
D
D
D
450
|
W
W
E
E
I
I
P
P
D
D
G
G
Q
Q
I
I
T
T
V
V
460
|
G
G
Q
Q
R
R
I
I
G
G
S
S
G
G
S
S
F
F
G
G
470
|
T
T
V
V
Y
Y
K
K
G
G
K
K
W
W
H
H
G
G
D
D
480
|
V
V
A
A
V
V
K
K
M
M
L
L
N
N
V
V
T
T
A
A
490
|
P
P
T
T
P
P
Q
Q
Q
Q
L
L
Q
Q
A
A
F
F
K
K
500
|
N
N
E
E
V
V
G
G
V
V
L
L
R
R
K
K
T
T
R
R
510
|
H
H
V
V
N
N
I
I
L
L
L
L
F
F
M
M
G
G
Y
Y
520
|
S
S
T
T
K
K
P
P
Q
Q
L
L
A
A
I
I
V
V
T
T
530
|
Q
Q
W
W
C
C
E
E
G
G
S
S
S
S
L
L
Y
Y
H
H
540
|
H
H
L
L
H
H
I
I
I
I
E
E
T
T
K
K
F
F
E
E
550
|
M
M
I
I
K
K
L
L
I
I
D
D
I
I
A
A
R
R
Q
Q
560
|
T
T
A
A
Q
Q
G
G
M
M
D
D
Y
Y
L
L
H
H
A
A
570
|
K
K
S
S
I
I
I
I
H
H
R
R
D
D
L
L
K
K
S
S
580
|
N
N
N
N
I
I
F
F
L
L
H
H
E
E
D
D
L
L
T
T
590
|
V
V
K
K
I
I
G
G
D
D
F
F
G
G
L
L
A
A
T
T
600
|
V
E
K
K
S
S
R
R
W
W
S
S
G
G
S
S
H
H
Q
Q
610
|
F
F
E
E
Q
Q
L
L
S
S
G
G
S
S
I
I
L
L
W
W
620
|
M
M
A
A
P
P
E
E
V
V
I
I
R
R
M
M
Q
Q
D
D
630
|
K
K
N
N
P
P
Y
Y
S
S
F
F
Q
Q
S
S
D
D
V
V
640
|
Y
Y
A
A
F
F
G
G
I
I
V
V
L
L
Y
Y
E
E
L
L
650
|
M
M
T
T
G
G
Q
Q
L
L
P
P
Y
Y
S
S
N
N
I
I
660
|
N
N
N
N
R
R
D
D
Q
Q
I
I
I
I
F
F
M
M
V
V
670
|
G
G
R
R
G
G
Y
Y
L
L
S
S
P
P
D
D
L
L
S
S
680
|
K
K
V
V
R
R
S
S
N
N
C
C
P
P
K
K
A
A
M
M
690
|
K
K
R
R
L
L
M
M
A
A
E
E
C
C
L
L
K
K
K
K
700
|
K
K
R
R
D
D
E
E
R
R
P
P
L
L
F
F
P
P
Q
Q
710
|
I
I
L
L
A
A
S
S
I
I
E
E
L
L
L
L
A
A
R
R
720
|
S
S
L
L
P
P
K
K
I
I
H
H
R
R
|
|||||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 | |||||||||
| Experiment for Molecule Alteration |
Whole exome sequencing; Targeted Exon Sequencing | ||||||||||||
| Experiment for Drug Resistance |
CellTiter-Glo assay; IC50 assay | ||||||||||||
| Key Molecule: Serine/threonine-protein kinase B-raf (BRAF) | [1] | ||||||||||||
| Sensitive Disease | Lymphatic system cancer [ICD-11: 2E81.1] | ||||||||||||
| Molecule Alteration | Complex-indel | p.N486_T491delinsK (c.1458_1472del15) |
|||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 | |||||||||
| Experiment for Molecule Alteration |
Whole exome sequencing; Targeted Exon Sequencing | ||||||||||||
| Experiment for Drug Resistance |
CellTiter-Glo assay; IC50 assay | ||||||||||||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
