Drug Information
Drug (ID: DG01270) and It's Reported Resistant Information
| Name |
Copanlisib
|
||||
|---|---|---|---|---|---|
| Synonyms |
Copanlisib; 1032568-63-0; BAY 80-6946; Aliqopa; BAY-80-6946; BAY80-6946; UNII-WI6V529FZ9; BAY 80-6946 (Copanlisib); 2-amino-N-[7-methoxy-8-(3-morpholin-4-ylpropoxy)-2,3-dihydroimidazo[1,2-c]quinazolin-5-yl]pyrimidine-5-carboxamide; WI6V529FZ9; Copanlisib (BAY 80-6946); 2-Amino-N-[2,3-dihydro-7-methoxy-8-[3-(4-morpholinyl)propoxy]imidazo[1,2-c]quinazolin-5-yl]-5-pyrimidinecarboxamide; 2-amino-N-(7-methoxy-8-(3-morpholinopropoxy)-2,3-dihydroimidazo[1,2-c]quinazolin-5-yl)pyrimidine-5-carboxamide; 2-amino-N-[7-methoxy-8-(3-morpholin-4-ylpropoxy)-2,3-dihydro-1H-imidazo[1,2-c]quinazolin-5-ylidene]pyrimidine-5-carboxamide; copanlisibum; Copanlisib tris-HCl; Copanlisib (USAN/INN); Copanlisib [USAN:INN]; GTPL7875; SCHEMBL1655478; BAY 80-6946; Copanlisib; BAY-80-6946 tris-HCl; Copanlisib; BAY-80-6946; CHEMBL3218576; SCHEMBL13084037; DTXSID00145728; CHEBI:173077; C23H28N8O4; BCP04754; EX-A2005; 2253AH; BDBM50204093; MFCD18633201; NSC760443; NSC800076; NSC809693; NSC816437; s2802; ZINC68247389; AKOS025290222; BAY-806946; CS-0741; DB12483; NSC-760443; NSC-800076; NSC-809693; NSC-816437; PB22956; VS-0128; NCGC00346457-01; NCGC00346457-02; NCGC00346457-04; 2-amino-N-(7-methoxy-8-(3-morpholinopropoxy)-2,3-dihydroimidazo(1,2-c)quinazolin-4-yl)pyrimidine-5-carboxamide; AC-28438; BC164810; HY-15346; QC-10511; D10867; Q19903876; 2-amino-N-[7-methoxy-8-(3-morpholin-4-ylpropoxy)-2,3-dihydroimidazo[1.2-c]quinazolin-5-yl]pyrimidine-5-carboxamide; 2-amino-N-{7-methoxy-8-[3-(morpholin-4-yl)propoxy]-2,3-dihydroimidazo[1,2-c]quinazolin-5-yl}pyrimidine-5-carboxamide; BAY-80-6946; ; ; 2-Amino-N-[7-methoxy-8-(3-morpholin-4-ylpropoxy)-2,3-dihydroimidazo[1,2-c]quinazolin-5-yl]pyrimidine-5-carboxamide
Click to Show/Hide
|
||||
| Indication |
In total 3 Indication(s)
|
||||
| Structure |
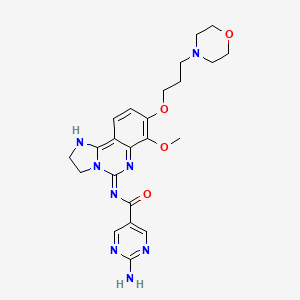
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Resistance Information Discovered by Cell Line Test for This Drug
(1 diseases)
[1]
|
||||
| Target | PI3-kinase alpha (PIK3CA) | PK3CA_HUMAN | [1] | ||
| PI3-kinase delta (PIK3CD) | PK3CD_HUMAN | [1] | |||
| PI3-kinase gamma (PIK3CG) | PK3CG_HUMAN | [1] | |||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
C23H28N8O4
|
||||
| IsoSMILES |
COC1=C(C=CC2=C3NCCN3C(=NC(=O)C4=CN=C(N=C4)N)N=C21)OCCCN5CCOCC5
|
||||
| InChI |
1S/C23H28N8O4/c1-33-19-17(35-10-2-6-30-8-11-34-12-9-30)4-3-16-18(19)28-23(31-7-5-25-20(16)31)29-21(32)15-13-26-22(24)27-14-15/h3-4,13-14,25H,2,5-12H2,1H3,(H2,24,26,27)
|
||||
| InChIKey |
MWYDSXOGIBMAET-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
| TTD Drug ID | |||||
| INTEDE ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-02: Benign/in-situ/malignant neoplasm
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Cyclin-dependent kinase 4 (CDK4) | [2] | |||
| Sensitive Disease | Acute promyelocytic leukemia [ICD-11: 2A60.2] | |||
| Molecule Alteration | Expression | Express CDK4 |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | MOLM-13 cells | Peripheral blood | Homo sapiens (Human) | CVCL_2119 |
| MOLM14 cells | Peripheral blood | Homo sapiens (Human) | CVCL_7916 | |
| MV-4-11 cells | Peripheral blood | Homo sapiens (Human) | CVCL_0064 | |
| Experiment for Molecule Alteration |
Protein component assay; Immunoblotting assay; Immunofluorescence staining assay | |||
| Experiment for Drug Resistance |
Cell viability assay; Synergistic effects assay; Cell growth rates assay | |||
| Mechanism Description | Considering the fact that for many tumour cells, inhibition of CDK4/6 can induce cellular quiescence or senescence, we evaluated whether CDK4 expression was affected by copanlisib alone or in combination with palbociclib. Copanlisib was selected as it was more effective than other PI3K inhibitors on its own. While the cells did not react to palbociclib by reducing the expression of CDK4, copanlisib lead to dose-dependent downregulation in CDK4 expression, especially when combined with palbociclib (Additional file 1: Fig. S8). Moreover, the cells did not express p-Akt following treatment with copanlisib (Additional file 1: Fig. S9). | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Interleukin 6 receptor (IL6R) | [1] | |||
| Resistant Disease | Non-Hodgkin lymphoma [ICD-11: 2A85.5] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | RWPE-1 cells | Prostate | Homo sapiens (Human) | CVCL_3791 |
| SW1116 cells | Colon | Homo sapiens (Human) | CVCL_0544 | |
| HCT15 cells | Colon | Homo sapiens (Human) | CVCL_0292 | |
| LS174T cells | Colon | Homo sapiens (Human) | CVCL_1384 | |
| NCI-H716 cells | Colon | Homo sapiens (Human) | CVCL_1581 | |
| SW948 cells | Colon | Homo sapiens (Human) | CVCL_0632 | |
| C4-2B cells | Prostate | Homo sapiens (Human) | CVCL_4784 | |
| OCI-Ly1 cells | Bone marrow | Homo sapiens (Human) | CVCL_1879 | |
| Riva cells | Pleural effusion | Homo sapiens (Human) | N.A. | |
| SU-DHL2 cells | Pleural effusion | Homo sapiens (Human) | CVCL_9550 | |
| U2932 (ABC-DLBCL) cells | Ascites | Homo sapiens (Human) | CVCL_1896 | |
| BJAB cells | Groin | Homo sapiens (Human) | CVCL_5711 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK-8 assay | |||
| Mechanism Description | Cytokine arrays revealed upregulation of interleukin (IL)-6 in both copanlisib- and duvelisib-resistant cell lines. Phosphorylated STAT5, AKT, p70S6K and MAPK were increased in copanlisib-resistant B-cell lymphoma cells, whereas phosphorylated STAT3 and NF-kappaB were increased in duvelisib-resistant T cell lymphoma cells. Conversely, depletion of IL-6 sensitized both resistant cell lines, and led to downregulation of phosphorylated STAT3 and STAT5 in copanlisib- and duvelisib-resistant cells, respectively. Moreover, combined treatment with a JAK inhibitor (BSK805) and a PI3K inhibitor circumvented the acquired resistance to PI3K inhibitors in lymphoma, and concurrent inhibition of the activated pathways produced combined effects.IL-6-induced STAT3 or STAT5 activation is a critical mechanism underlying PI3K inhibitor resistance in lymphoma, supporting the utility of IL-6 as an effective biomarker to predict therapeutic response to PI3K inhibitors. | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
