Drug Information
Drug (ID: DG01101) and It's Reported Resistant Information
| Name |
Midecamycin
|
||||
|---|---|---|---|---|---|
| Synonyms |
Midecamycin; Rubimycin; Espinomycin A; Platenomycin B1; Turimycin P3; Medecamycin A1; Mydecamycin; 35457-80-8; Midecamycin A1; Medemycin; UNII-N34Z0Y5UH7; Antibiotic SF 837; N34Z0Y5UH7; SF 837; Madecacine; Midecamicina; Midecamycine; Midecamycinum; YL 704 B1; Macro-Dil; Antibiotic SF-837; Turimycin P(sub 3); Midecamycin A(sub 1); Antibiotic SF 837 A1; Antibiotic YL 704 B1; Midecamycine [INN-French]; Midecamycinum [INN-Latin]; NSC 154011; Midecamicina [INN-Spanish]; Midecamycin [INN:DCF:JAN]; Midecamycin,(S); Medemycin (TN); NCGC00016830-01; EINECS 252-578-0; CAS-35457-80-8; Midecamycin (JP17/INN); DSSTox_CID_25463; DSSTox_RID_80894; DSSTox_GSID_45463; SCHEMBL141581; CHEMBL444963; DTXSID5045463; CHEBI:31845; C41H67NO15; (4R,5S,6S,7R,9R,10R,11E,13E,16R)-6-(((2S,3R,4R,5S,6R)-4-(dimethylamino)-3-hydroxy-5-(((2S,4R,5S,6S)-4-hydroxy-4,6-dimethyl-5-(propionyloxy)tetrahydro-2H-pyran-2-yl)oxy)-6-methyltetrahydro-2H-pyran-2-yl)oxy)-10-hydroxy-5-methoxy-9,16-dimethyl-2-oxo-7-(2-oxoethyl)oxacyclohexadeca-11,13-dien-4-yl propionate; ACT02621; HY-B1908; Tox21_110635; s5560; AKOS022185298; ZINC169368401; CCG-270507; CS-5909; DB13456; Leucomycin V, 3,4(sup B)-dipropanoate; Leucomycin V, 3,4B-dipropanoate (9CI); D01339; H10500; Midecamycin), Antibiotic for Culture Media Use Only; Q2636110; W-106669; 7-(Formylmethyl)-4,10-dihydroxy-5-methoxy-9,16-dimethyl-2-oxooxacyclohexadeca-11,13-dien-6-yl 3,6-dideoxy-4-O-(2,6-dideoxy-3-C-methyl-alpha-L-ribo-hexopyranosyl)-3-(dimethylamino)-beta-D-glucopyranoside 4',4''-dipropionate (ester); 7-(Formylmethyl)-4,10-dihydroxy-5-methoxy-9,16-dimethyl-2-oxooxacyclohexadeca-11,13-dien-6-yl 3,6-dideoxy-4-O-(2,6-dideoxy-3-C-methyl-alpha-L-ribo-hexopyranosyl)-3-(dimethylamino)-beta-D-glucopyranoside 4',4'-dipropionate (ester)
Click to Show/Hide
|
||||
| Indication |
In total 1 Indication(s)
|
||||
| Structure |
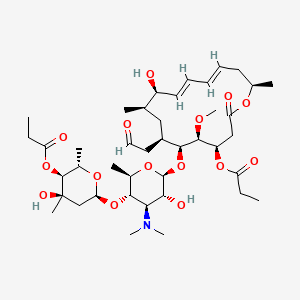
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Resistance Information Discovered by Cell Line Test for This Drug
(1 diseases)
[2]
Disease(s) with Resistance Information Validated by in-vivo Model for This Drug
(4 diseases)
[3]
[1]
[1]
[1]
|
||||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
C41H67NO15
|
||||
| IsoSMILES |
CCC(=O)O[C@@H]1CC(=O)O[C@@H](C/C=C/C=C/[C@@H]([C@@H](C[C@@H]([C@@H]([C@H]1OC)O[C@H]2[C@@H]([C@H]([C@@H]([C@H](O2)C)O[C@H]3C[C@@]([C@H]([C@@H](O3)C)OC(=O)CC)(C)O)N(C)C)O)CC=O)C)O)C
|
||||
| InChI |
1S/C41H67NO15/c1-11-30(45)54-29-21-32(47)51-24(4)16-14-13-15-17-28(44)23(3)20-27(18-19-43)37(38(29)50-10)57-40-35(48)34(42(8)9)36(25(5)53-40)56-33-22-41(7,49)39(26(6)52-33)55-31(46)12-2/h13-15,17,19,23-29,33-40,44,48-49H,11-12,16,18,20-22H2,1-10H3/b14-13+,17-15+/t23-,24-,25-,26+,27+,28+,29-,33+,34-,35-,36-,37+,38+,39+,40+,41-/m1/s1
|
||||
| InChIKey |
DMUAPQTXSSNEDD-QALJCMCCSA-N
|
||||
| PubChem CID | |||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-01: Infectious/parasitic diseases
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Oleandomycin glycosyltransferase oleD (OLED) | [1] | |||
| Resistant Disease | Bacillus intestinalis infection [ICD-11: 1A00-1C4Z] | |||
| Molecule Alteration | Expression | Acquired |
||
| Experimental Note | Discovered Using In-vivo Testing Model | |||
| In Vitro Model | Bacillus intestinalis strain T30 | 1963032 | ||
| Experiment for Molecule Alteration |
SDS-PAGE analysis | |||
| Experiment for Drug Resistance |
Broth microdilution antifungal susceptibility test assay | |||
| Key Molecule: Oleandomycin glycosyltransferase oleD (OLED) | [1] | |||
| Resistant Disease | Bacillus intestinalis infection [ICD-11: 1A00-1C4Z] | |||
| Molecule Alteration | Missense mutation | p.Q327A |
||
| Experimental Note | Discovered Using In-vivo Testing Model | |||
| In Vitro Model | Bacillus intestinalis strain T30 | 1963032 | ||
| Experiment for Molecule Alteration |
SDS-PAGE analysis | |||
| Experiment for Drug Resistance |
Broth microdilution antifungal susceptibility test assay | |||
| Key Molecule: Oleandomycin glycosyltransferase oleD (OLED) | [1] | |||
| Resistant Disease | Bacillus intestinalis infection [ICD-11: 1A00-1C4Z] | |||
| Molecule Alteration | Missense mutation | p.Q327F |
||
| Experimental Note | Discovered Using In-vivo Testing Model | |||
| In Vitro Model | Bacillus intestinalis strain T30 | 1963032 | ||
| Experiment for Molecule Alteration |
SDS-PAGE analysis | |||
| Experiment for Drug Resistance |
Broth microdilution antifungal susceptibility test assay | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Oleandomycin glycosyltransferase oleD (OLED) | [1] | |||
| Resistant Disease | Staphylococcus aureus infection [ICD-11: 1B54.0] | |||
| Molecule Alteration | Expression | Acquired |
||
| Experimental Note | Discovered Using In-vivo Testing Model | |||
| In Vitro Model | Pseudomonas aeruginosa strain B-2099/18 | 287 | ||
| Experiment for Molecule Alteration |
SDS-PAGE analysis | |||
| Experiment for Drug Resistance |
Broth microdilution antifungal susceptibility test assay | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: 50S ribosomal protein L4 (RplD) | [2] | |||
| Resistant Disease | Acinetobacter meningitis [ICD-11: 1D01.1] | |||
| Molecule Alteration | Missense mutation | G214A; G215T |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | M. pneumoniae M129 | 2093 | ||
| Experiment for Molecule Alteration |
GeneSeq assay; PCR | |||
| Experiment for Drug Resistance |
Antimicrobial susceptibility assay | |||
| Mechanism Description | Since the secondary treatment choice for pediatric patients is very limited, we decided to look for potential new treatment strategies in macrolide drugs and investigate possible new mechanisms of resistance. We performed an in vitro selection of mutants resistant to five macrolides (erythromycin, roxithromycin, azithromycin, josamycin, and midecamycin) by inducing the parent M. pneumoniae strain M129 with increasing concentrations of the drugs. The evolving cultures in every passage were tested for their antimicrobial susceptibilities to eight drugs and mutations known to be associated with macrolide resistance by PCR and sequencing. The final selected mutants were also analyzed by whole-genome sequencing. Results showed that roxithromycin is the drug that most easily induces resistance (at 0.25 mg/L, with two passages, 23 days), while with midecamycin it is most difficult (at 5.12 mg/L, with seven passages, 87 days). Point mutations C2617A/T, A2063G, or A2064C in domain V of 23S rRNA were detected in mutants resistant to the 14- and 15-membered macrolides, while A2067G/C was selected for the 16-membered macrolides. Single amino acid changes (G72R, G72V) in ribosomal protein L4 emerged during the induction by midecamycin. Genome sequencing identified sequence variations in dnaK, rpoC, glpK, MPN449, and in one of the hsdS (MPN365) genes in the mutants. Mutants induced by the 14- or 15-membered macrolides were resistant to all macrolides, while those induced by the 16-membered macrolides (midecamycin and josamycin) remained susceptible to the 14- and 15-membered macrolides. In summary, these data demonstrated that midecamycin is less potent in inducing resistance than other macrolides, and the induced resistance is restrained to the 16-membered macrolides, suggesting a potential benefit of using midecamycin as a first treatment choice if the strain is susceptible. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Oleandomycin glycosyltransferase oleD (OLED) | [1] | |||
| Resistant Disease | Sepsis [ICD-11: 1G40.0] | |||
| Molecule Alteration | Expression | Acquired |
||
| Experimental Note | Discovered Using In-vivo Testing Model | |||
| In Vitro Model | T7 express cells | Lung | Mus musculus (Mouse) | CVCL_4314 |
| Experiment for Molecule Alteration |
SDS-PAGE analysis | |||
| Experiment for Drug Resistance |
Broth microdilution antifungal susceptibility test assay | |||
ICD-10: Ear/mastoid process diseasess
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Oleandomycin glycosyltransferase oleD (OLED) | [1] | |||
| Resistant Disease | Pseudomonas aeruginosa infection [ICD-11: 1A00-1C4Z] | |||
| Molecule Alteration | Expression | Acquired |
||
| Experimental Note | Discovered Using In-vivo Testing Model | |||
| In Vitro Model | Prostate cancer tissue | N.A. | ||
| Experiment for Molecule Alteration |
SDS-PAGE analysis | |||
| Experiment for Drug Resistance |
Broth microdilution antifungal susceptibility test assay | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
